Abstract
Background
Previous studies of brain structure abnormalities in conduct disorder and attention-deficit/hyperactivity disorder (ADHD) samples have been limited owing to cross-comorbidity, preventing clear understanding of which structural brain abnormalities might be specific to or shared by each disorder. To our knowledge, this study was the first direct comparison of grey and white matter volumes in diagnostically “pure” (i.e., no comorbidities) conduct disorder and ADHD samples.
Methods
Groups of adolescents with noncormobid conduct disorder and with noncomorbid, combined-subtype ADHD were compared with age- and sex-matched controls using DARTEL voxel-based analysis of T1-weighted brain structure images. Analysis of variance with post hoc analyses compared whole brain grey and white matter volumes among the groups.
Results
We included 24 adolescents in each study group. There was an overall 13% reduction in grey matter volume in adolescents with conduct disorder, reflecting numerous frontal, temporal, parietal and subcortical deficits. The same grey matter regions typically were not abnormal in those with ADHD. Deficits in frontal lobe regions previously identified in studies of patients with ADHD either were not detected, or group differences from controls were not as strong as those between the conduct disorder and control groups. White matter volume measurements did not differentiate conduct disorder and ADHD.
Limitations
Our modest sample sizes prevented meaningful examination of individual features of ADHD or conduct disorder, such as aggression, callousness, or hyperactive versus inattentive symptom subtypes.
Conclusion
The evidence supports theories of frontotemporal abnormalities in adolescents with conduct disorder, but raises questions about the prominence of frontal lobe and striatal structural abnormalities in those with noncomorbid, combined-subtype ADHD. The latter point is clinically important, given the widely held belief that ADHD is associated with numerous frontal lobe structural deficits, a conclusion that is not strongly supported following direct comparison of diagnostically pure groups. The results are important for future etiological studies, particularly those seeking to identify how early expression of specific brain structure abnormalities could potentiate the risk for antisocial behaviour.
Introduction
Neuroimaging has been used to identify neurobiological factors expressed at early ages that might confer risk for disruptive behaviour disorders, such as attention-deficit/hyperactivity disorder (ADHD) and conduct disorder.1,2 As defined by the DSM-IV, ADHD is marked by persistent symptoms of inattention, hyperactivity and impulsivity that cause clinically important impairment, whereas conduct disorder reflects a chronic pattern of aggression, deceitfulness/theft, property destruction and rules violation arising before age 18.3 Current theories emphasize distinct frontostriatal/frontocerebellar (ADHD4) or frontotemporal (conduct disorder1,5) disorder conceptualizations. These theories are built in large part on dozens of studies of brain structure abnormalities in samples with each disorder (for meta-analytic and qualitative reviews, see Valera and colleagues,2 Yang and Raine,5 Ellison-Wright and colleagues,6 Hutchison and colleagues7 and Seidman and colleagues8). Brain volume abnormalities are important indicators of pathophysiological processes that likely reflect disorder etiology. Although results vary somewhat across antisocial disorder studies of different age groups, there is consistent evidence for reduced grey matter volume in the temporal lobe, including lateral and medial (e.g., amygdala, hippocampus) regions, in patients with conduct disorder.9 Meta-analyses have shown that brain volume deficits in patients with ADHD are consistently found in the cerebellum, the corpus callosum splenium, total brain volume, right cerebral volume and right caudate.2 Many brain structure studies report evidence of frontal lobe volume reductions in samples of youth with primary diagnoses of either conduct disorder5 or ADHD,8 but studies vary in their specific findings. However, it is commonly believed that frontal lobe abnormalities — both structural and functional — contribute to behavioural impairments associated with both disorders.
Although there are inconsistencies among studies in which brain regions are found to be abnormal for both patients with conduct disorder and those with ADHD, the prefrontal cortex findings are particularly variable. The reasons for these inconsistencies are not clear, but might be due to the frequent comorbidity of ADHD and conduct disorder in the samples examined. Although the 2 diagnoses are behaviourally distinct, about 65% of clinic-referred youth with conduct disorder diagnoses also meet diagnostic criteria for ADHD.10 Although this association is not as strong in community-recruited youth with conduct disorder (i.e., about 7%), epidemiological studies find that about 48% of community-recruited youth with ADHD also have conduct disorder.11 Perhaps unsurprisingly, every published brain structure study of youth with conduct disorder examined samples who either entirely or mostly had comorbid ADHD.12–16 Conversely, careful review of published ADHD brain structure reports reveals that nearly half of these studies (12 of 26; 46%) extensively sampled youth with comorbid antisocial or oppositional diagnoses,16–27 and another 5 studies (19%) did not report on antisociality.28–32 The remaining 9 (35%) studies33–41 with confirmed “pure” (i.e., no comorbidities) ADHD samples typically reported reduced volume only in the small handful of brain regions (i.e., total grey volume, caudate, somatosensory/premotor cortices, cerebellum) identified by meta-analysis.2 However, these pure ADHD studies typically mixed together DSM-defined clinical subtypes (e.g., predominantly inattentive v. combined)34,35,37,38,40,41 or did not specify subtype,33,34 possibly adding to variability across studies. More important to the present study, none of the published meta-analyses of ADHD or conduct disorder addressed the possible impact of antisocial behaviour on ADHD brain structure findings2,6,7 or vice versa.5 Only Seidman and colleagues8 discussed the presence of psychiatric comorbidities in ADHD brain structure studies in their qualitative review. They noted that most studies did not control for comorbidities, whereas the 2 pure ADHD studies and 1 study that statistically controlled for about 43% of comorbid oppositional defiant/conduct disorders that were published at that time reported only summary volume measures of cortex or frontal lobe regions.24
In sum, previous studies have not clearly addressed which specific frontal lobe brain regions are structurally abnormal in samples with diagnostically pure ADHD or conduct disorder, or whether any abnormalities differed between the 2 disorders. Our review also raises an important question about whether lateral frontal lobe volume deficits commonly attributed to ADHD might instead be more directly linked to conduct disorder, given the apparent absence of evidence for specific, regional prefrontal cortex deficits in studies of ADHD samples without antisocial disorders. Functional neuroimaging evidence has emphasized somewhat different frontal lobe abnormalities in noncomorbid samples of conduct disorder42–44 and ADHD (e.g., a ventral prefrontal surface versus dorsolateral surface dissociation for conduct disorder and ADHD, respectively45). Clearer understanding of disorder etiology also requires careful comparison of brain structures between the 2 disorders to confirm which regional abnormalities are specific to each. To our knowledge, no studies to date have directly compared ADHD and conduct disorder brain volume abnormalities. Several studies of comorbid conduct disorder/ADHD samples have statistically adjusted results for symptom severity in attempts to isolate abnormalities to one disorder or the other, but this approach has produced mixed results. On the one hand, some evidence from studies using such statistical control suggests that medial orbitofrontal and anterior cingulate volume deficits might not be linked to ADHD,13 and analysis can reveal greater ADHD structural deficits when covarying for conduct disorder symptoms.46 On the other hand, most attempts to use covariance or post hoc analyses to tease apart diagnostic specificity actually linked putative conduct disorder or ADHD volume deficits to symptom severity for the other disorder.12,14,18 This has reduced, not improved, clarity. Only a direct comparison of well-characterized noncomorbid samples can effectively determine whether there are uniquely affected brain regions or shared volume abnormalities in each disorder.
The purpose of our study was to identify grey or white matter volume regions that differed between groups of adolescents with pure combined-subtype ADHD, conduct disorder and matched controls. Our broad prediction was that direct comparison would identify deficits specific to each disorder. Consequently, any overlap of abnormalities between the disorders would identify brain regions potentially representing shared etiological factors. We hypothesized that adolescents with conduct disorder diagnoses would show reduced volume in medial and lateral temporal lobe and ventral frontal lobe surface regions (e.g., ventromedial, orbitofrontal cortex, insular cortex, rostral cingulate), as found in previous reports. A key question was whether the conduct disorder and ADHD groups would differ in lateral prefrontal region volume, which has been found in studies of both disorders, albeit in samples with high cross-comorbidity. We hypothesized lateral prefrontal cortex volume differences between both diagnostic groups and controls, but planned comparisons among study groups to ascertain specific and evaluate relative deficit severity. We also hypothesized that cerebellum and basal ganglia deficits would be found in the ADHD group. The lack of previous evidence implicating white matter in antisocial groups9,47,48 and mixed evidence in patients with ADHD8 prevented detailed hypotheses for group white matter differences, but we expected ADHD-specific corpus callosum deficits, as indicated by meta-analytic review.2 A secondary aim was to determine if conduct disorder or ADHD symptom severity was linearly associated with any abnormal volumetric finding.
Methods
Participants
The conduct disorder and ADHD study groups each comprised right-handed, medically healthy adolescents (ages 12–18) recruited for a National Institute of Mental Health (NIMH)-funded project (K23 MH070036) aimed at differentiating the neural correlates of each diagnosis. Participants were recruited using a combination of community advertisements and letters sent to families of youth on probation following arrest in the Connecticut Court Support Services Division. Groups were matched by sex and mean age to control participants without diagnosable DSM-IV disorders or health problems. We obtained parental permission and informed consent to participate in the study jointly from the participants and their parent/legal guardian. All study procedures were approved by the Hartford Hospital Institutional Review Board.
Clinical diagnoses for research purposes were made using the Kiddie-Schedule for Affective Disorder and Schizophrenia — Present and Lifetime Version (K-SADS-PL)49 conducted by trained bachelor’s- and master’s-level staff working under the supervision of a licensed clinical psychologist. The K-SADS-PL, based on DSM-IV, is a validated, reliable and widely used semistructured clinical research interview. Interviews were performed separately for both adolescents and parents. Information was synthesized and diagnoses confirmed in weekly research group meetings. By design, we excluded youth with comorbid disorders. All ADHD participants met criteria for DSM-IV combined-subtype.
Verbal ability was estimated using the Wide Range Achievement Test 3 (WRAT3)50 reading scale score because numerous participants with conduct disorder did not exert adequate effort on the more challenging Weschler Intelligence Scale for Children, third edition, or the Weschler Adult Intelligence Scale, third edition, vocabulary subtests originally intended to estimate verbal–conceptual ability. The WRAT3 reading scores are strongly correlated with verbal IQ51 and likely better represent the well-known verbal impairments in people with conduct disorder.52
MRI data collection and data preparation
T1-weighted brain structure images were collected using a 3-dimentional magnetization-prepared rapid-acquisition gradient echo (3-D MPRAGE) pulse sequence (repetition time [TR] 2300 ms, echo time [TE] 2.74 ms, inversion time [TI] 900 ms, flip angle 8°, field of view [FOV] 176 × 256 mm, matrix 176 × 256 × 176, voxel size 1 × 1 × 1 mm, pixel bandwidth 190 Hz, total scan time 7:09 min) using a Siemens 3-T Allegra magnetic resonance imaging (MRI) scanner at the Olin Neuropsychiatry Research Center, The Institute of Living/Hartford Hospital. Head movement was controlled using padded cushions to restrict head motion. Board-certified radiologists confirmed that all scans were free of gross structural abnormality. Visual inspection plus signal-to-noise metrics confirmed scan quality.
Data were prepared for analysis using the DARTEL tool-box,53 implemented in SPM8 (www.fil.ion.ucl.ac.uk/spm/software/spm8/), which is a variant of widely used voxel-based morphometry methods. The toolbox is a suite of MATLAB programs that achieves highly accurate intersubject registration of brain images54 using an algorithm for diffeomorphic 3-D image registration that considers nonlinear registration as a local optimization problem that is solved using a Levenberg–Marquardt strategy. Data preparation involved several steps, including nonlinear noise reduction (www.fmrib.ox.ac.uk/analysis/research/susan), segmentation of each participant’s T1-weighed brain structure image in native space into grey and white matter and cerebrospinal fluid (CSF) maps,55 import of segmented images to SPM8 to create a series of rigidly aligned tissue class images, and creation of flow fields generated by nonlinear registration of each image to a highly precise, iteratively generated template of all participants. Participant images then were warped to the template, interpolated to a 1.5 mm isotropic voxel resolution and used to create Jacobian-scaled (i.e., modulated) warped tissue class images. Whereas unmodulated images represent the relative concentration of a tissue class (i.e., grey and white matter, CSF), modulated images have adjusted these values by the deformation field produced by spatial normalization. This adjustment permits inferences about volume differences instead of the harder to interpret concept of concentration. The Jacobian-modulated images were spatially normalized into standardized Montreal Neurological Institute (MNI) space to facilitate anatomic localization and smoothed using an 8 mm full-width at half-maximum Gaussian kernel before random-effects analyses.
Statistical analysis
Because the SPM unified segmentation method produces total volume measurements (mL) for grey and white matter and CSF tissue classes, we first compared the study groups’ total grey and white matter volume using analysis of variance (ANOVA) and post hoc t tests in SPSS software version 17.0. We calculated percent reduction of grey matter volume in conduct disorder and ADHD groups relative to the control group.
Primary study hypotheses for volume differences among groups were evaluated separately for grey and white matter using SPM8 factorial ANOVA. Because there were no group differences in total intracranial volume (see the “Whole brain volume differences between conduct disorder and control groups” section), these tests used absolute (not relative) volumetric values. To achieve accurate statistical inference given the nonuniform smoothness of brain structure images used in voxel-based morphometry,56 we used a non-stationary correction to adjust voxel-wise results parametrically based on their local smoothness using random field theory.57 We reported group differences if they were significant at a false discovery rate (FDR) of q < 0.05, corrected, for the whole brain.58 Planned comparisons involved Dunn–Sidak corrections for multiple tests (t statistic equivalent of p < 0.05 was 2.47) to determine significant pairwise study group differences within regions identified by ANOVA omnibus-F. Pairwise group differences were tabulated and converted to Cohen d effect sizes59 to assist interpretation. Importantly, the ANOVA models were redone to covary for total intracranial volume, sex or WRAT3 reading score without altering the overall SPM8 voxel-wise results or pairwise group comparisons for individual brain regions. Supplemental correlation analysis determined linear associations between grey or white matter volume and K-SADS-PL DSM-IV symptom severity separately for the ADHD and conduct disorder groups. The sum of inattentive (ADHD-I) and hyperactive–impulsive (ADHD-H/I) symptoms represented overall ADHD severity. Results are reported at a statistical threshold of p < 0.05, uncorrected. Finally, in recognition of our modest sample sizes, we conducted exploratory pairwise group comparisons using SPM8 t tests, with results considered to be significant at p < 0.05, uncorrected.
All SPM8 analyses used explicit masks of grey and white matter calculated from participants’ data using a method that optimized the binary distinction between tissue and nonbrain areas.60 To help visualize the primary study results, we created volume renderings (http://surfer.nmr.mgh.harvard.edu/). This approach projected statistical maps onto a brain template to depict significant group volume differences that occurred on gyri and within sulci. Other results were depicted using activation maps overlaid on brain slices (http://ric.uthscsa.edu/mango/). Identification of brain regions that had significant effects was guided by MNI stereotactic brain anatomy atlas labels.
Results
Participants
The conduct disorder, ADHD and control groups each comprised 24 participants. The sample demographic and clinical characteristics are listed in Table 1. Not only did no participants with conduct disorder or ADHD meet the criteria for the other disorder, they typically had no suprathreshold (i.e., K-SADS-PL symptom rating = 3) comorbid symptoms. Only 3 participants with ADHD had 1 conduct disorder symptom. Likewise, a single participant with conduct disorder had 1 inattentive/1 hyperactive–impulsive symptom whereas 1 other participant had 2 inattentive symptoms. One participant in the conduct disorder group met criteria for cannabis abuse. Because cannabis use likely had minimal impact on our study aims, it did not warrant exclusion. There were no other lifetime psychiatric/substance disorder comorbidities. All participants tested negative for the presence of marijuana, cocaine and heroin on a urine drug screen on the day of the MRI scan.
Table 1.
Demographic and clinical characteristics of the sample
| Group; mean (SD) | ||||
|---|---|---|---|---|
|
|
||||
| Characteristic | Control | CD | ADHD | p value |
| Age, yr | 16.0 (1.47) | 16.0 (1.29) | 15.7(1.55) | 0.64 |
| Sex, male:female | 16:8 | 16:8 | 19:5 | 0.55 |
| WRAT-3 reading scale score | 97.4 (7.94) | 91.3 (13.18) | 98.3(15.04) | 0.14 |
| K-SADS-PL | ||||
| CD symptoms* | 0.0 (0.20) | 5.5 (1.95) | 0.2(0.38) | < 0.001 |
| ADHD | ||||
| Hyperactivity/impulsivity | 0.0 (0.20) | 0.1 (0.48) | 7.0(0.95) | < 0.001 |
| Inattentive | 0.1 (0.45) | 0.3 (0.80) | 7.6(1.21) | < 0.001 |
ADHD = attention-deficit/hyperactivity disorder; CD = conduct disorder; K-SADS-PL = Kiddie-Schedule for Affective Disorder and Schizophrenia — Present and Lifetime Version;49 SD = standard deviation; WRAT3 = Wide Range Achievement Test 3.50
Conduct disorder symptom counts were not reliably documented for 2 participants.
The χ2 test revealed no sex differences among groups, and 1-way ANOVA revealed no group differences in age or WRAT3 score. As designed, the number of conduct disorder (F2,67 = 172.824, p < 0.001), ADHD-I (F2,68 = 556.242, p < 0.001) and ADHD-H/I symptoms (F2,68 = 920.340, p < 0.001) significantly differed among groups. The mean K-SADS-PL symptom count for each diagnosis exceeded the minimum DSM-IV cut-offs, suggesting the symptoms were at least of moderate clinical severity. Exact conduct disorder symptom counts for 2 participants were lost to file following diagnostic consensus and are not reported. These participants were retained in the group MRI analysis, but omitted from supplemental correlations between brain structure and symptom severity.
Whole brain volume differences between conduct disorder and control groups
Whole brain grey matter volume was significantly different across study groups. Post hoc comparisons revealed a 13% overall reduction in grey matter volume in participants with conduct disorder relative to controls (mean [standard deviation (SD)] volume 532.1 [104.20] mL in the conduct disorder group v. 611.5 [103.75] mL in the control group, p = 0.017), as compared with a nonsignificant 3% difference between the ADHD (592.9 [83.2] mL, p = 0.50) and control groups. We detected no group differences for CSF, intracranial or white matter volume with ANOVA.
Regional volume differences between conduct disorder, ADHD and control groups
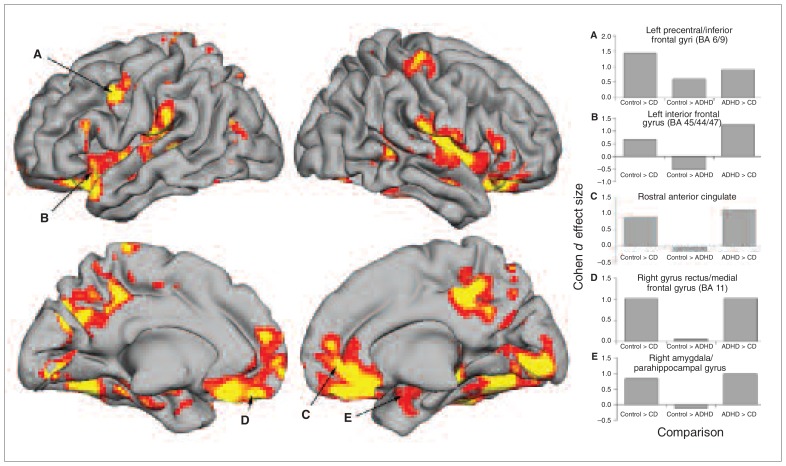
Table 2 lists and Figure 1 depicts the results of the SPM8 ANOVA for grey matter volume. As hypothesized, we found many group differences detected at an FDR of q < 0.05 (F2,69 = 5.40) in frontal and temporal lobe regions, but other brain structures also differed across groups. Table 2 lists post hoc pairwise group comparison results and effect sizes for each significant effect (p < 0.05, uncorrected) above Cohen d = 0.50. These comparisons revealed that adolescents with conduct disorder had less absolute grey matter volume than controls and adolescents with ADHD in nearly every region detected by ANOVA omnibus-F. The comparisons in which we found participants with conduct disorder had reduced grey matter volume relative to controls — the left inferior frontal gyrus, right/middle frontal gyri, left amygdala/parahippocampus, right parahippocampus/fusiform gyri, paracentral lobule/cingulate gyrus and right caudate — failed to meet corrections for multiple comparisons. Interestingly, adolescents with ADHD had evidence for reduced grey matter volume relative to controls only in the left precentral/inferior frontal gyri (Brodmann area [BA] 6, 9) at a threshold of p < 0.05, uncorrected. A few regions showed greater grey matter volume in participants with ADHD than in controls (albeit at the same uncorrected thresholds, representing medium effect sizes). This included greater right caudate and left ventrolateral prefrontal cortex volume in the ADHD than in the control or conduct disorder groups.
Table 2.
Total intracranial volume and specific brain regions that differ among ADHD, conduct disorder and control groups*
| Brain region (Brodmann area) | MNI peak coordinate | ANOVA omnibus-F | Cohen d effect size | ||||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| x | y | z | CD < ADHD | CD < control | ADHD < control | ||
| Total intracranial volume | 2.764 | 0.51 | — | — | |||
| Total grey matter volume | 4.351 | — | 0.71 | — | |||
| Total white matter volume | 0.715 | — | — | — | |||
| Total CSF volume | 1.107 | — | — | — | |||
| Frontal lobe | |||||||
| Left precentral/inferior frontal gyri (BA 6, 9) | −54 | 6 | 40.5 | 12.33 | 0.91† | 1.43† | 0.60‡ |
| Left inferior frontal gyrus (BA 45, 44, 47) | −60 | 18 | 16.5 | 9.10 | 1.24† | 0.68‡ | −0.53‡ |
| Rostral anterior cingulate | 9 | 36 | −4.5 | 7.86 | 1.10† | 0.89† | — |
| Medial frontal gyrus (BA 10) | 4.5 | 52.5 | 7.5 | 8.43 | 1.08† | 1.01† | — |
| Superior/medial frontal gyri (BA 10) | −9 | 70.5 | −6 | 7.59 | 1.00† | 0.99† | — |
| Left gyrus rectus/medial frontal gyrus (BA 11, 25) | 6 | 40.5 | −19.5 | 9.37 | 1.15† | 1.05† | — |
| Right gyrus rectus/medial frontal gyrus (BA 11) | −7.5 | 49.5 | −19.5 | 8.10 | 1.03† | 1.02† | — |
| Right inferior/middle frontal gyri | 48 | 42 | −18 | 8.07 | 1.17† | 0.69‡ | — |
| Right inferior/middle frontal gyri (lateral orbitofrontal BA 47, 11) | 30 | 37.5 | −9 | 14.23 | 1.54† | 0.99† | −0.50‡ |
| Left inferior/middle frontal gyri/(lateral orbitofrontal BA 47, 11) | −30 | 27 | −16.5 | 10.07 | 1.23† | 1.02† | — |
| Right inferior/middle frontal gyri (orbitofrontal BA 47, 11) | 24 | 24 | −19.5 | 13.26 | 1.34† | 1.28† | — |
| Left insular cortex (BA 13) | −39 | 1.5 | −10.5 | 6.77 | 1.03† | 0.79† | — |
| Temporal lobe | |||||||
| Left superior/transverse temporal gyri (BA 41, 40) | −49.5 | −31.5 | 6 | 10.50 | 1.19† | 1.09† | — |
| Right superior temporal gyrus | 37.5 | −3 | −16.5 | 8.84 | 1.09† | 1.05† | — |
| Left amygdala/hippocampus/parahippocampal gyrus | −19.5 | −3 | −19.5 | 6.38 | 1.04† | 0.56‡ | — |
| Right amygdala/hippocampus/parahippocampal gyrus | 24 | −1.5 | −24 | 6.49 | 0.99† | 0.83† | — |
| Left parahippocampal/fusiform gyri (BA 36, 35) | −22.5 | −39 | −10.5 | 8.35 | 1.18† | 0.74† | — |
| Right parahippocampal/fusiform gyri (BA 27, 36, 35) | 21 | −34.5 | −9 | 10.39 | 1.33† | 0.70‡ | −0.59‡ |
| Right fusiform/inferior temporal gyri (BA 20, 37) | 52.5 | −37.5 | −25.5 | 8.78 | 1.07† | 1.06† | — |
| Parietal lobe | |||||||
| Right precuneus/cingulate gyrus (BA 31, 5) | 6 | −42 | 48 | 12.69 | 1.28† | 1.28† | — |
| Right insular cortex (Rolandic operculum BA 13, 6) | 45 | −10.5 | 16.5 | 12.37 | 1.34† | 1.17† | — |
| Paracentral lobule/cingulate gyrus (SMA BA 6, 24) | −1.5 | −10.5 | 51 | 6.67 | 1.06† | 0.66‡ | — |
| Left postcentral/precentral gyri | −22.5 | −30 | 73.5 | 7.68 | 0.84‡ | 1.10† | — |
| Right postcentral/precentral gyri | 43.5 | −28.5 | 57 | 9.02 | 1.17† | 0.96† | — |
| Right supramarginal gyrus | 69 | −19.5 | 28.5 | 7.44 | 0.90‡ | 1.05† | — |
| Left inferior parietal lobule/supramarginal gyrus | −67.5 | −30 | 28.5 | 7.02 | 1.05† | 0.80† | — |
| Occipital lobe | |||||||
| Left fusiform/lingual/parahippocampal gyri (BA 37, 19, 18) | −30 | −69 | −10.5 | 9.40 | 1.14† | 1.07† | — |
| Right fusiform/lingual/parahippocampal gyri (BA 19, 18, 37) | 25.5 | −63 | −9 | 17.21 | 1.39† | 1.58† | — |
| Right lingual/cuneus (BA 18) | 3 | −84 | −4.5 | 11.69 | 1.25† | 1.22† | — |
| Left precuneus | −1.5 | −69 | 27 | 7.38 | 1.09† | 0.78† | — |
| Subcortical region | |||||||
| Right caudate (head) | 9 | 22.5 | −1.5 | 6.68 | 1.06† | — | −0.58‡ |
ADHD = attention-deficit/hyperactivity disorder; ANOVA = analysis of variance; BA = Brodmann area; CSF = cerebrospinal fluid; MNI = Montreal Neurological Institute; SMA = supplementary motor area.
Determined through SPM8 1–way ANOVA. Distinct regions that significantly differ (q < 0.05 false discovery rate) are listed. The last columns report the Cohen d effect size estimate from planned post hoc pairwise comparisons between study groups. Effect sizes are listed if they surpass a “medium” effect (Cohen d = 0.50).
Denotes pairwise group difference that survives Dunn–Sidak correction for 3 2–tailed tests among study groups.
Denotes pairwise group difference at p < 0.05, uncorrected, for multiple comparisons.
Fig. 1.
Medial and lateral view of brain renderings of regions that significantly differed across conduct disorder, attention-deficit/hyperactivity disorder and control groups. Results are thresholded at a false discovery rate of q < 0.05, corrected, for the whole brain. (A–E) Several frontal and temporal lobe brain regions hypothesized a priori to differ among study groups along with Cohen d effect size estimates of the differences between study groups. BA = Brodmann area
Analysis of variance comparisons of white matter volume among study groups did not detect any significant differences.
Supplemental correlation analysis of grey matter volume with symptom severity in conduct disorder and ADHD groups
We queried voxels of peak group differences reported in Table 1 separately for each diagnostic group to determine whether they had a significant association with the severity of conduct disorder or ADHD symptoms. Lesser grey matter volume in the right precuneus, left sensorimotor cortex and left amygdala in the ADHD group was related to greater symptom severity. For conduct disorder, greater grey matter volume in the right ventrolateral/lateral orbitofrontal cortex, bilateral amygdala and both left and right lateral temporal lobe cortex was associated with greater symptom severity. Appendix 1, Table S1, available at cma.ca/jpn, lists brain regions with significant (p < 0.05, uncorrected) exploratory effects.
Exploratory pairwise group differences: ADHD versus control and conduct disorder versus control groups
Because, to our knowledge, this was the first direct comparison of pure conduct disorder and ADHD, we further explored pairwise study group differences at a threshold of p < 0.05, uncorrected, to identify any other disorder-specific differences. Interesting ADHD findings included grey matter volume deficits in several cerebellar regions and the left ventrolateral prefrontal and parietal cortices. There was also some evidence for greater grey matter volume, and both deficits and excesses of white matter volume in the ADHD group. Exploration of conduct disorder at liberal thresholds revealed evidence for grey matter volume deficits in subcortical structures (thalamus and caudate), the cerebellum and occipital lobe. Additional white matter volume deficits were found in numerous regions in participants with conduct disorder. Details of these results are available in Appendix 1, Tables S2 and S3; however, because they are exploratory, they are not discussed in depth here and have a limited impact on study conclusions.
Discussion
To our knowledge, the present study was the first direct comparison of brain structure among adolescents with pure DSM-IV conduct disorder, pure combined-subtype ADHD and matched controls. The total brain grey matter volume deficit in youth with conduct disorder was 13%, compared with a nonsignificant 3% reduction in those with ADHD. Our estimate of ADHD total grey matter volume deficit is smaller than in previous whole brain studies in samples with pure ADHD (typically reporting 7%–8%37–39,41), but our results are consistent with the only other whole brain study of adolescents with ADHD, which reported a 4% deficit in total grey matter volume.35 Consistent with our hypotheses, ANOVA identified numerous specific regional volumetric differences between the study groups. Most interesting was the observation that all grey matter regions detected as different among study groups in all lobes were of larger magnitude in youth with conduct disorder than in youth with ADHD or were found only in those with conduct disorder. The average regional grey matter deficit effect size was large59 (Cohen d = 1.14, range 0.84–1.54) in the conduct disorder group, whereas that in the ADHD group was medium (range 0.58–0.72). Moreover, the greater relative deficits in the conduct disorder group distinguished the 2 disorders in almost every region assessed in the post hoc test. Notably, we only found a single regional grey matter deficit in the frontal lobe (left dorsolateral/precentral prefrontal) in the ADHD sample, which is inconsistent with several previous reports2 of brain volume in ADHD samples. However, nearly all these previous studies that detected prefrontal cortex volume deficits included patients with comorbid conduct disorder. Also, most previous studies reporting prefrontal cortex deficits in ADHD samples used summary frontal lobe volume measurements23,26,31,36–38,41 rather than querying more localized regions. Whereas it is possible that only select, small regional volume deficits (e.g., left dorsolateral premotor/prefrontal identified in this study) in ADHD samples were responsible for previous findings from total volume summary measures, it seems more likely that most previously reported frontal lobe deficits in ADHD samples were the result of comorbid conduct disorder. Alternatively, it remains possible that global frontal lobe grey matter volume deficits in people with ADHD might reflect a unique, informative pathophysiological process worthy of further investigation.
The striking differences in the profile of brain structure abnormalities between noncomorbid conduct disorder and ADHD suggest the need to re-evaluate our understanding of regional volume deficits in people with ADHD, particularly deficits in frontal lobe grey matter. We realize that this conclusion is surprising, and perhaps somewhat provocative; however, given the absence of careful, direct comparison of noncomorbid conduct disorder and ADHD in the literature, it is not unreasonable to tentatively conclude that
some volume abnormalities previously attributed to ADHD are actually better linked to conduct disorder, and
the etiological factors that resulted in the different profiles and magnitude of volume deficits in the conduct disorder and ADHD groups likely differ for these 2 disorders.
Indeed, the only evidence for a shared structural abnormality was found for left dorsolateral/precentral gyri (BA 9), where both diagnostic groups had deficits (albeit of different magnitudes) compared with controls. It is unlikely that this abnormality reflects a shared trait-like behavioural disinhibition, because functional MRI (fMRI) has not linked trait impulsivity to functional abnormalities in this region,61 and the 2 disorders have markedly different clinical presentations. This region is noted for its role in many types of executive cognition,62 suggesting a common neural liability of cognitive control over behaviour. A key question for future studies is whether this putatively shared deficit represents a specific type of impulsivity or disinhibition63,64 as a direct etiological factor (i.e., dorsolateral prefrontal structural deficits are present from birth and causally influence symptom expression) that is common to both disorders. Alternatively, perhaps volumetric deficits in this region arise from different, possibly indirect etiological factors (i.e., brain volume deficits emerge over time as a consequence of disrupted neural development in one group and from dysfunctional mechanisms proximal to disorder etiology in the other group). For this particular dorsolateral brain region, the latter possibility seems more reasonable, given that fMRI studies of pure ADHD65–68 more consistently find ventrolateral (but not dorsolateral) prefrontal activity deficits in people with ADHD. A factor complicating the interpretation of our results is that functional deficits do not always occur in regions with structural abnormalities. Emerging conceptualizations of some psychiatric illnesses as disorders of large-scale distributed network integration (for example, see Makris and colleagues4 and Banaschewski and colleagues69) have begun to emphasize more greatly the idea that deficits in one region (e.g., structural deficits in the striatum, cerebellum or specific abnormal white matter connections in people with ADHD) could plausibly exert “downstream” effects that alter functional engagement of other regions in certain cognitive contexts. In addition, it is increasingly asked whether more than one form of pathophysiology can be isolated using neuroimaging that gives rise to ADHD symptoms (for example, see Sonuga-Barke and Halperin70 and Cherkasova and Hechtman71). It is possible that the failure to find stronger volumetric differences between the ADHD and control groups in the present study might simply reflect an admixture of different ADHD causal mechanisms. In particular, to our knowledge, no previous studies of noncomorbid ADHD have distinguished between ADHD clinical subtypes as defined by DSM-IV. Therefore, our findings might be specific to combined-subtype ADHD, whereas different results in the frontal lobe might be found for predominantly inattentive youth. Given all these different possibilities, we hope that the present study’s findings serve as an important point of comparison for future research seeking to better understand the numerous, complicated influences on brain structure in ADHD samples with meaningfully different behavioural presentations.
Another of our study findings was the lack of strong white matter volume differences among the diagnostic groups. Only exploratory analyses at liberal statistical thresholds detected white matter abnormalities specific to conduct disorder or ADHD. Although each diagnostic group had noteworthy deficits relative to controls in supplemental analyses at more liberal statistical thresholds (Cohen d = 0.53–0.98), the overall ANOVA could not confirm that these differed across study groups. At the very least, this suggests that white matter volume is not as important a potential differentiator of the 2 diagnoses as grey matter volume. A final finding was evidence for greater grey matter volume in the right caudate, left ventrolateral prefrontal, lateral orbitofrontal and parahippocampal gyri in the ADHD group. Previous reports have occasionally noted greater regional volumes in people with ADHD, but specific effects are inconsistent and do not typically overlap with our findings.28,34 However, previous research has linked psychostimulant treatment to the left ventrolateral prefrontal cortex, with treatment normalizing ADHD cortical thickness in this area.72
For conduct disorder, our findings of numerous medial (anterior cingulate, ventral part of medial frontal gyrus) and lateral (dorso/ventrolateral, insular, frontopolar) frontal lobe deficits and temporal lobe deficits are consistent with those of previous studies73 and current frontotemporal neurocognitive theories of antisociality.1,74 For example, previous fMRI studies of pure conduct disorder noted temporal lobe activation deficits in response to emotional stimuli in participants with conduct disorder, but not in those with ADHD.43 Detection of these findings likely was obscured by the ADHD sampling comorbidity in previous brain volume examinations of people with conduct disorder. Theories of antisocial disorder pathophysiology should attempt to incorporate these findings. We also observed an association between conduct disorder symptom severity and ventrolateral prefrontal and temporal lobe abnormalities. Although this positive association was in the opposite direction that many might have anticipated, it was roughly consistent in directionality with the findings of 2 recent psychopathy trait studies13,75 and implicated some of the same regions linked to abnormal emotional/motivational valuation, amoral cognition and attention to emotion.76 Because we did not measure callous/unemotional traits, it was not possible to examine this idea further, which might better explain the positive association. Future studies should ascertain whether this represents disrupted normative developmental volume reductions throughout adolescence, as others have argued,13 possibly specific to the proposed DSM-V callous/unemotional conduct disorder trait.77 However, it is important to keep in mind that the field has not reached consensus on whether greater or lesser grey matter volume in adolescence necessarily translates to better functioning, as the relationships between neural development, brain structure/function, cognition and clinical status are complicated and require additional study.
Limitations
Although the rigorously confirmed lack of disorder comorbidity is the key strength of this study, the focus on combined-subtype ADHD and purposeful exclusion of comorbid DSM-IV diagnoses prevents generalization to typical clinical groups who often present with complex psychopathology. A comparable study of younger children might produce different findings of equal importance to developmental theories of each disorder. Another study limitation is our modest sample size. We could not attempt meaningful sex comparisons, confidently examine possible associations between brain abnormalities and specific subtypes of symptoms (e.g., aggression, hyperactivity), explore possible effects of medication history on volume, or make firm conclusions about exploratory evidence for grey matter volume deficits unique to the ADHD group or various white matter abnormalities noted in both the ADHD and conduct disorder groups that were not found by the primary ANOVA. However, it is likely that many deficits detected by our supplemental analyses were meaningful. A supplemental power analysis indicated effective control over type II error in the ANOVA only for medium-to-large effect sizes,59 which are somewhat larger than the pairwise group differences found in our supplemental analyses. However, all the ANOVA results tell us is that other, smaller-magnitude abnormalities in these other brain regions fail to distinguish the 3 study groups, not that they are without import. Indeed, most grey matter volume deficits observed at liberal statistical thresholds in the ADHD group (Appendix 1, Table S1) replicated deficits frequently found in studies of pure ADHD samples.33–41 These deficits in people with ADHD included medium-to-large effect size deficits in the cerebellum, ventrolateral prefrontal and somatosensory/premotor cortices. A final limitation is that our use of an IQ estimate based on reading ability, rather than IQ itself, to confirm possible verbal–conceptual ability differences did not influence the results, particularly in the conduct disorder sample. However, this measure was arguably more relevant to cognitive deficits often found in people with conduct disorder.
Conclusion
Despite its limitations, this study makes several contributions to our understanding of brain structure abnormality diagnostic specificity. First, to our knowledge, it demonstrates for the first time striking differences between ADHD and conduct disorder in specific regional grey matter volume abnormalities. Importantly, we failed to find evidence for strong grey matter volume deficits in adolescents with ADHD in most specific frontal lobe regions that have variably been implicated in previous studies, even when using liberal statistical thresholds. Moreover, these results held regardless of individual or group differences in total intracranial volume, sex or level of academic achievement. This suggests that much of the previously reported evidence for specific prefrontal volume deficits in ADHD samples was the result of comorbid conduct disorder. There is a widely held belief, supported by a meta-analysis2 of dozens of studies, that ADHD involves prefrontal cortex brain structure abnormality. However, the present study failed to differentiate samples of pure ADHD, conduct disorder and controls in nearly all prefrontal regions, calling into question this belief and raising the need to revisit etiological theories of ADHD that depend on volumetric abnormality in the prefrontal cortex. Such findings require replication; it would be useful to learn whether reanalysis of the largest published studies of ADHD samples would support or contradict these findings. Second, the study determined that the single prefrontal volume deficit shared by both diagnostic groups was greater in the conduct disorder than the ADHD group. If this abnormality is related to the same trait, it raises questions about expression of this trait or possibly different genetic contributions. Third, the study confirms previously reported structural brain abnormalities in people with conduct disorder in samples with comorbid ADHD. To our knowledge, ours was the first-ever study of brain volume deficits in adolescents with diagnoses of noncomorbid conduct disorder, as all previously reported samples all or almost all had comorbid ADHD. We report many specific abnormal regions in adolescents with conduct disorder that are the same as those that function abnormally in antisocial disorder samples.45 Finally, we found several novel regional volume deficits in the conduct disorder sample that might be used to refine frontotemporal theories. Many theorists have increasingly considered distributed network dysfunction models of ADHD and antisocial disorders (for example, see Makris and colleagues4 and Banaschewski and colleagues69) instead of seeking to identify single pathognomonic core deficits. Better understanding of unique versus shared structural deficits should facilitate new research seeking to understand disorder-specific proximate causes of whole brain dysfunction that lead to symptoms.
Acknowledgements
This study was funded by National Institute of Menatl Health grant K23 MH070036 (M.C. Stevens) and supported in part by R01 MH080956 (M.C. Stevens). Appreciation is offered to the Connecticut Court Support Services Division program and research staff who collected and helped to prepare the data for analysis.
Footnotes
Competing interests: As above for M.C. Stevens. None declared for E. Haney-Caron.
Contributors: M.C. Stevens designed the study, acquired the data and reviewed the article. Both he and E. Haney-Caron analyzed the data, wrote the article and approved its publication.
References
- 1.Gao Y, Glenn AL, Schug RA, et al. The neurobiology of psychopathy: a neurodevelopmental perspective. Can J Psychiatry. 2009;54:813–23. doi: 10.1177/070674370905401204. [DOI] [PubMed] [Google Scholar]
- 2.Valera EM, Faraone SV, Murray KE, et al. Meta-analysis of structural imaging findings in attention-deficit/hyperactivity disorder. Biol Psychiatry. 2007;61:1361–9. doi: 10.1016/j.biopsych.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 3.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed. Washington: The Association; 2000. text revised. [Google Scholar]
- 4.Makris N, Biederman J, Monuteaux MC, et al. Towards conceptualizing a neural systems-based anatomy of attention-deficit/hyperactivity disorder. Dev Neurosci. 2009;31:36–49. doi: 10.1159/000207492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang Y, Raine A. Prefrontal structural and functional brain imaging findings in antisocial, violent, and psychopathic individuals: a meta-analysis. Psychiatry Res. 2009;174:81–8. doi: 10.1016/j.pscychresns.2009.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ellison-Wright I, Ellison-Wright Z, Bullmore E. Structural brain change in attention deficit hyperactivity disorder identified by meta-analysis. BMC Psychiatry. 2008;8:51. doi: 10.1186/1471-244X-8-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hutchinson AD, Mathias JL, Banich MT. Corpus callosum morphology in children and adolescents with attention deficit hyperactivity disorder: a meta-analytic review. Neuropsychology. 2008;22:341–9. doi: 10.1037/0894-4105.22.3.341. [DOI] [PubMed] [Google Scholar]
- 8.Seidman LJ, Valera EM, Makris N. Structural brain imaging of attention-deficit/hyperactivity disorder. Biol Psychiatry. 2005;57:1263–72. doi: 10.1016/j.biopsych.2004.11.019. [DOI] [PubMed] [Google Scholar]
- 9.Vloet TD, Konrad K, Huebner T, et al. Structural and functional MRI-findings in children and adolescents with antisocial behavior. Behav Sci Law. 2008;26:99–111. doi: 10.1002/bsl.794. [DOI] [PubMed] [Google Scholar]
- 10.Abikoff H, Klein RG. Attention-deficit hyperactivity and conduct disorder: comorbidity and implications for treatment. J Consult Clin Psychol. 1992;60:881–92. doi: 10.1037//0022-006x.60.6.881. [DOI] [PubMed] [Google Scholar]
- 11.Angold A, Costello EJ, Erkanli A. Comorbidity. J Child Psychol Psychiatry. 1999;40:57–87. [PubMed] [Google Scholar]
- 12.Huebner T, Vloet TD, Marx I, et al. Morphometric brain abnormalities in boys with conduct disorder. J Am Acad Child Adolesc Psychiatry. 2008;47:540–7. doi: 10.1097/CHI.0b013e3181676545. [DOI] [PubMed] [Google Scholar]
- 13.De Brito SA, Mechelli A, Wilke M, et al. Size matters: increased grey matter in boys with conduct problems and callous-unemotional traits. Brain. 2009;132:843–52. doi: 10.1093/brain/awp011. [DOI] [PubMed] [Google Scholar]
- 14.Sterzer P, Stadler C, Poustka F, et al. A structural neural deficit in adolescents with conduct disorder and its association with lack of empathy. Neuroimage. 2007;37:335–42. doi: 10.1016/j.neuroimage.2007.04.043. [DOI] [PubMed] [Google Scholar]
- 15.Kruesi MJ, Casanova MF, Mannheim G, et al. Reduced temporal lobe volume in early onset conduct disorder. Psychiatry Res. 2004;132:1–11. doi: 10.1016/j.pscychresns.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 16.Bussing R, Grudnik J, Mason D, et al. ADHD and conduct disorder: an MRI study in a community sample. World J Biol Psychiatry. 2002;3:216–20. doi: 10.3109/15622970209150624. [DOI] [PubMed] [Google Scholar]
- 17.Overmeyer S, Bullmore ET, Suckling J, et al. Distributed grey and white matter deficits in hyperkinetic disorder: MRI evidence for anatomical abnormality in an attentional network. Psychol Med. 2001;31:1425–35. doi: 10.1017/s0033291701004706. [DOI] [PubMed] [Google Scholar]
- 18.McAlonan GM, Cheung V, Cheung C, et al. Mapping brain structure in attention deficit-hyperactivity disorder: a voxel-based MRI study of regional grey and white matter volume. Psychiatry Res. 2007;154:171–80. doi: 10.1016/j.pscychresns.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 19.Mataró M, Garcia-Sanchez C, Junque C, et al. Magnetic resonance imaging measurement of the caudate nucleus in adolescents with attention-deficit hyperactivity disorder and its relationship with neuropsychological and behavioral measures. Arch Neurol. 1997;54:963–8. doi: 10.1001/archneur.1997.00550200027006. [DOI] [PubMed] [Google Scholar]
- 20.Lyoo IK, Noam GG, Lee CK, et al. The corpus callosum and lateral ventricles in children with attention-deficit hyperactivity disorder: a brain magnetic resonance imaging study. Biol Psychiatry. 1996;40:1060–3. doi: 10.1016/s0006-3223(96)00349-6. [DOI] [PubMed] [Google Scholar]
- 21.Hynd GW, Semrud-Clikeman M, Lorys AR, et al. Corpus callosum morphology in attention deficit-hyperactivity disorder: morphometric analysis of MRI. J Learn Disabil. 1991;24:141–6. doi: 10.1177/002221949102400302. [DOI] [PubMed] [Google Scholar]
- 22.Giedd JN, Castellanos FX, Casey BJ, et al. Quantitative morphology of the corpus callosum in attention deficit hyperactivity disorder. Am J Psychiatry. 1994;151:665–9. doi: 10.1176/ajp.151.5.665. [DOI] [PubMed] [Google Scholar]
- 23.Durston S, Hulshoff Pol HE, Schnack HG, et al. Magnetic resonance imaging of boys with attention-deficit/hyperactivity disorder and their unaffected siblings. J Am Acad Child Adolesc Psychiatry. 2004;43:332–40. doi: 10.1097/00004583-200403000-00016. [DOI] [PubMed] [Google Scholar]
- 24.Castellanos FX, Lee PP, Sharp W, et al. Developmental trajectories of brain volume abnormalities in children and adolescents with attention-deficit/hyperactivity disorder. JAMA. 2002;288:1740–8. doi: 10.1001/jama.288.14.1740. [DOI] [PubMed] [Google Scholar]
- 25.Castellanos FX, Giedd JN, Berquin PC, et al. Quantitative brain magnetic resonance imaging in girls with attention-deficit/hyperactivity disorder. Arch Gen Psychiatry. 2001;58:289–95. doi: 10.1001/archpsyc.58.3.289. [DOI] [PubMed] [Google Scholar]
- 26.Castellanos FX, Giedd JN, Marsh WL, et al. Quantitative brain magnetic resonance imaging in attention-deficit hyperactivity disorder. Arch Gen Psychiatry. 1996;53:607–16. doi: 10.1001/archpsyc.1996.01830070053009. [DOI] [PubMed] [Google Scholar]
- 27.Castellanos FX, Giedd JN, Eckburg P, et al. Quantitative morphology of the caudate nucleus in attention deficit hyperactivity disorder. Am J Psychiatry. 1994;151:1791–6. doi: 10.1176/ajp.151.12.1791. [DOI] [PubMed] [Google Scholar]
- 28.Wang J, Jiang T, Cao Q, et al. Characterizing anatomic differences in boys with attention-deficit/hyperactivity disorder with the use of deformation-based morphometry. AJNR Am J Neuroradiol. 2007;28:543–7. [PMC free article] [PubMed] [Google Scholar]
- 29.Sowell ER, Thompson PM, Welcome SE, et al. Cortical abnormalities in children and adolescents with attention-deficit hyperactivity disorder. Lancet. 2003;362:1699–707. doi: 10.1016/S0140-6736(03)14842-8. [DOI] [PubMed] [Google Scholar]
- 30.McAlonan GM, Cheung V, Chua SE, et al. Age-related grey matter volume correlates of response inhibition and shifting in attention-deficit hyperactivity disorder. Br J Psychiatry. 2009;194:123–9. doi: 10.1192/bjp.bp.108.051359. [DOI] [PubMed] [Google Scholar]
- 31.Hynd GW, Semrud-Clikeman M, Lorys AR, et al. Brain morphology in developmental dyslexia and attention deficit disorder/hyperactivity. Arch Neurol. 1990;47:919–26. doi: 10.1001/archneur.1990.00530080107018. [DOI] [PubMed] [Google Scholar]
- 32.Baumgardner TL, Singer HS, Denckla MB, et al. Corpus callosum morphology in children with Tourette syndrome and attention deficit hyperactivity disorder. Neurology. 1996;47:477–82. doi: 10.1212/wnl.47.2.477. [DOI] [PubMed] [Google Scholar]
- 33.Berquin PC, Giedd JN, Jacobsen LK, et al. Cerebellum in attention-deficit hyperactivity disorder: a morphometric MRI study. Neurology. 1998;50:1087–93. doi: 10.1212/wnl.50.4.1087. [DOI] [PubMed] [Google Scholar]
- 34.Brieber S, Neufang S, Bruning N, et al. Structural brain abnormalities in adolescents with autism spectrum disorder and patients with attention deficit/hyperactivity disorder. J Child Psychol Psychiatry. 2007;48:1251–8. doi: 10.1111/j.1469-7610.2007.01799.x. [DOI] [PubMed] [Google Scholar]
- 35.Carmona S, Vilarroya O, Bielsa A, et al. Global and regional gray matter reductions in ADHD: a voxel-based morphometric study. Neurosci Lett. 2005;389:88–93. doi: 10.1016/j.neulet.2005.07.020. [DOI] [PubMed] [Google Scholar]
- 36.Filipek PA, Semrud-Clikeman M, Steingard RJ, et al. Volumetric MRI analysis comparing subjects having attention-deficit hyperactivity disorder with normal controls. Neurology. 1997;48:589–601. doi: 10.1212/wnl.48.3.589. [DOI] [PubMed] [Google Scholar]
- 37.Hill DE, Yeo RA, Campbell RA, et al. Magnetic resonance imaging correlates of attention-deficit/hyperactivity disorder in children. Neuropsychology. 2003;17:496–506. doi: 10.1037/0894-4105.17.3.496. [DOI] [PubMed] [Google Scholar]
- 38.Mostofsky SH, Cooper KL, Kates WR, et al. Smaller prefrontal and premotor volumes in boys with attention-deficit/hyperactivity disorder. Biol Psychiatry. 2002;52:785–94. doi: 10.1016/s0006-3223(02)01412-9. [DOI] [PubMed] [Google Scholar]
- 39.Pineda DA, Restrepo MA, Sarmiento RJ, et al. Statistical analyses of structural magnetic resonance imaging of the head of the caudate nucleus in Colombian children with attention-deficit hyperactivity disorder. J Child Neurol. 2002;17:97–105. doi: 10.1177/088307380201700202. [DOI] [PubMed] [Google Scholar]
- 40.Semrud-Clikeman M, Filipek PA, Biederman J, et al. Attention-deficit hyperactivity disorder: magnetic resonance imaging morphometric analysis of the corpus callosum. J Am Acad Child Adolesc Psychiatry. 1994;33:875–81. doi: 10.1097/00004583-199407000-00014. [DOI] [PubMed] [Google Scholar]
- 41.Wolosin SM, Richardson ME, Hennessey JG, et al. Abnormal cerebral cortex structure in children with ADHD. Hum Brain Mapp. 2009;30:175–84. doi: 10.1002/hbm.20496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jones AP, Laurens KR, Herba CM, et al. Amygdala hypoactivity to fearful faces in boys with conduct problems and callous-unemotional traits. Am J Psychiatry. 2009;166:95–102. doi: 10.1176/appi.ajp.2008.07071050. [DOI] [PubMed] [Google Scholar]
- 43.Herpertz SC, Huebner T, Marx I, et al. Emotional processing in male adolescents with childhood-onset conduct disorder. J Child Psychol Psychiatry. 2008;49:781–91. doi: 10.1111/j.1469-7610.2008.01905.x. [DOI] [PubMed] [Google Scholar]
- 44.Passamonti L, Fairchild G, Goodyer IM, et al. Neural abnormalities in early-onset and adolescence-onset conduct disorder. Arch Gen Psychiatry. 2010;67:729–38. doi: 10.1001/archgenpsychiatry.2010.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rubia K. “Cool” inferior frontostriatal dysfunction in attention-deficit/hyperactivity disorder versus “hot” ventromedial orbitofrontallimbic dysfunction in conduct disorder: a review”. Biol Psychiatry. 2011;69:e69–87. doi: 10.1016/j.biopsych.2010.09.023. [DOI] [PubMed] [Google Scholar]
- 46.Sasayama D, Hayashida A, Yamasue H, et al. Neuroanatomical correlates of attention-deficit-hyperactivity disorder accounting for comorbid oppositional defiant disorder and conduct disorder. Psychiatry Clin Neurosci. 2010;64:394–402. doi: 10.1111/j.1440-1819.2010.02102.x. [DOI] [PubMed] [Google Scholar]
- 47.Wahlund K, Kristiansson M. Aggression, psychopathy and brain imaging. Review and future recommendations. Int J Law Psychiatry. 2009;32:266–71. doi: 10.1016/j.ijlp.2009.04.007. [DOI] [PubMed] [Google Scholar]
- 48.Weber S, Habel U, Amunts K, et al. Structural brain abnormalities in psychopaths-a review. Behav Sci Law. 2008;26:7–28. doi: 10.1002/bsl.802. [DOI] [PubMed] [Google Scholar]
- 49.Kaufman J, Birmaher B, Brent D, et al. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36:980–8. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- 50.Jastak J, Wilkinson G. WRAT-3: Wide range achievement test administration manual. Lutz (FL): Wide Range, Inc; 1993. [Google Scholar]
- 51.Strauss E, Sherman EMS, Spreen O. A compendium of neuropsychological tests: administration, norms and commentary. 3rd ed. New York: Oxford University Press; 2006. [Google Scholar]
- 52.Moffitt TE. Adolescence-limited and life-course-persistent antisocial behavior: a developmental taxonomy. Psychol Rev. 1993;100:674–701. [PubMed] [Google Scholar]
- 53.Ashburner J. A fast diffeomorphic image registration algorithm. Neuroimage. 2007;38:95–113. doi: 10.1016/j.neuroimage.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 54.Klein A, Andersson J, Ardekani BA, et al. Evaluation of 14 nonlinear deformation algorithms applied to human brain MRI registration. Neuroimage. 2009;46:786–802. doi: 10.1016/j.neuroimage.2008.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ashburner J, Friston KJ. Unified segmentation. Neuroimage. 2005;26:839–51. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 56.Ashburner J, Friston KJ. Voxel-based morphometry–the methods. Neuroimage. 2000;11:805–21. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- 57.Hayasaka S, Phan KL, Liberzon I, et al. Nonstationary cluster-size inference with random field and permutation methods. Neuroimage. 2004;22:676–87. doi: 10.1016/j.neuroimage.2004.01.041. [DOI] [PubMed] [Google Scholar]
- 58.Genovese CR, Lazar NA, Nichols T. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage. 2002;15:870–8. doi: 10.1006/nimg.2001.1037. [DOI] [PubMed] [Google Scholar]
- 59.Cohen J. Statistical power analysis for the behavioral sciences. 2nd ed. Hillsdale (NJ): L. Erlbaum Associates; 1988. [Google Scholar]
- 60.Ridgway GR, Omar R, Ourselin S, et al. Issues with threshold masking in voxel-based morphometry of atrophied brains. Neuroimage. 2009;44:99–111. doi: 10.1016/j.neuroimage.2008.08.045. [DOI] [PubMed] [Google Scholar]
- 61.Horn NR, Dolan M, Elliott R, et al. Response inhibition and impulsivity: an fMRI study. Neuropsychologia. 2003;41:1959–66. doi: 10.1016/s0028-3932(03)00077-0. [DOI] [PubMed] [Google Scholar]
- 62.Tanji J, Hoshi E. Role of the lateral prefrontal cortex in executive behavioral control. Physiol Rev. 2008;88:37–57. doi: 10.1152/physrev.00014.2007. [DOI] [PubMed] [Google Scholar]
- 63.Birkley EL, Smith GT. Recent advances in understanding the personality underpinnings of impulsive behavior and their role in risk for addictive behaviors. Curr Drug Abuse Rev. 2011;4:215–27. doi: 10.2174/1874473711104040215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kim S, Lee D. Prefrontal cortex and impulsive decision making. Biol Psychiatry. 2011;69:1140–6. doi: 10.1016/j.biopsych.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rubia K, Halari R, Cubillo A, et al. Disorder-specific inferior prefrontal hypofunction in boys with pure attention-deficit/hyperactivity disorder compared to boys with pure conduct disorder during cognitive flexibility. Hum Brain Mapp. 2010;31:1823–33. doi: 10.1002/hbm.20975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rubia K, Halari R, Smith AB, et al. Shared and disorder-specific prefrontal abnormalities in boys with pure attention-deficit/hyperactivity disorder compared to boys with pure CD during interference inhibition and attention allocation. J Child Psychol Psychiatry. 2009;50:669–78. doi: 10.1111/j.1469-7610.2008.02022.x. [DOI] [PubMed] [Google Scholar]
- 67.Rubia K, Halari R, Smith AB, et al. Dissociated functional brain abnormalities of inhibition in boys with pure conduct disorder and in boys with pure attention deficit hyperactivity disorder. Am J Psychiatry. 2008;165:889–97. doi: 10.1176/appi.ajp.2008.07071084. [DOI] [PubMed] [Google Scholar]
- 68.Rubia K, Smith AB, Halari R, et al. Disorder-specific dissociation of orbitofrontal dysfunction in boys with pure conduct disorder during reward and ventrolateral prefrontal dysfunction in boys with pure ADHD during sustained attention. Am J Psychiatry. 2009;166:83–94. doi: 10.1176/appi.ajp.2008.08020212. [DOI] [PubMed] [Google Scholar]
- 69.Banaschewski T, Hollis C, Oosterlaan J, et al. Towards an understanding of unique and shared pathways in the psychopathophysiology of ADHD. Dev Sci. 2005;8:132–40. doi: 10.1111/j.1467-7687.2005.00400.x. [DOI] [PubMed] [Google Scholar]
- 70.Sonuga-Barke EJ, Halperin JM. Developmental phenotypes and causal pathways in attention deficit/hyperactivity disorder: potential targets for early intervention? J Child Psychol Psychiatry. 2010;51:368–89. doi: 10.1111/j.1469-7610.2009.02195.x. [DOI] [PubMed] [Google Scholar]
- 71.Cherkasova MV, Hechtman L. Neuroimaging in attention-deficit hyperactivity disorder: beyond the frontostriatal circuitry. Can J Psychiatry. 2009;54:651–64. doi: 10.1177/070674370905401002. [DOI] [PubMed] [Google Scholar]
- 72.Shaw P, Sharp WS, Morrison M, et al. Psychostimulant treatment and the developing cortex in attention deficit hyperactivity disorder. Am J Psychiatry. 2009;166:58–63. doi: 10.1176/appi.ajp.2008.08050781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fairchild G, Passamonti L, Hurford G, et al. Brain structure abnormalities in early-onset and adolescent-onset conduct disorder. Am J Psychiatry. 2011;168:624–33. doi: 10.1176/appi.ajp.2010.10081184. [DOI] [PubMed] [Google Scholar]
- 74.Glenn AL, Raine A. The neurobiology of psychopathy. Psychiatr Clin North Am. 2008;31:463–75. doi: 10.1016/j.psc.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 75.Glenn AL, Raine A, Yaralian PS, et al. Increased volume of the striatum in psychopathic individuals. Biol Psychiatry. 2010;67:52–8. doi: 10.1016/j.biopsych.2009.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Blair RJ. The amygdala and ventromedial prefrontal cortex in morality and psychopathy. Trends Cogn Sci. 2007;11:387–92. doi: 10.1016/j.tics.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 77.Moffitt TE, Arseneault L, Jaffee SR, et al. Research review: DSM-V conduct disorder: research needs for an evidence base. J Child Psychol Psychiatry. 2008;49:3–33. doi: 10.1111/j.1469-7610.2007.01823.x. [DOI] [PMC free article] [PubMed] [Google Scholar]