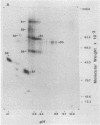
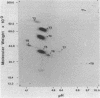
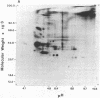
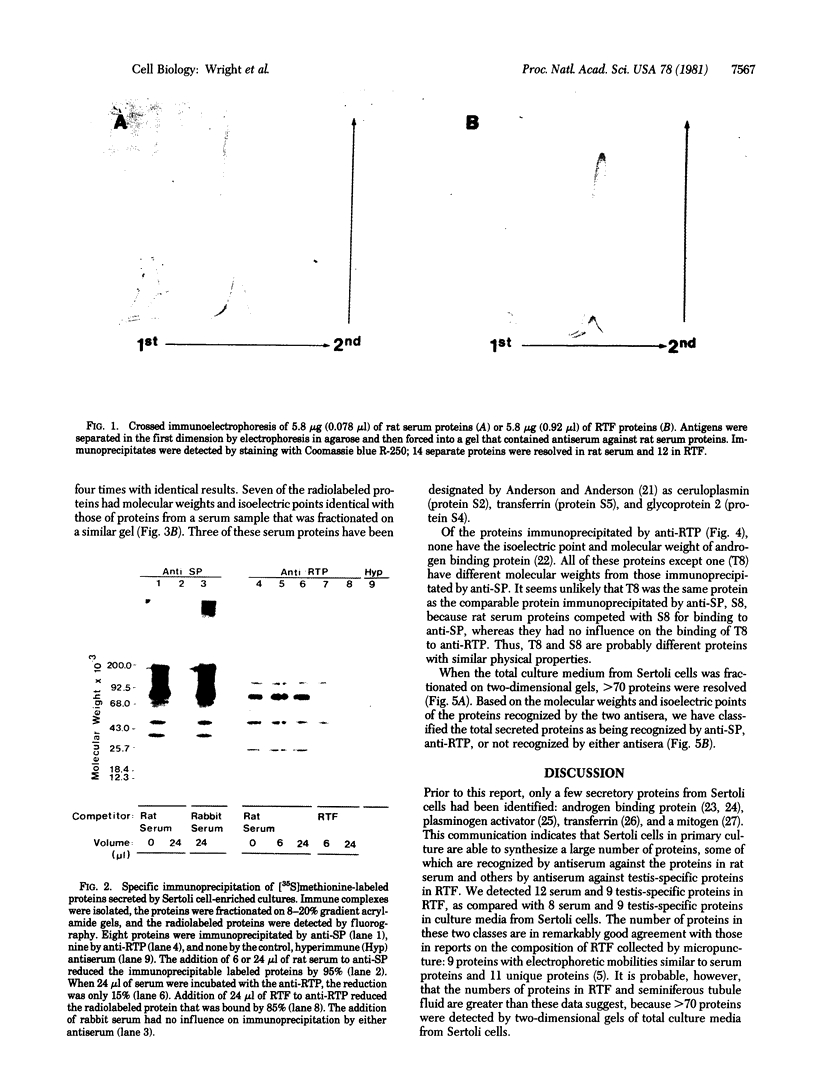
Abstract
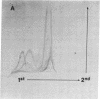
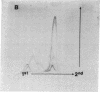
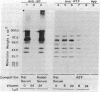
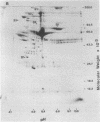
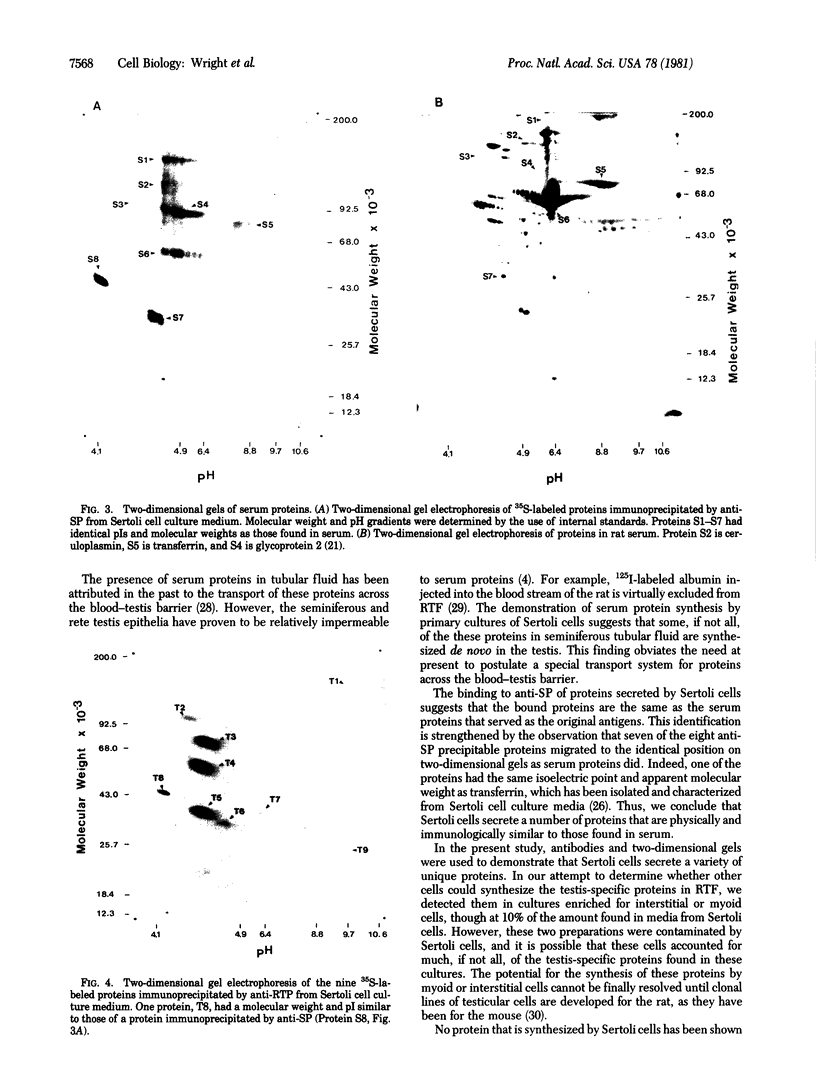
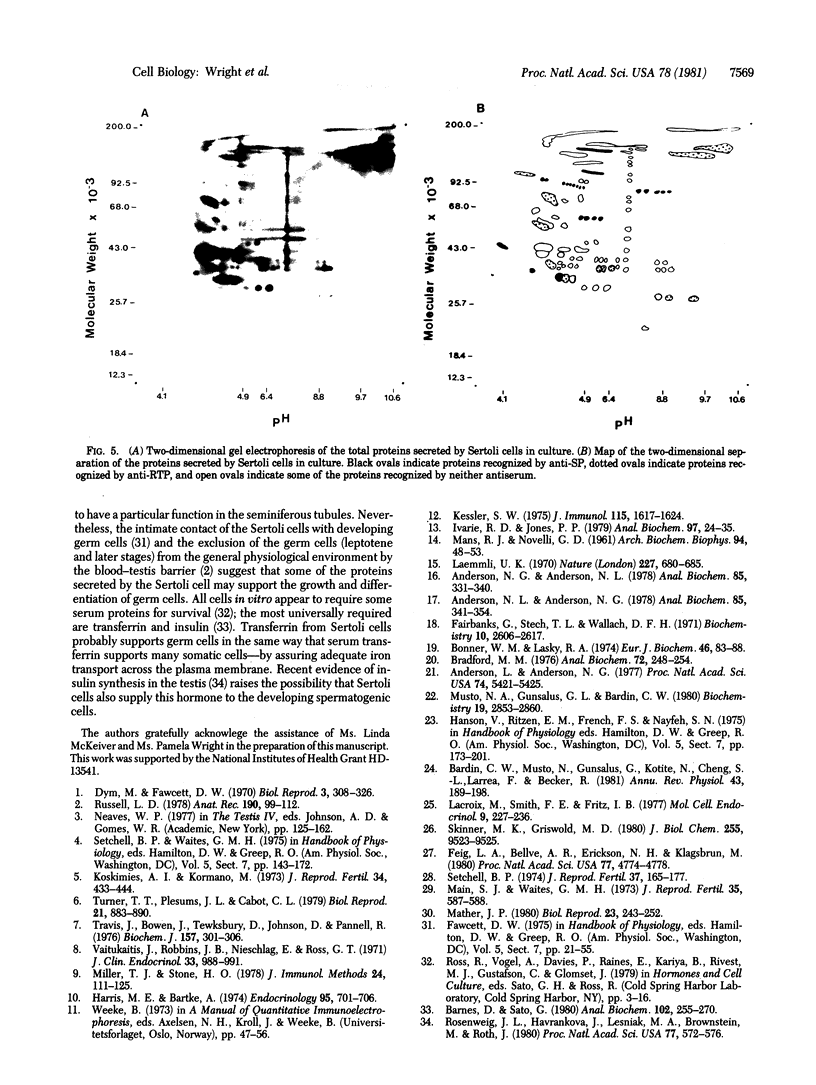
The secretions of the Sertoli cell were examined with two polyvalent antisera--one prepared against proteins in rat serum and the other against testis-specific proteins in rete testis fluid. These antisera detected 12 serum and 9 testis-specific proteins in rete testis fluid. To determine the origin of these proteins, primary cultures enriched in Sertoli cells were incubated with [35S]methionine, and the radiolabeled proteins in the medium were immunoprecipitated. Gel electrophoresis of the two immunoprecipitates resolved eight serum and nine testis-specific proteins. These two sets of proteins were specifically bound to their respective antiserum and were immunologically distinct. Medium from Sertoli cell cultures contained 10 times more of the testis-specific proteins than did cultures enriched for testicular myoid or interstitial cells. The concentration of the serum proteins in Sertoli cell medium was 5 and 10 times greater, respectively, than in myoid or interstitial cell preparations. The proteins from Sertoli cells were next characterized on two-dimensional gels. Seven of the proteins recognized by antiserum against serum proteins had identical molecular weights and isoelectric points as serum proteins. Three of these proteins were ceruloplasmin, transferrin, and glycoprotein 2. In addition to the proteins immunoprecipitated by the two antisera, more than 60 other proteins were detected on two-dimensional gels of the total secretory proteins. We conclude that the Sertoli cell secretes many proteins, some of which are specific to the testis and others of which are similar to serum proteins.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson L., Anderson N. G. High resolution two-dimensional electrophoresis of human plasma proteins. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5421–5425. doi: 10.1073/pnas.74.12.5421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson N. G., Anderson N. L. Analytical techniques for cell fractions. XXI. Two-dimensional analysis of serum and tissue proteins: multiple isoelectric focusing. Anal Biochem. 1978 Apr;85(2):331–340. doi: 10.1016/0003-2697(78)90229-4. [DOI] [PubMed] [Google Scholar]
- Anderson N. L., Anderson N. G. Analytical techniques for cell fractions. XXII. Two-dimensional analysis of serum and tissue proteins: multiple gradient-slab gel electrophoresis. Anal Biochem. 1978 Apr;85(2):341–354. doi: 10.1016/0003-2697(78)90230-0. [DOI] [PubMed] [Google Scholar]
- Bardin C. W., Musto N., Gunsalus G., Kotite N., Cheng S. L., Larrea F., Becker R. Extracellular androgen binding proteins. Annu Rev Physiol. 1981;43:189–198. doi: 10.1146/annurev.ph.43.030181.001201. [DOI] [PubMed] [Google Scholar]
- Barnes D., Sato G. Methods for growth of cultured cells in serum-free medium. Anal Biochem. 1980 Mar 1;102(2):255–270. doi: 10.1016/0003-2697(80)90151-7. [DOI] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Dym M., Fawcett D. W. The blood-testis barrier in the rat and the physiological compartmentation of the seminiferous epithelium. Biol Reprod. 1970 Dec;3(3):308–326. doi: 10.1093/biolreprod/3.3.308. [DOI] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Feig L. A., Bellvé A. R., Erickson N. H., Klagsbrun M. Sertoli cells contain a mitogenic polypeptide. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4774–4778. doi: 10.1073/pnas.77.8.4774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris M. E., Bartke A. Concentration of testosterone in testis fluid of the rat. Endocrinology. 1974 Sep;95(3):701–706. doi: 10.1210/endo-95-3-701. [DOI] [PubMed] [Google Scholar]
- Ivarie R. D., Jones P. P. A rapid sensitive assay for specific protein synthesis in cells and in cell-free translations: use of Staphylococcus aureus as an adsorbent for immune complexes. Anal Biochem. 1979 Aug;97(1):24–35. doi: 10.1016/0003-2697(79)90322-1. [DOI] [PubMed] [Google Scholar]
- Kessler S. W. Rapid isolation of antigens from cells with a staphylococcal protein A-antibody adsorbent: parameters of the interaction of antibody-antigen complexes with protein A. J Immunol. 1975 Dec;115(6):1617–1624. [PubMed] [Google Scholar]
- Koskimies A. I., Kormano M. The proteins in fluids from the seminiferous tubules and rete testis of the rat. J Reprod Fertil. 1973 Sep;34(3):433–434. doi: 10.1530/jrf.0.0340433. [DOI] [PubMed] [Google Scholar]
- Lacroix M., Smith F. E., Fritz I. B. Secretion of plasminogen activator by Sertoli cell enriched cultures. Mol Cell Endocrinol. 1977 Dec;9(2):227–236. doi: 10.1016/0303-7207(77)90124-1. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Main S. J., Waites G. M. Proceedings: Studies on some effects of temperature on the blood-testis barrier in rats. J Reprod Fertil. 1973 Dec;35(3):587–588. doi: 10.1530/jrf.0.0350587. [DOI] [PubMed] [Google Scholar]
- Mather J. P. Establishment and characterization of two distinct mouse testicular epithelial cell lines. Biol Reprod. 1980 Aug;23(1):243–252. doi: 10.1095/biolreprod23.1.243. [DOI] [PubMed] [Google Scholar]
- Miller T. J., Stone H. O. The rapid isolation of ribonuclease-free immunoglobulin G by protein A-sepharose affinity chromatography. J Immunol Methods. 1978;24(1-2):111–125. doi: 10.1016/0022-1759(78)90092-3. [DOI] [PubMed] [Google Scholar]
- Musto N. a., Gunsalus G. L., Bardin C. W. Purification and characterization of androgen binding protein from the rat epididymis. Biochemistry. 1980 Jun 24;19(13):2853–2860. doi: 10.1021/bi00554a006. [DOI] [PubMed] [Google Scholar]
- Rosenzweig J. L., Havrankova J., Lesniak M. A., Brownstein M., Roth J. Insulin is ubiquitous in extrapancreatic tissues of rats and humans. Proc Natl Acad Sci U S A. 1980 Jan;77(1):572–576. doi: 10.1073/pnas.77.1.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell L. D. The blood-testis barrier and its formation relative to spermatocyte maturation in the adult rat: a lanthanum tracer study. Anat Rec. 1978 Jan;190(1):99–111. doi: 10.1002/ar.1091900109. [DOI] [PubMed] [Google Scholar]
- Setchell B. P. Secretions of the testis and epididymis. J Reprod Fertil. 1974 Mar;37(1):165–177. doi: 10.1530/jrf.0.0370165. [DOI] [PubMed] [Google Scholar]
- Skinner M. K., Griswold M. D. Sertoli cells synthesize and secrete transferrin-like protein. J Biol Chem. 1980 Oct 25;255(20):9523–9525. [PubMed] [Google Scholar]
- Travis J., Bowen J., Tewksbury D., Johnson D., Pannell R. Isolation of albumin from whole human plasma and fractionation of albumin-depleted plasma. Biochem J. 1976 Aug 1;157(2):301–306. doi: 10.1042/bj1570301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner T. T., Plesums J. L., Cabot C. L. Luminal fluid proteins of the male rat reproductive tract. Biol Reprod. 1979 Nov;21(4):883–890. doi: 10.1095/biolreprod21.4.883. [DOI] [PubMed] [Google Scholar]
- Vaitukaitis J., Robbins J. B., Nieschlag E., Ross G. T. A method for producing specific antisera with small doses of immunogen. J Clin Endocrinol Metab. 1971 Dec;33(6):988–991. doi: 10.1210/jcem-33-6-988. [DOI] [PubMed] [Google Scholar]