Abstract
Background
Schizophrenia is characterized by deficits in executive control and impairments in emotion processing. This study assessed the nature and extent of potential alterations in the neural substrates supporting the interaction between cognitive control mechanisms and emotion attribution processes in people with schizophrenia.
Methods
Functional magnetic resonance imaging was performed during a verbal emotional go/no-go task. People with schizophrenia and healthy controls responded to word stimuli of a prespecified emotional valence (positive, negative or neutral) while inhibiting responses to stimuli of a different valence.
Results
We enrolled 20 people with schizophrenia and 23 controls in the study. Healthy controls activated an extensive dorsal prefrontal–parietal network while inhibiting responses to negative words compared to neutral words, but showed deactivation of the midcingulate cortex while inhibiting responses to positive words compared to neutral words. People with schizophrenia failed to activate this network during response inhibition to negative words, whereas during response inhibition to positive words they did not deactivate the cingulate, but showed increased responsivity in the frontal cortex.
Limitations
Sample heterogeneity is characteristic of studies of schizophrenia and may have contributed to more variable neural responses in the patient sample despite the care taken to control for potentially confounding variables.
Conclusion
Our results showed that schizophrenia is associated with aberrant modulation of neural responses during the interaction between cognitive control and emotion processing. Failure of the frontal circuitry to regulate goal-directed behaviour based on emotion attributions may contribute to deficits in psychosocial functioning in daily life.
Introduction
Schizophrenia is characterized by a diverse set of symptoms, including positive symptoms (e.g., hallucinations, delusions) and negative symptoms (e.g., lack of initiative, avolition). In addition, cognitive impairments are now considered to be a core feature of the illness, frequently manifesting before the onset of other symptoms.1 Although a generalized neuropsychological deficit is often recognized,2 poor performance is most apparent in executive control processes, such as response inhibition and working memory.3,4 These processes have been particularly linked to anterior cingulate and dorsolateral prefrontal cortex (DLPFC) function.5,6 A large number of neuroimaging studies have reported aberrant prefrontal activation in people with schizophrenia, although the exact nature of the deficit remains a subject of debate.7–12
There is also an abundance of evidence of emotional processing abnormalities in people with schizophrenia,13 with impairments shown during emotion recognition,14 emotional memory15 and the experience of affective states.16,17 For instance, a large number of studies have reported evidence of dysfunction in the recognition of facial expressions in people with schizophrenia, especially for negative emotions18,19 (for a recent review, see Morris and colleagues20). Some evidence also suggests that people with schizophrenia have similar difficulties when producing and interpreting the emotional content of linguistic material,21,22 which again suggests more impairment for negative material.23 There is evidence of reduced activation in brain regions associated with emotional processing, such as the orbitofrontal and cingulate cortices, amygdala and insula, during emotion processing in people with schizophrenia.24
Interestingly, cognitive control processes supported by the dorsal prefrontal cortex also contribute to the interpretation, regulation and modulation of emotions, as well as the translation of emotionally charged motivational states into goal-directed behaviour.25 That is, the processes of cognition and emotion have been shown to be tightly linked and interactive. In fact, emotion processing in ventral (para)limbic brain regions and goal-directed response selection guided by dorsal executive brain areas are thought to comprise reciprocally interactive neural systems.26–29 This hypothesis derives from the observation that stimuli that engage dorsal prefrontal cognitive control circuits simultaneously induce deactivation or suppression of limbic regions and ventral prefrontal regions, whereas activation of limbic regions by affective stimuli appears to suppress activity in dorsal prefrontal regions.30 In terms of emotion–cognition interaction processes, a recent study31 suggested that people with schizophrenia are predominantly impaired in cognitive control and the maintenance of emotional representations rather than in the evaluation of the emotional content itself. Given the evidence of prefrontal dysfunction in people with schizophrenia, the prefrontal cortex’s role in cognitive control and the suggestion of cognition–emotion interaction deficits, the present study aims to further delineate potential impairments in the neural substrates subserving the interactive processes of emotion and cognitive control by incorporating the attribution of emotional content and cognitive control demands in a single task. In the emotional go/no-go task used in the present study, response selection is guided by emotional content of the stimuli: it requires participants to withhold responses to emotional words of a particular valence (positive or negative) while responding to neutral stimuli and vice versa. This design allows us to test the hypothesis that people with schizophrenia will display abnormal prefrontal activation when top–down cognitive control processes are required to guide task-relevant behaviour based on the subjective attribution of emotional content.
Previous research in healthy individuals has shown that the emotional go/no-go task activates cognitive control areas, such as the dosolateral prefrontal regions, dorsal cingulate and inferior parietal cortex, as well as ventral areas, such as the inferior frontal and orbitofrontal cortices. We hypothesized deficits in the neural circuitry underlying cognitive control during emotion inhibition in people with schizophrenia and expected to find aberrant activity in the cortical regions encompassing the dorsal executive control system. As has been noted in healthy participants,32 this verbal paradigm is unlikely to elicit intense affective feeling states in the same way that emotionally evocative scenes or emotional face expressions tend to do. Hence we anticipated that we may not find very robust activation of affect-related (para)limbic areas. However, ventral and medial regions of the prefrontal cortex, including the inferior and medial orbitofrontal cortices, have been linked to emotion regulation33 and are likely to be involved in the interaction between emotion and cognition. Inadequate modulation of these regions is also expected in people with schizophrenia.
Methods
Participants
We recruited right-handed adults with a DSM-IV diagnosis of schizophrenia or schizoaffective disorder by distributing leaflets within the local area health service and via a national TV segment. We also recruited healthy controls without a personal or family history of mental illness matched for age, sex and handedness via advertisements in local newspapers and on notice boards. All potential participants were initially screened against exclusion criteria using a standardized telephone interview, and patient diagnosis was confirmed using a Structured Clinical Interview for DSM-IV-TR Axis I Disorders (SCID).34 We excluded all individuals with a concurrent Axis I diagnosis, a history of recent (≤ 5 yr) substance abuse, uncontrolled diabetes or hypertension, or head injury with loss of consciousness. Current symptom severity in people with schizophrenia was assessed via a structured interview during which a trained neuropsychologist or research assistant administered the Positive and Negative Syndrome Scale (PANSS).35 All participants were assessed with the Wechsler Test of Adult Reading (WTAR)36 to obtain an estimate of premorbid intellectual functioning and with a 4-subtest version of the Wechsler Adult Intelligence Scale, 3rd edition, (WAIS-III)37 to assess current intellectual functioning. In addition, all participants completed the Edinburgh Handedness Inventory (EHI),38 and 2 self-report questionnaires: one assessing general health and well-being (SF-36v2)39 and one assessing quality of life (Schizophrenia Quality of Life Scale; SQLS).40 Written informed consent was obtained from all participants. Ability to give informed consent was assessed independently by the patient’s own primary care physician and during a routine medical examination conducted by one of the study physicians (R.L. or another psychiatrist). We assessed IQ to ensure absence of intellectual disability that would compromise ability to provide informed consent. All procedures were approved by the University of New South Wales and the South East Sydney and Illawarra Area Health Service Human Research Ethic Committees.
Procedures
Emotional stimulus ratings
The words used in the emotional go/no-go task were selected from the Affective Norms for English Words41 stimulus set, which provides normative valence and arousal ratings. We selected 40 neutral, 20 positive and 20 negative words from the normative lists and matched them for length and frequency, with positive and negative words also matched for arousal. Prior to scanning, participants rated the word stimuli as positive, negative or neutral using a tick box questionnaire format.
Emotional go/no-go task
All participants received detailed verbal instructions before the functional magnetic resonance imaging (fMRI) scan, and the experimenter (A.V. or another experimenter) verified that the participant had an adequate comprehension of the procedure. The emotional go/no-go task included 4 conditions:
attend negative (responding to negative words while inhibiting responses to neutral ones),
inhibit negative (responding to neutral words while in-hibiting responses to negative ones),
attend positive (responding to positive words while inhibiting responses to neutral ones), and
inhibit positive (responding to neutral words while inhibiting responses to positive ones).
“Negative target:neutral distracter” and “neutral target: negative distracter” conditions (i.e., conditions 1 and 2, respectively) were identical with respect to stimuli; likewise for the “positive target:neutral distracter” and “neutral target: positive distracter” conditions (i.e., conditions 3 and 4, respectively). For the fMRI scan, participants were instructed to respond as quickly as possible to stimuli of the specified valence (targets), but to ignore stimuli of other valences (distracters). At the start of each condition, the words “neutral,” “positive” or “negative” appeared in capital letters to indicate which valence the participant was to attend to. Each trial consisted of a fixation cross for 300 ms, a word stimulus presented in the centre of the screen for 300 ms and a 900 ms response interval. The 4 conditions were presented in pseudo-randomized order in a block design. Each block consisted of 10 stimulus presentations, and each condition was presented 4 times, for a total of 160 stimulus presentations. There were 30 seconds of fixation to a crosshair at the initiation and conclusion of the task. Stimuli were presented on a computer screen that participants viewed in a mirror, and responses were recorded using a fibre optic response pad (Lumina Systems) that collected accuracy (percent correct) and reaction time (ms) measures.
Functional MRI
Magnetic resonance imaging was performed using a 3-T Phillips Achieva MRI scanner at Neuroscience Research Australia, Randwick, Australia. We obtained a T1-weighted, high-resolution anatomical scan for each participant for registration purposes and to screen for anatomical abnormalities (repetition time [TR] 5.4 ms, echo time [TE] 2.4 ms, field of view [FOV] 256 mm, matrix 256 × 256, sagittal plane, slice thickness 1 mm, 180 slices). Functional T2*-weighted images were obtained using a gradient echo-planar imaging sequence (TR 3000 ms, TE 30 m, 32 interleaved slices, covering the whole brain, 3 mm thickness, 1 mm gap, voxel size 3 × 3 × 3 mm, 212 scan repetitions, flip angle 90°, FOV 24 cm).
Data analysis
Emotional stimulus ratings
The collection of individual subjective stimulus ratings allowed us to, first, assess whether their ratings deviated from the published normative valences, and second, assess whether there were group differences in subjective ratings of the affective stimuli between people with schizophrenia and our healthy sample. We performed repeated-measures analysis of variance (ANOVA) to assess the accuracy of the valence ratings (concordance between participants’ stimulus classification compared with the published normative valence) with group as a between-subjects variable and valence as a within-subjects variable.
Emotional go/no-go task
As we obtained subjective stimulus ratings for each participant, this allowed us to take into account individual differences in stimulus ratings in the analysis of the behavioural data acquired during fMRI scanning. Thus, for analyses of task performance in the fMRI environment, each participant’s responses were scored based on their individual ratings of the stimuli. For instance, when a normative neutral stimulus was rated as negative by a particular participant, a button press response following that stimulus during the fMRI task was scored as correct on a negative block and as an error on a neutral or positive block. We performed repeated-measures ANOVAs on the mean percentage correct and on the average reaction times on go trials, with group as the between-subjects variable and task condition (attend negative, inhibit negative, attend positive, inhibit positive) as the within-subjects variable. To assess group differences in failures to inhibit responses, we also conducted a repeated-measures ANOVA on the number of false alarm errors, with group as a between-subjects variable and task condition as a within-subjects variable.
Functional MRI
Preprocessing and statistical analyses were conducted with SPM5 (Wellcome Trust Centre for Neuroimaging) running under MATLAB version 2006b. We realigned functional images to the first image in the time series. Four dummy scans were obtained before each fMRI data acquisition to allow the equilibration of the MRI signal. Functional images were then coregistered with the anatomical image. The images were manually reoriented to optimize the normalization process and normalized to the Montreal Neurological Institute (MNI) anatomical template using a nonlinear 12-parameter affine transformation. Images were smoothed with a 10-mm full-width at half-maximum Gaussian kernel. All data sets were screened for excessive motion artifacts and successful normalization.
At the first level of analysis, we performed contrast analyses for each participant to assess the magnitude of difference in blood oxygen level–dependent (BOLD) signal between conditions of interest. Two contrasts were defined: condition 2 (attend neutral:inhibit negative) minus condition 1 (attend negative:inhibit neutral) and condition 4 (attend neutral: inhibit positive) minus condition 3 (attend positive:inhibit neutral) to arrive at the relative activation during inhibition of negative and positive stimuli, respectively. At the second level, we conducted a whole-brain analysis using within- and between-groups t tests on these contrasts to assess task-related and diagnostic differences in brain activation. To correct for false-positive results, we used the double-threshold approach advocated by Forman and colleagues,42 imposing both an activation threshold and a cluster size threshold. Unless otherwise indicated, activation clusters were considered significant if they reached or exceeded a threshold of p = 0.001, uncorrected, with an extent greater than 18 voxels corresponding to a whole brain correction of p < 0.05, corrected (www2.bc.edu/~slotnics/scripts.htm). As we observed an overall poorer performance among people with schizophrenia compared with healthy controls for between-group comparisons, behavioural performance (defined as mean percentage correct on the relevant task conditions) was added as a nuisance covariate. In addition, we included the WAIS-III IQ, education level and sex as nuisance covariates because the groups differed on these variables and because general cognitive ability is thought to contribute to task performance and brain activation differences.
Results
Participants
We initially recruited 31 right-handed adults with schizophrenia or schizoaffective disorder and 29 controls for participation in the study. Data were excluded owing to movement or other MRI artifacts (9 patients and 3 controls) or the incidental finding of gross abnormalities on the structural MRI (2 patients and 1 control). Two additional controls were excluded from subsequent analyses owing to a history of depression or dyslexia. Thus, the total number of participants whose data were analyzed in this study was 20 people with schizophrenia (15 men) and 23 controls (11 men). The people with schizophrenia had a mean age of 34.4 (standard deviation [SD] 7.8) years, a mean age at illness onset of 22.0 (SD 3.0) years and a mean duration of illness of 12.0 (SD 7.2) years. The controls had a mean age of 33.3 (SD 7.1) years. All people with schizophrenia were taking antipsychotic medication for at least 1 year before participation.
Demographic, neuropsychological and symptoms measures
A summary of the demographic, clinical and neuropsychological characteristics of the sample is presented in Table 1. A χ2 test revealed a trend-level difference in sex distribution between the groups. The groups did not differ in terms of mean age. As expected, healthy controls had a significantly higher education level than people with schizophrenia and a significantly higher estimated full-scale IQ. Estimated premorbid intellectual functioning, as assessed by the WTAR, was also significantly higher in the healthy controls than participants with schizophrenia, although the patients’ mean WTAR score was within the normal range. The discrepancy between WAIS-III and WTAR scores in people with schizophrenia is indicative of some illness-related impairment in current intellectual functioning.3 As expected, compared with controls, people with schizophrenia reported a lower level of general health based on the SF-36v2, and a lower quality of life indicated by higher scores on the motivation, symptoms of mental illness and psychosocial skills subscales of the SQLS. The PANSS scores for people with schizophrenia were mild to moderately severe (Table 1).
Table 1.
Demographic, neuropsychological and clinical characteristics of healthy controls and patients with schizophrenia
| Group; mean (SD)* | ||||
|---|---|---|---|---|
|
|
||||
| Characteristic | Control, n = 23 | Schizophrenia,† n = 20 | Statistic | p value |
| Age, yr | 33.3 (7.1) | 34.4 (7.8) | t41 = −0.5 | 0.65 |
| Sex, no. male:female | 11:12 | 15:5 | χ21 = 3.3 | 0.07 |
| Education level, yr | 15.7 (1.8) | 13.3 (2.6) | t41 = 3.5 | 0.001 |
| WAIS-III IQ score | 113.6 (15.4) | 91.7 (13.6) | t41 = 5.1 | < 0.001 |
| WTAR score | 112.1 (4.8) | 105.4 (7.6) | t41 = 3.5 | 0.001 |
| SF-36 total score | 141.5 (8.1) | 111.9 (17.7) | t41 = 7.18 | < 0.001 |
| SQLS score | ||||
| Motivation | 7.1 (2.5) | 13.0 (3.0) | t41 = −6.9 | < 0.001 |
| Symptoms | 3.4 (3.4) | 8.3 (3.8) | t41 = −4.4 | < 0.001 |
| Psychosocial | 14.8 (7.4) | 27.3 (9.2) | t41 = −4.9 | < 0.001 |
| Total | 25.3 (9.8) | 48.5 (12.6) | t41 = −6.4 | < 0.001 |
| PANSS score | ||||
| Positive | 15.9 (5.9) | |||
| Negative | 16.1 (7.5) | |||
| General | 35.4 (11.6) | |||
| Total | 67.4 (21.8) | |||
PANSS = Positive and Negative Syndrome Scale;35 SD = standard deviation; SF-36v2 = short-form health survey;39 SQLS = Schizophrenia Quality of Life Scale;40 WAIS-III = Wechsler Adult Intelligence Scale 3rd ed.;37 WTAR = Wechsler Test of Adult Reading.36
Unless otherwise indicated.
The schizophrenia group comprised patients with the following diagnoses: schizoaffective disorder (n = 4; depressed subtype [n = 2], bipolar subtype [n = 2]) and schizophrenia (n = 16; paranoid subtype [n = 10], disorganized subtype [n = 2], residual subtype [n = 1], undifferentiated subtype [n = 3]). Patients were on the following antipsychotic medications: clozapine (n = 9), clozapine + aripiprazole (n = 1), olanzapine (n = 4), aripiprazole (n = 1), risperidone (n = 2), quetiapine + zuclopenthixol (n = 1), quetiapine + ziprasidone (n = 1) and amisulpride (n = 1).
Behavioural measures
Emotional stimulus ratings
Word rating data were missing for 1 participant. Mean accuracy of valence ratings (i.e., concordance rates with the normative valence) for healthy controls was 90% for negative words, 81% for positive words and 53% for neutral words. In people with schizophrenia, mean accuracy for valence ratings was 78% for negative words, 72% for positive words and 51% for neutral words. We observed a significant main effect of valence (F2,80 = 29.02, p < 0.001), indicating that accurate perception of valence was highest in the negative, intermediate in the positive and lowest in the neutral stimuli in both groups (see Appendix 1, available at cma.ca/jpn). There was a non-significant trend toward reduced concordance with normative ratings in the schizophrenia group (F1,40 = 3.52, p = 0.07). There was no significant group × valence interaction.
Emotional go/no-go task performance
Reaction times
Performance measures of participants during the scan are presented in Table 2. For reaction times to go stimuli, the ANOVA revealed a significant main effect of group, indicating slower go responses in people with schizophrenia (F1,41 = 8.7, p = 0.005). The significant main effect of condition indicated slower reaction times on conditions requiring inhibition of responses to positive or negative stimuli in both groups (F3,123 = 22.8, p < 0.001). We also found a significant group × condition interaction (F1,41 = 3.1, p = 0.027). This was driven by a greater slowing of reaction times when responding to neutral stimuli in the context of negative rather than positive distracters in controls. This differential context effect was not observed in people with schizophrenia.
Table 2.
Performance measures of the emotional go/no-go task during fMRI scanning of healthy controls and patients with schizophrenia
| Group; task condition; mean (SD) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
||||||||||
| Control | Schizophrenia | |||||||||
|
|
|
|||||||||
| Measure | Attend neg, inhibit neu | Inhibit neg | Attend pos, inhibit neu | Inhibit pos | Grand mean | Attend neg, inhibit neu | Inhibit neg | Attend pos, inhibit neu | Inhibit pos | Grand mean |
| Reaction time on go trials, ms | 765 (108) | 942 (163) | 740 (100) | 869 (133) | 829 | 886 (130) | 980 (203) | 905 (166) | 990 (192) | 940 |
| Accuracy, % | 87 (13) | 82 (10) | 86 (10) | 73 (13) | 82 | 77 (17) | 69 (19) | 78 (14) | 64 (13) | 72 |
| No. of false alarms | 5 (5.3) | 4 (3.0) | 3 (2.9) | 6 (5.3) | 4.5 | 7 (6.3) | 6 (4.3) | 4 (3.2) | 8 (4.9) | 6.25 |
fMRI = functional magnetic resonance imaging; neg = negative; neu = neutral; pos = positive; SD = standard deviation.
Accuracy
For the mean percentage correct, there was a significant main effect of group, indicating that people with schizophrenia were generally less accurate than healthy controls (F1,41 = 10.6, p = 0.002). The main effect of condition was also significant, indicating that across participant groups, participants were less accurate when asked to inhibit responses to positive or negative stimuli relative to the conditions requiring inhibition of responses to neutral stimuli (F3,123 = 19.5, p < 0.001). The group × condition interaction was not significant, indicating that the 2 groups showed similar response patterns over the 4 conditions. Regarding false alarm errors, there was a main effect of condition (F3,123 = 6.1, p < 0.001). False alarm errors were rare in the attend positive condition compared with the other conditions. There was a main effect of group: patients generally made more false alarm errors (F1,41 = 4.8, p = 0.035). However, the group × condition interaction was not significant (F < 1), indicating a similar error pattern across groups.
Functional MRI measures
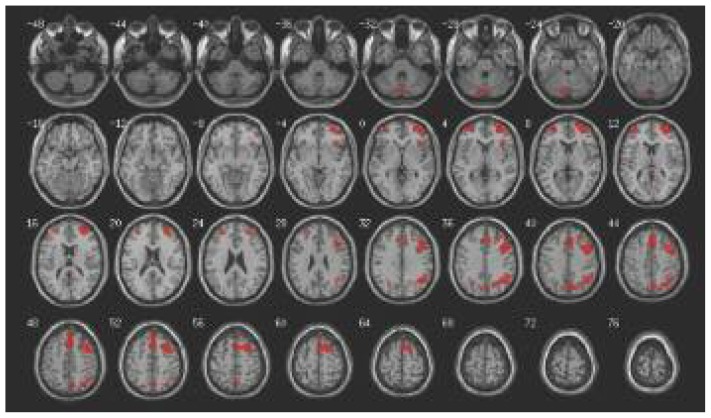
Table 3 lists the brain areas activated for each of the contrasts of interest within each diagnostic group. The single sample t test in healthy controls revealed increased activation in a prefrontal–parietal network when they were asked to inhibit responses to negative stimuli compared with neutral ones (Fig. 1). The same contrast revealed no significant clusters in people with schizophrenia. The opposite contrast, examining the inhibition of responses to neutral stimuli compared with negative ones, revealed no regions of activation at the p = 0.001 level in either group. Healthy controls did not show increased activation when inhibiting responses to positive stimuli compared with neutral ones at p = 0.001, whereas people with schizophrenia showed a small cluster of increased activation in the right middle frontal gyrus. Increased activation during inhibition of responses to neutral stimuli compared with positive ones was observed in the mid anterior cingulate and a region in the left posterior cerebellum in healthy participants, but no significant clusters were evident in people with schizophrenia.
Table 3.
Regions of activation on comparison of responses to stimuli in healthy controls and patients with schizophrenia
| Comparison; group | p value, uncorrected | Peak MNI coordinate | Brain region | Brodmann area | t value | Voxels | ||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| x | y | z | ||||||
| Inhibit negative > inhibit neutral | ||||||||
| Schizophrenia | < 0.001 | No suprathreshold voxels | ||||||
| Control | < 0.001 | −4 | −82 | −30 | Left posterior lobe cerebellum | 6.52 | 334 | |
| 36 | −82 | −18 | Right occipital lobe–fusiform gyrus | 19 | 4.07 | 20 | ||
| 36 | 6 | 52 | Right middle frontal | 6.36 | 4909 | |||
| −30 | 28 | −6 | Left inferior frontal gyrus | 4.35 | 45 | |||
| 38 | 22 | 2 | Right interior frontal gyrus–insula | 47–13 | 4.87 | 179 | ||
| −38 | 56 | 4 | Left middle to superior frontal | 10 | 5.57 | 488 | ||
| −46 | 44 | 20 | Left middle frontal | 46 | 4.24 | 66 | ||
| 2 | −40 | 14 | Right posterior cingulate | 4.36 | 63 | |||
| 26 | −66 | 38 | Right superior occipital | 39–40 | 5.40 | 1166 | ||
| −26 | −66 | 34 | Left precuneus | 4.50 | 74 | |||
| 8 | −74 | 44 | Right precuneus | 5.03 | 532 | |||
| Inhibit negative < inhibit neutral | ||||||||
| Schizophrenia | < 0.001 | No suprathreshold voxels | ||||||
| Control | < 0.001 | No suprathreshold voxels | ||||||
| Inhibit positive > inhibit neutral | ||||||||
| Schizophrenia | < 0.001 | 28 | 56 | 4 | Right middle frontal | 10 | 4.46 | 61 |
| Control | < 0.001 | No suprathreshold voxels | ||||||
| Inhibit positive < inhibit neutral | ||||||||
| Schizophrenia | < 0.001 | No suprathreshold voxels | ||||||
| Control | < 0.001 | −8 | −72 | −16 | Left posterior lobe cerebellum | 4.90 | 252 | |
| < 0.001 | 12 | 2 | 34 | Right middle cingulate gyrus | 4.85 | 38 | ||
MNI = Montreal Neurological Institute.
Fig. 1.
Areas of increased activation during the inhibition of responses to negative words compared with neutral words in healthy controls, p = 0.001, uncorrected, voxel extent = 18, shown on transverse sections.
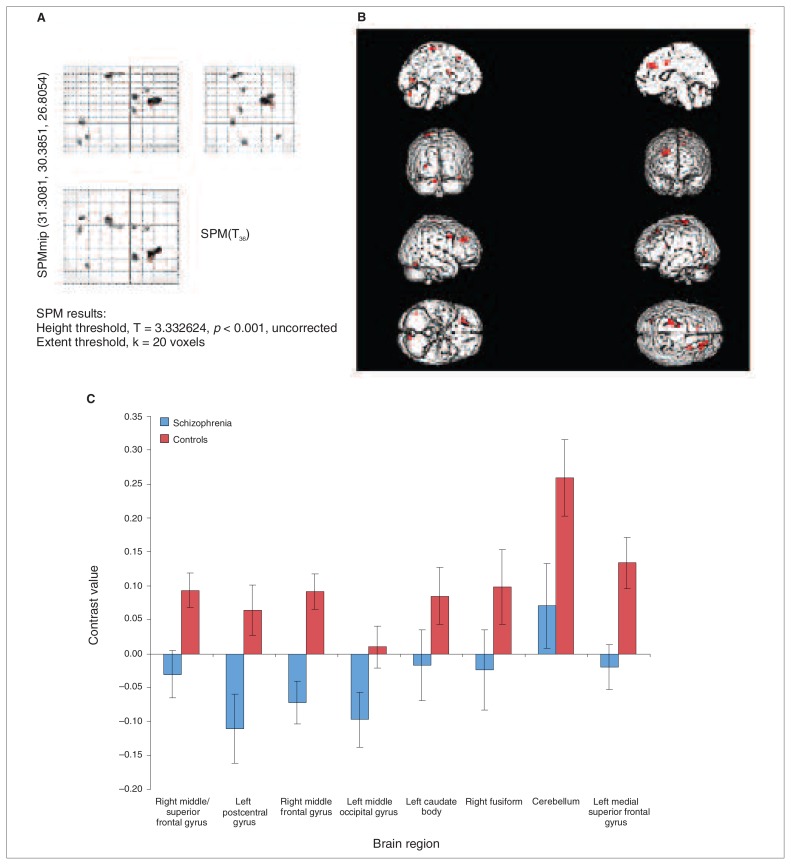
To examine the activation differences between the groups at the whole-brain level, we performed 2-sample t tests, which showed increased activation in controls compared with people with schizophrenia in a predominantly dorsal prefrontal and superior frontal network that also included some clusters in the occipitotemporal area (fusiform gyrus), the caudate and cerebellum during the inhibition of responses to negative stimuli versus neutral stimuli (Fig. 2 and Table 4). There were no significant between-group differences for the inhibit positive versus inhibit neutral comparison.
Fig. 2.
Whole brain analysis of between group differences, p = 0.001, uncorrected, voxel extent = 18. The figure shows a glass brain (panel A) and a rendered image (panel B), demonstrating the increased activation in healthy controls compared with people with schizophrenia during response inhibition to negative compared with neutral words. Parameter estimates for the contrast of interest at each of the significantly activated cluster peaks are provided in panel C.
Table 4.
Between-group comparisons showing areas of significant difference between healthy controls and patients with schizophrenia in response to specific stimuli
| Comparison; group comparison | Peak MNI coordinate | Brain region | Brodmann area | t value | Voxels | ||
|---|---|---|---|---|---|---|---|
|
| |||||||
| x | y | z | |||||
| Inhibit negative > inhibit neutral | |||||||
| Control > schizophrenia | 26 | 30 | 32 | Right mid/superior frontal gyrus | 9 | 4.54 | 255 |
| −24 | −32 | 70 | Left postcentral gyrus | 4.01 | 108 | ||
| 26 | 8 | 38 | Right middle frontal gyrus | 3.97 | 87 | ||
| −26 | −74 | 0 | Left middle occipital gyrus | 3.69 | 49 | ||
| −12 | 6 | 18 | Left caudate body | 3.57 | 39 | ||
| 42 | −66 | −20 | Right fusiform gyrus | 19 | 3.54 | 26 | |
| −10 | −76 | −28 | Left cerebellum crus posterior | 3.52 | 49 | ||
| −10 | 26 | 48 | Left medial superior frontal gyrus | 3.49 | 29 | ||
| Schizophrenia > control | No suprathreshold voxels | ||||||
| Inhibit positive > inhibit neutral | |||||||
| Control > schizophrenia | No suprathreshold voxels | ||||||
| Schizophrenia > control | No suprathreshold voxels | ||||||
MNI = Montreal Neurological Institute.
Discussion
The primary finding of our study was that, compared with healthy controls, people with schizophrenia showed significantly attenuated neural responsiveness in the dorsal frontal cortex, occipitotemporal regions and caudate body during go/no-go conditions requiring the inhibition of responses to negative words compared with neutral words. In controls, inhibiting responses to negative words elicited increased activation in a network of prefrontal, cingulate and parietal cortices, but inhibiting responses to positive words did not. The latter condition induced less activation in the middle cingulate cortex compared with response inhibition to neutral words. In contrast, people with schizophrenia exhibited no increased activation during response inhibition to negative words, but did show hyperactivation in an anterior section of the DLPFC during response inhibition to positive words. These findings suggest that schizophrenia is associated with abnormal modulation of the neural response in dorsal regions during inhibition processes associated with negative material and more anterior regions during inhibition in association with positive material. Aberrant function of the neural substrates of emotion–cognition interaction could have important consequences for adaptive behaviour, especially in psychosocial functioning.
In healthy people, inhibiting responses to negative stimuli results in increased brain responses in a fronto–temporo–parietal network corresponding to the circuitry typically activated during inhibitory control.43 These findings imply that both dorsal and ventral frontal brain regions, which are associated with cognitive control and emotion regulation, respectively, are involved during emotional go/no-go task performance, as found in a previous study using a similar verbal paradigm in healthy individuals.32 Thus, the presence of negative emotional distracters requires additional resources of the cognitive control and emotion regulation network in healthy controls.44,45 Interestingly, a recent study46 observed enhanced recruitment of a similar network of brain regions during attempts at suppressing recollection of negative versus neutral learned word pairs. This suggests that these neural resources are increasingly tapped into when negative emotional valence increases processing demands. In comparison, as evidenced by the contrast values (Fig. 2), people with schizophrenia showed attenuated effects of negative distracters and failed to modulate activation in these brain regions, showing limited or no change in activation when inhibiting responses to negative versus neutral stimuli in most regions, which is in accordance with previous observations of hypoactivation in monitoring/control and emotion regulation regions during negative emotion–cognition interaction.47 In a subset of areas, including the right DLPFC, people with schizophrenia showed an opposite brain response compared to controls, with increased activation during response inhibition to neutral stimuli versus response inhibition to negative stimuli. Other studies have similarly observed stronger prefrontal activation to neutral situations in patients with schizophrenia.48 One additional consideration is that the differential response in patients could in part result from changes in the evaluation of the emotional stimuli themselves (i.e., boundaries between perceived stimulus valences may be relatively indistinct in people with schizophrenia), which was suggested by a greater divergence from the normative ratings in patients (Appendix 1, available at cma.ca/jpn).
When response inhibition was guided by positive affective content, healthy controls did not activate the response inhibition network as they did during inhibition of responses to negative words. Careful stimulus selection based on published norms ensured that emotional stimuli were matched for word frequency, and the fact that we took into account individual stimulus ratings argues against an explanation in terms of stimulus selection bias. Our results suggest that processing subjectively negative and positive material poses inherently differential processing requirements on cognitive control mechanisms.49 In fact, healthy controls showed a reduction in middle cingulate activation during response inhibition to positive stimuli. Interestingly, the cingulate cortex is concerned with attention orienting, conflict monitoring and response selection.5,50 The fact that the cingulate (albeit more posteriorly) was more engaged during response inhibition to negative emotional content than neutral content and less engaged during response inhibition to positive emotional content suggests that negative stimuli have inherent attention-grabbing properties.51 They may be more likely to prompt target responses and require additional conflict monitoring. Our data support previous observations that positive and negative emotions may induce opposite activation patterns in the (right) cingulate cortex in healthy individuals.52 We observed a different pattern of brain responses in people with schizophrenia: no neural response modulation in the cingulate cortex was observed, which would be consistent with a relative failure of the attention orienting and error monitoring systems. In patients, response inhibition to positive content was associated with increased activation in the anterior right middle frontal gyrus (Brodmann area 10). A similar finding has been observed in patients with bipolar disorder during a response inhibition task and may be suggestive of a compensatory strategy.53 Nevertheless, our findings do point toward a comparatively more robust effect of negative stimuli on cognitive control mechanisms in healthy controls and a more pronounced deficit in neural processes that depend on negative, as opposed to positive emotion evaluation in people with schizophrenia.54–56
Direct comparisons between patients and controls using a whole-brain approach revealed decreased activation during response inhibition to negative compared with neutral material in dorsal (pre)frontal regions and the occipital cortex, where controls showed an increase in activity. This analysis also revealed a lack of brain response in the medial superior frontal cortex, the caudate and the fusiform gyrus in people with schizophrenia compared with healthy controls. Frontal and basal ganglia hypoactivation suggests a relative dysfunction in the cortical control mechanisms associated with go/no-go task performance and the frontostriatal pathways, which play a crucial role in involuntary action control, including the ability to inhibit motor response. Although it has been linked to emotional face processing, the fusiform gyrus also activates more strongly during suppression of negative compared with neutral memories46 and has been implicated as an additional neural mediator of the interaction between inhibitory control and negative stimulus processing.45 In all, these findings suggest a fairly comprehensive neural deficit in people with schizophrenia during response inhibition challenges in an emotional context. The integrative network supporting cognitive control mechanisms during emotional challenges would be critical in the production of adaptive behaviour in many real-life situations. Even subtle impairments may have functional consequences in more complex behavioural settings and may contribute to the psychosocial deficits observed in people with schizophrenia.
Limitations
Heterogeneity in the pathophysiology associated with schizophrenia may contribute to more variable neural responses. Patients may also have developed individually variable neural pathways to ensure that some functioning of cognition–emotion interaction is maintained, which results in less consistent neural patterns when assessed at the group level. The absence of a robust task effect in the people with schizophrenia could thus be owing to a more variable neural response rather than a consistent failure to activate the relevant circuitry seen in healthy controls. Prefrontal response in people with schizophrenia has also been characterized as “fractionized” and unfocused, resulting from increased residual noise variance of the BOLD response, and the inconsistency of the response also correlates with severity of psychotic symptoms.57,58 However, our patient sample was fairly homogeneous in that all patients received pharmacotherapy with second-generation antipsychotics; were chronically ill, but clinically stable, community-dwellers; and were within a relatively small age range.
Nevertheless, the patient group was characterized by a general cognitive deficit, and performed more poorly on the task across conditions. Such behavioural and cognitive differences between patients and controls can have important modulating effects on the neural substrates of any cognitive task. Given that cognitive changes are a core feature of the illness, a priori matching based on IQ may reduce generalizability of the findings to the schizophrenia population as a whole. We therefore chose to statistically control for task accuracy and general cognitive ability, but still observed a robust activation decrease in people with schizophrenia, suggesting that general cognitive deficits are insufficient to account for the attenuated brain response in schizophrenia. Overall, this suggests that emotional go/no-go conditions elicit abnormal brain responses that are linked to the illness process rather than confounding variables. Under more complex, real-life conditions, these abnormal activation patterns may well lead to observable behavioural changes.
Finally, the sex balance was skewed toward men in the patient sample, and some studies suggest that men and women may differ in the neural substrates of cognitive appraisal of affective content, as well as emotion regulation processes, which are relevant to the affective go/no-go paradigm.59,60 As sex effects were not of primary interest to us, we treated sex as a nuisance covariate, but another productive approach would be to explicitly assess whether differential responses are observed in men and women during emotional go/no-go conditions and whether these putative sex differences are present to a similar degree in people with schizophrenia.
Conclusion
Our findings indicate that schizophrenia is associated with aberrant brain responses in the neural network involved in cognition–emotion interaction, particularly when faced with negatively valenced material. These findings point to neurophysiological alterations in brain networks associated with cognitive control over emotion processing, which may underlie impairments in many aspects of goal-directed behaviour in people with schizophrenia.
Acknowledgements
This work was supported by the University of New South Wales School of Psychiatry, NHMRC Project Grant no. 568807, Neuroscience Research Australia and the Australian Schizophrenia Research Bank, which is supported by the National Health and Medical Research Council of Australia, the Pratt Foundation, Ramsay Health Care and the Viertal Charitable Foundation. C.S. Weickert is also supported by the Schizophrenia Research Institute, utilizing infrastructure funding from NSW Health, and funding from the Macquarie Group Foundation.
Footnotes
Competing interests: None declared for R. Morris, M.J. Green, R. Lenroot and V.J. Carr. As above for A. Vercammen, J. Kulkarni, C.S. Weickert and T.W. Weickert. A. Vercammen also declares having received travel support from the International Congress on Schizophrenia Research. J. Kulkarni serves on the advisory boards for Lundbeck, Janssen Cilag, Asta Zeneca and Eli Lilly; declares having received insitutional grant support from Astra Zeneca, Janssen Cilag, Eli Lilly, Amgen and Roche; has received lectures fees from Jansen Cilag, Astra Zeneca and Eli Lilly; has a patent pending with EVestG; receives royalties from Cambridge University Press; and has received payment from Medimark Australia for preparing educational material on women and schizophrenia.
Contributors: M.J. Green, R. Lenroot, C.S. Weickert and T.W. Weickert designed the study. A. Vercammen and R. Morris acquired the data. A. Vercammen, R. Morris, R. Lenroot, J. Kulkarni, V.J. Carr, C.S. Weickert and T.W. Weickert analyzed the data. A. Vercammen, C.S. Weickert and T.W. Weickert wrote the article. All authors reviewed the article and approved its publication.
References
- 1.Heinrichs RW, Zakzanis KK. Neurocognitive deficit in schizophrenia: a quantitative review of the evidence. Neuropsychology. 1998;12:426–45. doi: 10.1037//0894-4105.12.3.426. [DOI] [PubMed] [Google Scholar]
- 2.Reichenberg A, Harvey PD. Neuropsychological impairments in schizophrenia: integration of performance-based and brain imaging findings. Psychol Bull. 2007;133:833–58. doi: 10.1037/0033-2909.133.5.833. [DOI] [PubMed] [Google Scholar]
- 3.Weickert TW, Goldberg TE, Gold JM, et al. Cognitive impairments in patients with schizophrenia displaying preserved and compromised intellect. Arch Gen Psychiatry. 2000;57:907–13. doi: 10.1001/archpsyc.57.9.907. [DOI] [PubMed] [Google Scholar]
- 4.Dollfus S, Lombardo C, Benali K, et al. Executive/attentional cognitive functions in schizophrenic patients and their parents: a preliminary study. Schizophr Res. 2002;53:93–9. doi: 10.1016/s0920-9964(01)00156-6. [DOI] [PubMed] [Google Scholar]
- 5.Kerns JG, Cohen JD, MacDonald AW, III, et al. Anterior cingulate conflict monitoring and adjustments in control. Science. 2004;303:1023–6. doi: 10.1126/science.1089910. [DOI] [PubMed] [Google Scholar]
- 6.Carter CS, Macdonald AMI, Stenger VA, et al. Dissociating the contributions of DLPFC and anterior cingulate to executive control: an event-related fMRI study. Brain Cogn. 2001;47:66–9. [Google Scholar]
- 7.Barch DM, Carter CS, Braver TS, et al. Selective deficits in pre-frontal cortex function in medication-naive patients with schizophrenia. Arch Gen Psychiatry. 2001;58:280–8. doi: 10.1001/archpsyc.58.3.280. [DOI] [PubMed] [Google Scholar]
- 8.Callicott JH, Ramsey NF, Tallent K, et al. Functional magnetic resonance imaging brain mapping in psychiatry: methodological issues illustrated in a study of working memory in schizophrenia. Neuropsychopharmacology. 1998;18:186–96. doi: 10.1016/S0893-133X(97)00096-1. [DOI] [PubMed] [Google Scholar]
- 9.Perlstein WM, Dixit NK, Carter CS, et al. Prefrontal cortex dysfunction mediates deficits in working memory and prepotent responding in schizophrenia. Biol Psychiatry. 2003;53:25–38. doi: 10.1016/s0006-3223(02)01675-x. [DOI] [PubMed] [Google Scholar]
- 10.Callicott JH, Bertolino A, Mattay VS, et al. Physiological dysfunction of the dorsolateral prefrontal cortex in schizophrenia revisited. Cereb Cortex. 2000;10:1078–92. doi: 10.1093/cercor/10.11.1078. [DOI] [PubMed] [Google Scholar]
- 11.Weinberger DR, Berman KF, Zec RF. Physiologic dysfunction of dorsolateral prefrontal cortex in schizophrenia. I. Regional cerebral blood flow evidence. Arch Gen Psychiatry. 1986;43:114–24. doi: 10.1001/archpsyc.1986.01800020020004. [DOI] [PubMed] [Google Scholar]
- 12.Callicott JH, Mattay VS, Verchinski BA, et al. Complexity of prefrontal cortical dysfunction in schizophrenia: more than up or down. Am J Psychiatry. 2003;160:2209–15. doi: 10.1176/appi.ajp.160.12.2209. [DOI] [PubMed] [Google Scholar]
- 13.Kohler CG, Martin EA. Emotional processing in schizophrenia. Cogn Neuropsychiatry. 2006;11:250–71. doi: 10.1080/13546800500188575. [DOI] [PubMed] [Google Scholar]
- 14.Habel U, Chechko N, Pauly K, et al. Neural correlates of emotion recognition in schizophrenia. Schizophr Res. 2010;122:113–23. doi: 10.1016/j.schres.2010.06.009. [DOI] [PubMed] [Google Scholar]
- 15.Gard DE, Cooper S, Fisher M, et al. Evidence for an emotion maintenance deficit in schizophrenia. Psychiat Res. 2011;187:24–9. doi: 10.1016/j.psychres.2010.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schneider F, Gur RC, Gur RE, et al. Emotional processing in schizophrenia: neurobehavioral probes in relation to psychopathology. Schizophr Res. 1995;17:67–75. doi: 10.1016/0920-9964(95)00031-g. [DOI] [PubMed] [Google Scholar]
- 17.Taylor SF, Phan KL, Britton JC, et al. Neural response to emotional salience in schizophrenia. Neuropsychopharmacology. 2005;30:984–95. doi: 10.1038/sj.npp.1300679. [DOI] [PubMed] [Google Scholar]
- 18.Kohler CG, Turner TH, Bilker WB, et al. Facial emotion recognition in schizophrenia: intensity effects and error pattern. Am J Psychiatry. 2003;160:1768–74. doi: 10.1176/appi.ajp.160.10.1768. [DOI] [PubMed] [Google Scholar]
- 19.Quintana J, Lee J, Marcus M, et al. Brain dysfunctions during facial discrimination in schizophrenia: selective association to affect decoding. Psychiatry Res. 2011;191:44–50. doi: 10.1016/j.pscychresns.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 20.Morris RW, Weickert CS, Loughland CM. Emotional face processing in schizophrenia. Curr Opin Psychiatry. 2009;22:140–6. doi: 10.1097/YCO.0b013e328324f895. [DOI] [PubMed] [Google Scholar]
- 21.Hoekert M, Kahn RS, Pijnenborg M, et al. Impaired recognition and expression of emotional prosody in schizophrenia: review and meta-analysis. Schizophr Res. 2007;96:135–45. doi: 10.1016/j.schres.2007.07.023. [DOI] [PubMed] [Google Scholar]
- 22.Roux P, Christophe A, Passerieux C. The emotional paradox: dissociation between explicit and implicit processing of emotional prosody in schizophrenia. Neuropsychologia. 2010;48:3642–9. doi: 10.1016/j.neuropsychologia.2010.08.021. [DOI] [PubMed] [Google Scholar]
- 23.Edwards J, Jackson HJ, Pattison PE. Emotion recognition via facial expression and affective prosody in schizophrenia: a methodological review. Clin Psychol Rev. 2002;22:789–832. doi: 10.1016/s0272-7358(02)00130-7. [DOI] [PubMed] [Google Scholar]
- 24.Russell TA, Reynaud E, Kucharska-Pietura K, et al. Neural responses to dynamic expressions of fear in schizophrenia. Neuropsychologia. 2007;45:107–23. doi: 10.1016/j.neuropsychologia.2006.04.026. [DOI] [PubMed] [Google Scholar]
- 25.Ochsner KN, Gross JJ. The cognitive control of emotion. Trends Cogn Sci. 2005;9:242–9. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 26.Dolcos F, Diaz-Granados P, Wang L, et al. Opposing influences of emotional and non-emotional distracters upon sustained pre-frontal cortex activity during a delayed-response working memory task. Neuropsychologia. 2008;46:326–35. doi: 10.1016/j.neuropsychologia.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 27.Dolcos F, LaBar KS, Cabeza R. Interaction between the amygdala and the medial temporal lobe memory system predicts better memory for emotional events. Neuron. 2004;42:855–63. doi: 10.1016/s0896-6273(04)00289-2. [DOI] [PubMed] [Google Scholar]
- 28.Dolcos F, McCarthy G. Brain systems mediating cognitive interference by emotional distraction. J Neurosci. 2006;26:2072–9. doi: 10.1523/JNEUROSCI.5042-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dolcos F, Miller B, Kragel P, et al. Regional brain differences in the effect of distraction during the delay interval of a working memory task. Brain Res. 2007;1152:171–81. doi: 10.1016/j.brainres.2007.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dichter GS, Bellion C, Casp M, et al. Impaired modulation of attention and emotion in schizophrenia. Schizophr Bull. 2010;36:595–606. doi: 10.1093/schbul/sbn118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ursu S, Kring AM, Gard MG, et al. Prefrontal cortical deficits and impaired cognition-emotion interactions in schizophrenia. Am J Psychiatry. 2011;168:276–85. doi: 10.1176/appi.ajp.2010.09081215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Elliott R, Rubinsztein JS, Sahakian BJ, et al. Selective attention to emotional stimuli in a verbal go/no-go task: an fMRI study. Neuroreport. 2000;11:1739–44. doi: 10.1097/00001756-200006050-00028. [DOI] [PubMed] [Google Scholar]
- 33.Quirk GJ, Beer JS. Prefrontal involvement in the regulation of emotion: convergence of rat and human studies. Curr Opin Neurobiol. 2006;16:723–7. doi: 10.1016/j.conb.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 34.First MB, Spitzer RL, Gibbon M, et al. Structured Clinical Interview for DSM-IV-TR Axis I Disorders-Patient Edition (SCID-I/P) New York: Biometric Research Department, New York State Psychiatric Institute; 2007. [Google Scholar]
- 35.Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–76. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- 36.Wechsler D. Wechsler Test of Adult Reading. San Antonio (TX): The Pscyhological Corporation; 2001. [Google Scholar]
- 37.Wechsler D. Wechsler Adult Intelligence Scale. 3rd ed. San Antonio (TX): The Psychological Corporation; 1997. [Google Scholar]
- 38.Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- 39.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30:473–83. [PubMed] [Google Scholar]
- 40.Wilkinson G, Hesdon B, Wild D, et al. Self-report quality of life measure for people with schizophrenia: the SQLS. Br J Psychiatry. 2000;177:42–6. doi: 10.1192/bjp.177.1.42. [DOI] [PubMed] [Google Scholar]
- 41.Bradley MM, Lang PJ. Affective norms for English words (ANEW): instruction manual and affective ratings. Gainesville (FL): The Centre for Research in Psychophysiology, University of Florida; 1999. Technical Report C-1. [Google Scholar]
- 42.Forman SD, Cohen JD, Fitzgerald M, et al. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magn Reson Med. 1995;33:636–47. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- 43.Garavan H, Ross TJ, Stein EA. Right hemispheric dominance of inhibitory control: an event-related functional MRI study. Proc Natl Acad Sci U S A. 1999;96:8301–6. doi: 10.1073/pnas.96.14.8301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shafritz KM, Collins SH, Blumberg HP. The interaction of emotional and cognitive neural systems in emotionally guided response inhibition. Neuroimage. 2006;31:468–75. doi: 10.1016/j.neuroimage.2005.11.053. [DOI] [PubMed] [Google Scholar]
- 45.Goldstein M, Brendel G, Tuescher O, et al. Neural substrates of the interaction of emotional stimulus processing and motor inhibitory control: an emotional linguistic go/no-go fMRI study. Neuroimage. 2007;36:1026–40. doi: 10.1016/j.neuroimage.2007.01.056. [DOI] [PubMed] [Google Scholar]
- 46.Butler AJ, James KH. The neural correlates of attempting to suppress negative versus neutral memories. Cogn Affect Behav Neurosci. 2010;10:182–94. doi: 10.3758/CABN.10.2.182. [DOI] [PubMed] [Google Scholar]
- 47.Habel U, Pauly K, Koch K, et al. Emotion-cognition interactions in schizophrenia. World J Biol Psychiatry. 2010;11:934–44. doi: 10.3109/15622975.2010.501820. [DOI] [PubMed] [Google Scholar]
- 48.Holt DJ, Lakshmanan B, Freudenreich O, et al. Dysfunction of a cortical midline network during emotional appraisals in schizophrenia. Schizophr Bull. 2011;37:164–76. doi: 10.1093/schbul/sbp067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kellermann TS, Sternkopf MA, Schneider F, et al. Modulating the processing of emotional stimuli by cognitive demand. Soc Cogn Affect Neurosci. 2011 Jan 22; doi: 10.1093/scan/nsq104. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci. 2000;4:215–22. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- 51.Pratto F, John OP. Automatic vigilance — the attention-grabbing power of negative social information. J Pers Soc Psychol. 1991;61:380–91. doi: 10.1037//0022-3514.61.3.380. [DOI] [PubMed] [Google Scholar]
- 52.Damasio AR, Grabowski TJ, Bechara A, et al. Subcortical and cortical brain activity during the feeling of self-generated emotions. Nat Neurosci. 2000;3:1049–56. doi: 10.1038/79871. [DOI] [PubMed] [Google Scholar]
- 53.Strakowski SM, Adler CM, Cerullo MA, et al. Magnetic resonance imaging brain activation in first-episode bipolar mania during a response inhibition task. Early Interv Psychiatry. 2008;2:225–33. doi: 10.1111/j.1751-7893.2008.00082.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bell M, Bryson G, Lysaker P. Positive and negative affect recognition in schizophrenia: a comparison of substance abuse and normal control subjects. Psychiatry Res. 1997;73:73–82. doi: 10.1016/s0165-1781(97)00111-x. [DOI] [PubMed] [Google Scholar]
- 55.An S, Jeon JH, Hwang SJ, et al. Emotional impairments in the patients with schizophrenia. Schizophr Res. 2003;60(Suppl):163. [Google Scholar]
- 56.An SK, Lee E, Kim JJ, et al. Greater impairment in negative emotion evaluation ability in patients with paranoid schizophrenia. Yonsei Med J. 2006;47:343–53. doi: 10.3349/ymj.2006.47.3.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Winterer G, Coppola R, Goldberg TE, et al. Prefrontal broadband noise, working memory, and genetic risk for schizophrenia. Am J Psychiatry. 2004;161:490–500. doi: 10.1176/appi.ajp.161.3.490. [DOI] [PubMed] [Google Scholar]
- 58.Winterer G, Musso F, Beckmann C, et al. Instability of prefrontal signal processing in schizophrenia. Am J Psychiatry. 2006;163:1960–8. doi: 10.1176/ajp.2006.163.11.1960. [DOI] [PubMed] [Google Scholar]
- 59.Welborn BL, Papademetris X, Reis DL, et al. Variation in or-bitofrontal cortex volume: relation to sex, emotion regulation and affect. Soc Cogn Affect Neurosci. 2009;4:328–39. doi: 10.1093/scan/nsp028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Koch K, Pauly K, Kellermann T, et al. Gender differences in the cognitive control of emotion: an fMRI study. Neuropsychologia. 2007;45:2744–54. doi: 10.1016/j.neuropsychologia.2007.04.012. [DOI] [PubMed] [Google Scholar]