Abstract
Background
Schizophrenia is considered to be a disorder of cerebral connectivity associated with disturbances of cortical development. Disturbances in connectivity at an early period of cortical maturation can result in widespread defects in gyrification. Investigating the anatomic distribution of gyrification defects can provide important information about neurodevelopment in patients with schizophrenia.
Methods
We undertook an automated surface-based morphometric assessment of gyrification on 3-dimensionally reconstructed cortical surfaces across multiple vertices that cover the entire cortex. We used a sample from our previous research of 57 patients (50 men) with schizophrenia and 41 controls (39 men) in whom we had tested a specific hypothesis regarding presence of both hypo-and hypergyria in the prefrontal cortex using a frontal region-of-interest approach.
Results
Regions with significant reductions in gyrification (hypogyria) were seen predominantly in the left hemisphere, involving the insula and several regions of the multimodal association cortex. Although the prefrontal hypergyria documented earlier did not survive the statistical correction required for a whole brain search (cluster inclusion at p = 0.0001), significant hypergyric frontal clusters emerged when the threshold was lowered (cluster inclusion at p = 0.05). In the insula, a reduction in gyrification was related to reduced cortical thickness in patients with schizophrenia.
Limitations
We studied a sample of patients taking antipsychotic medications, which could have confounded the results. Our sample was predominantly male, limiting the generalizability of our findings.
Conclusion
Our observations suggest that the disturbances in cortical gyrification seen in patients with schizophrenia might be related to a disrupted interaction between the paralimbic and the multimodal association cortex and thus might contribute to the pathogenesis of the illness.
Introduction
Schizophrenia is regarded as a disorder of connectivity associated with neurodevelopmental abnormality.1 Despite accumulating evidence for disturbances in functional connectivity,2 it is unclear how these findings are related to defective cortical development.
Developmental aberrations can affect several characteristic anatomic features of the grey matter surface. Cortical development is constrained by the need to develop a cost-efficient wiring scheme wherein signal transmission is quick and effective3 and by the limitations on the total brain size partly to facilitate parturition.4,5 This is facilitated by a substantial expansion of surface area along with a high degree of cortical folding despite a relatively minor gain in cortical thickness during evolutionary development.6 This remarkable dissociation between thickness and surface area persists in adult human brains.7
Axonal connections in the developing brain are considered to be one of several factors that influence the morphology of the cortical surface.8 In particular, a widely accepted model of cortical morphogenesis suggests that the appearance of cortical convolutions is dependent on the underlying neuronal connectivity.9 Disturbances in regional cortical gyrification can be a surrogate marker for disruptions in neuronal connectivity during development.9,10
Most studies examining neuroanatomical changes in patients with schizophrenia have employed voxel-based morphometry (VBM) and report consistent volume reduction bilaterally in the insula and anterior cingulate cortex (ACC). Compared with the number of studies investigating volumetric defects, relatively few studies have attempted to locate gyrification defects within the entire cortex in patients with schizophrenia (see White and Hilgetag10 for a detailed review). Most previous investigations have quantified differences in gyrification at preselected regions of interest (ROI), especially the cingulate11,12 and the prefrontal cortex.13–15 As a result, unlike the robust evidence localizing volumetric changes in patients with schizophrenia to the insula, the spatial distribution of focal gyrification abnormalities in these patients is currently unclear. If widespread abnormalities are present, this might reflect a deviation of the neurodevelopmental processes that produce gyrification; abnormalities confined to specific pathways might indicate a more focal defect that affects regions that are mutually connected. In particular, the insula is a region of specific interest while investigating cortical folding in patients with schizophrenia, not only owing to its relevance to the illness,16 but also because it has been shown to be one of the earliest brain regions where gyrification occurs,17–19 with an accelerated growth rate compared to the surrounding cortical plate during fetal development.20
The search for the brain region with the most prominent gyrification defect in patients with schizophrenia is hampered by several methodological issues.21 Zilles and colleagues’ gyrification index (the ratio between the inner folded contour and the outer curvature),22 though commonly used,10 does not capture the regional changes associated with subtle deviations in cortical connectivity. Its use is further limited by 2-dimensional slices whose orientation and thickness could bias the measurements, leading to inconclusive results.
To address these issues, we undertook a blinded automated assessment of gyrification in multiple vertices across 3-dimensionally reconstructed cerebral surfaces in a sample of 98 individuals. In the same sample, we have previously reported abnormal frontal gyrification using an ROI approach to test the specific hypothesis of lateralized changes in pre-frontal gyrification,23 as the laterality and the location of hypergyria within the prefrontal cortex were controversial. For that study we used an ROI analysis, as it has much greater power than a vertex-based examination of the entire cortical surface and hence is the preferred method for addressing a specific a priori hypothesis regarding a spatially localized abnormality. In the present study we analysed the entire cortical surface using a vertex-based approach. On account of the need for stringent correction for multiple comparisons when undertaking a whole brain search, this study has much less statistical power and hence would only be anticipated to detect regions with a large effect size of between-group differences. Nonetheless, this method offers the possibility of determining whether or not substantial abnormalities of gyrification occur in multiple brain regions in patients with schizophrenia. Further, to investigate whether cortical thinning was associated with the pathogenetic processes resulting in abnormal gyrification, we also studied the cortical thickness in regions showing gyrification abnormalities in patients with schizophrenia.
Methods
Participants
A detailed description of the sample and imaging procedures has been reported in our previous study of cortical area in patients with schizophrenia24 and in a study examining lateralized changes in frontal lobe gyrification.23 Briefly, the sample consisted of 57 patients (50 men) satisfying DSM-IV criteria for schizophrenia and 41 healthy controls. The North Notting-hamshire Research and Ethics Committee (REC) approved the study, and all participants provided written informed consent. Clinicians in community mental health teams, including the early intervention in psychosis teams, initially referred the patients for this study. The responsible clinicians confirmed that the patients were capable of giving informed consent, and written consent was obtained following the procedure approved by the REC. The diagnosis of schizophrenia was made in a clinical consensus meeting in accordance with the procedure of Leckman and colleagues,25 using all available information, including a review of case files and a standardized clinical interview (Symptoms and Signs in Psychotic Illness [SSPI]26). All patients were in a stable phase of schizophrenia (defined as a change of no more than 10 points in their Global Assessment of Function [GAF, DSM-IV]) score, assessed 6 weeks prior to and immediately before study participation), and the mean duration of illness was 4.3 years. Individuals with neurologic disorders, current substance dependence, IQ less than 70 using Quick Test27 and diagnosis of any other Axis I disorder were excluded. All patients were receiving treatment with antipsychotics and had no change in their prescriptions in the 6 weeks preceding the study. The average dose in chlorpromazine equivalents was 288.7 mg (range 100–1200 mg). Patients with schizophrenia were interviewed on the same day of the scans, and symptom scores were assigned according to the SSPI.26 Healthy controls were recruited from the local community via advertisements and included 41 individuals (39 men) free of any psychiatric or neurologic disorder, group-matched for age and parental socioeconomic status (measured using National Statistics —Socio Economic Classification28) to the patient group. Controls had similar exclusion criteria to patients; in addition, individuals with personal or family history of psychotic illness were excluded. A clinical interview by a research psychiatrist was employed to ensure that the controls were free from current Axis I disorders and history of either psychotic illness or neurologic disorder. From the original sample of 42 controls, 1 person was excluded owing to a movement artifact in the magnetic resonance imaging (MRI) scan that precluded volumetric computations.
Image acquisition
The MRI scans were collected using a Philips 3-T imaging system equipped with an 8-channel phased array head coil. The scanning protocol included a single high-resolution 3-dimensional T1-weighted magnetization-prepared rapid-acquisition gradient echo (MPRAGE) volume with 160 slices of isotropic voxel size 1 × 1 × 1 mm3, flip angle 8°, field of view 256 × 256 × 160 mm3. Head motion was minimized using cushion pads and by providing reassurance at the beginning of the procedure. A quality check to exclude motion artifacts was carried out by 2 researchers independently using predefined criteria (see Appendix 1, available at cma.ca/jpn).
Surface extraction
Surface extraction was completed using FreeSurfer version 4.5.0.29 The preprocessing was performed as described by Dale and colleagues29 and was reported in detail for this sample in our previous study.23 Cortical thickness values were computed using the methods developed by Fischl and Dale.30
Gyrification index
We obtained local gyrification indices (LGIs) using the method of Schaer and colleagues,31 with images reconstructed through the Freesurfer pipeline. This method is a vertex-wise extension of Zilles and colleagues’ gyrification index, which gives a ratio of the inner folded contour to the outer perimeter of the cortex.22 Using the grey–white interface constructed via surface registration and cortical inflation using Freesurfer, a pial surface is first obtained by constructing a set of lines perpendicular to the grey–white interface. In the second step, an outer “hull” surface is generated by means of a morphological closing operation, which ensures that the local curvature at all points on the outer hull surface is less than the curvature of a 15-mm radius sphere (a radius chosen to ensure that the hull surface does not dip into the sulci).
This hull surface acts as the outer perimeter while the original pial surface provides the inner perimeter. Both inner and outer surfaces are tessellated with numerous vertices formed by the meeting points of triangles. For each vertex (j) on the outer surface, a spherical ROI is created with the vertex as the centre and a standard 25-mm radius. This sphere yields 2 area measures for each vertex. The outer measure (AreajO) is the area of that part of the hull defined by the intersection of this sphere with the hull surface. To measure the corresponding pial surface area, the pial ROI for the given vertex on the outer hull surface is determined as follows. Initially all vertices within AreajO (on the hull surface) are identified. Next, the nearest pial vertex to each of these hull vertices is identified. These pial vertices define the outline of pial mesh, whose area is then calculated using the sum of areas of all included triangular tessellations (AreajP). The ratio of the pial surface area to the outer surface area gives the local gyrification index for each vertex on the outer surface (AreajP:AreajO). These outer surface values are redistributed to the pial surface using a weighted sum of all outer surface LGIs to which each pial vertex contributed during the prior computation. The weighting was inversely proportional to the distance of the hull vertex from the pial vertex. Thus the LGI for each vertex on the pial surface reflects the amount of cortex buried in its locality.
Statistical analysis
Whole-brain cortical maps
Each vertex-wise LGI measurement of the participants’ surface was mapped on a common spherical coordinate system (fsaverage) using a spherical transformation. Maps were smoothed using a 5 mm Gaussian kernel. We used a general linear model controlling for the effect of age and total cortical surface area to estimate differences in gyrification between the groups at each vertex of the right and left hemispheric surfaces. Total surface area was chosen as a covariate as it has a linear relationship with gyrification,32 and we have previously noted a significant difference between patients and controls in total surface area.24 This model allowed for the possibility that the slope for the relationship between LGI and total surface area may be different in different brain regions.32 We used the Query Design Estimate Contrast tool in the Freesurfer program to generate the contrasts. We undertook Monte Carlo permutation cluster analyses with 10 000 simulations to identify significant clusters with vertex-wise group differences (cluster inclusion threshold p = 0.0001). To examine the effect of sex, we carried out the same analysis after excluding the 9 female participants. To produce a visual display of the group comparison (t maps), we used the reconstructed grey–white boundary of the fsaverage image, which allows anatomic landmarks to be illustrated clearly.
Cortical thickness
Using analyses of covariance (ANCOVA), the mean values of cortical thickness from each of the significant clusters obtained from the gyrification analysis were compared between the 2 groups. We used age, sex and global mean thickness as covariates. The significance levels of group comparison of mean thickness within clusters were Bonferroni-corrected to allow for the number of clusters examined. To relate LGI to cortical thickness within regions that showed abnormalities in both thickness and LGI, we computed the Pearson correlation between mean LGI and cortical thickness values after removing the variance owing to age, sex and appropriate global covariates for thickness (global mean thickness) and LGI (global mean LGI) in the entire sample. We then performed Fisher r-to-z transformation to compare the correlation in the 2 groups. Correlations were also sought between the thickness and gyrification measures and antipsychotic dose in chlorpromazine equivalents in the patient group.
Results
There were no significant differences in demographic features including age (t1,96 = −1.32, p = 0.17) and parental socio-economic status (Mann–Whitney U test, z = −1.46, p = 0.16) between the 2 groups (Table 1). The mean total symptom score on the SSPI was 10.3 out of a maximum of 80 (range 0–29), indicating a low symptom burden. The mean score on reality distortion (delusions and hallucinations) in the patient group was 3 (range 0–7). The mean score on the psychomotor poverty dimension was 2.9 (range 0–9) and on the disorganization dimension was 0.74 (range 0–4).
Table 1.
Demographic characteristics of patients with schizophrenia and healthy controls
| Characteristic | Patients | Controls |
|---|---|---|
| No. | 57 | 41 |
| Sex, male:female | 50:7 | 39:2 |
| Handedness, right:left | 52:5 | 34:7 |
| Age, mean (SD) [range] yr | 26.1 (7.5) [19–47] | 28.0 (6.6) [18–44] |
| Parental NS-SEC, mean (SD) | 2.5 (1.6) | 2.0 (1.4) |
NS-SEC = National Statistics — Socio Economic Status;28 SD = standard deviation.
Whole-brain cortical maps
Whole-brain analysis revealed 4 clusters in the left hemisphere and a single cluster in the right hemisphere with significant reduction in gyrification in patients compared with controls. The largest cluster included the left insula extending to the pars opercularis and the superior temporal gyrus. Other clusters are shown in Figure 1 and Table 2. The right hemispheric cluster included the junction between the caudal superior temporal and inferior parietal regions. No regions with increased gyrification in the patients were noted at this threshold, but bilateral frontomarginal hypergyria emerged when the statistical threshold was more lenient (Table 3 and Figure 2). The exclusion of the 9 female participants did not alter the results substantially.
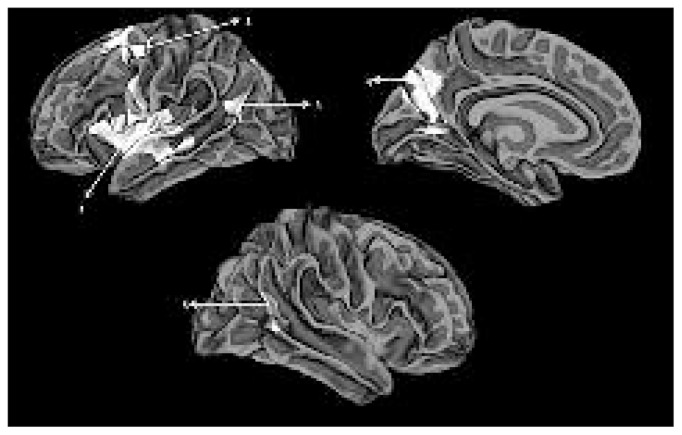
Fig. 1.
Clusters showing hypogyria in patients with schizophrenia compared with healthy controls displayed on a reconstructed average white matter surface (fsaverage). All displayed clusters survived multiple testing using Monte Carlo simulation with a cluster inclusion criterion of p = 0.0001. The left hemisphere (top) shows (1) the left insula extending to the inferior frontal gyrus and superior temporal gyrus, (2) the left caudal middle and superior frontal cluster, (3) the superior temporal/inferior parietal cluster and (4) the left parieto-occipital cluster extending to precuneus. The right hemisphere (bottom) shows (5) the right superior temporal/inferior parietal cluster.
Table 2.
Clusters showing group differences in gyrification*
| Cortical region | Talaraich coordinate of the centroid | Cluster size, mm2 | Cluster- wise probability | ||
|---|---|---|---|---|---|
|
| |||||
| x | y | z | |||
| Left insula | −37 | −1 | 1 | 6094 | 0.0001 |
| Left caudal superior and middle frontal region | −23 | 4 | 47 | 2345 | 0.0001 |
| Left parieto-occipital sulcus | −11 | −65 | 31 | 2339 | 0.0001 |
| Left superior temporal/inferior parietal junction | −43 | −61 | 14 | 698 | 0.0001 |
| Right superior temporal/inferior parietal junction | 49 | −53 | 11 | 543 | 0.0001 |
Threshold for inclusion in a cluster was p = 0.0001.
Table 3.
Clusters showing increased gyrification in patients at a lower threshold for inclusion in a cluster, p = 0.05
| Cortical region | Talaraich coordinate of the centroid | Cluster size, mm2 | Cluster- wise probability | ||
|---|---|---|---|---|---|
|
| |||||
| x | y | z | |||
| Right frontomarginal region | 32 | 50 | 1 | 144 | 0.039 |
| Left frontomarginal region | −29 | 48 | 1 | 260 | 0.030 |
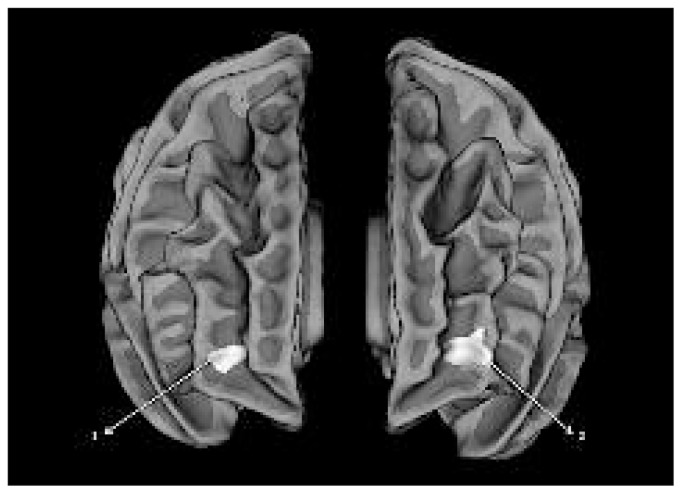
Fig. 2.
Clusters showing hypergyria in patients with schizophrenia compared with healthy controls. The clusters are displayed on the anterior aspect of a reconstructed average white matter surface (fsaverage). All displayed clusters survived multiple testing using Monte Carlo simulation with a cluster inclusion criterion of p = 0.05. (1) Right frontomarginal cluster. (2) Left frontomarginal cluster. No other hypergyric clusters were observed in the vertex-wise analysis.
Gyrification and thickness
The average reduction in gyrification within clusters ranged from 7.84% to 3.67% (Table 3). The greatest degree of hypogyria was seen in the left insula. We observed significant cortical thinning only in the left insula (F1,93 = 30.1, p = 0.007 × 10−6) with a trend toward thinning in the right temporal cluster (F1,93 = 6.06, corrected p = 0.08) in patients (Table 4). In the left insula, there was a significant correlation between gyrification and thickness for the whole sample (r = 0.22, p = 0.028, n = 98), with a significant difference between patients and controls in this relationship (r[patients] = 0.30, r[controls] = −0.17, Fisher r-to-z test p = 0.023). The correlation was significant in patients but not in controls. There were no significant correlations between the gyrification or thickness values and current antipsychotic dose or total disease duration in any of the examined clusters.
Table 4.
Percentage reduction in gyrification and thickness in patients with schizophrenia compared with healthy controls
| LGI | Thickness | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Cortical region | Patients, mean (SD) | Controls, mean (SD) | Percentage reduction in patients | Patients, mean (SD) | Controls, mean (SD) | Percentage reduction in patients |
| Left insula | 4.47 (0.30) | 4.85 (0.47) | 7.84* | 2.61 (0.12) | 2.75 (0.11) | 5.09† |
| Left caudal superior and middle frontal region | 2.88 (0.19) | 3.07 (0.15) | 5.88* | 2.42 (0.15) | 2.41 (0.16) | −0.41‡ |
| Left parieto-occipital sulcus | 3.02 (0.23) | 3.21 (0.23) | 5.94* | 2.24 (0.16) | 2.28 (0.13) | 1.75‡ |
| Left superior temporal/inferior parietal junction | 3.28 (0.18) | 3.43 (0.15) | 4.67* | 2.45 (0.19) | 2.53 (0.16) | 3.16‡ |
| Right superior temporal/inferior parietal junction | 3.42 (0.15) | 3.55 (0.19) | 3.67* | 2.47 (0.21) | 2.60 (0.20) | 5.00§ |
LGI = local gyrification index (no units); SD = standard deviation.
Cluster-wise significance at p = 0.0001 (permutation test n = 10 000).
Bonferroni-corrected p < 0.0001,
p > 0.1 and
p < 0.1.
Asymmetry index
Given the prominent hypogyria noted in the left insular cluster, we undertook a post hoc analysis of hemispheric differences in gyrification and thickness. By drawing ROI labels guided by landmarks on the fsaverage surface, we generated the homologous mask on the right insula. To ensure comparability, we used the parcellation scheme by Destrieux and colleagues (Destrieux atlas)33 and visually inspected the contiguous region included in the respective ROI labels from each hemisphere. This right insula mask was automatically mapped back onto the surface of each participant using the same spherical coordinate system used for the initial analysis. The right homologous insular cluster showed a 4.13% reduction in LGI patients with a trend toward statistical significance (mean [SD] LGI 4.84 [0.45] in controls v. 4.64 [0.63] in patients, uncorrected p = 0.08). Comparison of asymmetry index (AI = [left − right] × 100/[left + right]) revealed a significant group difference in the asymmetry of insular gyrification (AI 0.16 L > R in controls v. −1.5 R > L in patients, t1,96 = 2.028, p = 0.045). A significant cortical thinning (4.61%) was also noted in the right homologous cluster in patients compared with controls (mean [SD] thickness 2.82 [0.13] mm in controls v. 2.69 [0.13] mm in patients, uncorrected p < 0.001). No significant hemispheric differences in thickness was notable between the groups (AI −1.17 R > L in controls v. −1.45 R > L in patients, t1,96 = 0.825, p = 0.412).
Sulcal and gyral thinning
Various observations suggest that in healthy controls, cerebral gyri are generally thicker than the sulci.8,34 Among many possible mechanisms that can produce such a difference, the variation in axonal tension during development is thought to be an important mechanism.9 We separated the major sulci from the gyri within the hypogyric insular cluster by overlaying the cluster mask on the Destrieux atlas,33 and we computed the mean thickness of 3 gyral (short insular gyrus, inferior frontal opercular gyrus and superior temporal gyrus) and 3 sulcal regions (anterior, superior and inferior circular sulci) included in the mask. We did not include long insular gyri because, owing to its inconsistent appearance, the central sulcus of the insula and the long insular gyri were grouped in the same label in the parcellation scheme. An ANCOVA with diagnosis as a between-subject factor, and region (sulcal v. gyral) as a within-subject factor, with age, sex and global thickness as covariates revealed no significant interaction between diagnosis and the sulcogyral division (F1,93 = 1.62, p = 0.21). Both sulcal (partial η = 0.16, p < 0.001) and gyral regions (partial η = 0.11, p = 0.001) showed significant thinning in patients with schizophrenia compared with controls.
Discussion
Using a surface-based vertex-wise morphometric approach, we observed a significant reduction in gyrification in patients with schizophrenia compared with healthy controls. The hypogyria was more pronounced in the left hemisphere, with the greatest reduction occurring in the left insula, extending onto the superior temporal gyrus and sulcus posteriorly and the Broca area anteriorly. These extensions of the insular cluster are larger than could be accounted for by the smoothing employed in our study, consistent with the concept that the frontoinsular cortex acts as a coordinated unit.35,36
To quantify gyrification, we used Schaer and colleagues’ LGI, which captures changes in both the frequency and the depth of sulcogyral transitions in the cortical surface.31 Thus LGI reflects the biological process of cortical folding more closely than measuring either the sulcal depth37 or frequency of curvature changes38 independently. Nonetheless, our results are comparable to those obtained by Cachia and colleagues37 using a sulcal-wise gyrification measure in a selected group of patients with persistent hallucinations. Though the defect was more pronounced on the left, consistent with many previous observations,10,37 we also found a trend toward hypogyria on the right insula along with a significant group difference in the hemispheric asymmetry. Patients with schizophrenia exhibited a reversal of the normal tendency for greater insula gyrification on the left.
Gyrification maps derived from the pial surface are different from cortical surface area maps24 derived from the grey–white boundary. In a previous study, we investigated the surface area of 3 large-scale networks in patients with schizophrenia using the boundary between grey and white matter.24 In contrast, the gyrification maps used in the present study contain information about distribution of cortical convolutions and resulting complexity based on the amount of grey matter buried in the neighbourhood of multiple vertices on the pial surface. The results from the study of surface area changes identified the most prominent reduction in the temporoparietal junction and precuneus in patients with schizophrenia,24 while the present gyrification analysis implicates the insula as the site of most prominent hypogyria. The most striking similarity between the 2 studies is the predominance of left-sided changes in the cortical surface anatomy. Insofar as the degree of folding across the different regions in the cortex varies with the rate of maturation of those regions,7,20,39,40 the gyrification metrics provide crucial information about neurodevelopmental aberrations.
The significant insular hypogyria might be partially explained using the tension-based morphogenetic theory,9 which suggests that cortical folding is a result of radial tension during brain development, wherein established axonal networks resist radial while allowing tangential expansion. It is likely that insular connections with more medial grey matter preclude outward expansion of its grey matter, leading to sequestration of the insula within the lateral fissure during normal development. Some support for the existence of such a medial tract comes from studies on the interoceptive system in primates41 and the olfactory system in other animals.42 Given that insular folding is deficient in patients with schizophrenia, it is likely that connectivity of the insula to more medial regions of grey matter, possibly the cingulate cortex, is impaired in these patients. The connections between the 2 paralimbic structures, insula and anterior cingulate constitutes the salience network.35 The insula has been implicated in the generation of a state of proximal salience during stimulus evaluation that enables switching between an internally focused default mode and an external task-processing mental state.16
We did not find a differential change in the thickness of gyri and sulci, which would be expected if the insular hypogyria were solely due to fewer axonal connections creating less axonal tension. However, it is probable that developmental factors other than axonal tension account for folding. The principal mechanical effect of cortical folding has been noted to produce differential thickness changes in the superficial and deep layers of cortical laminae of the sulci and gyri,8,43 the examination of which requires a cytoarchitectural study. Our observation of combined gyral and sulcal thinning in the hypogyric insular cluster suggest that in addition to reduced connectivity, other mechanisms contributing to the development of the cortex may be affected in patients with schizophrenia. Goldman-Rakic and Rakic44 examined the effect of the timing of prenatal cortical lesions on subsequent gyrification and suggested that neuronal migration, which eventually contributes to the thickness of the cortical sheet, predates gyrification. Investigating the gyrification of regions showing cortical thinning in adolescents with schizophrenia, Janssen and colleagues45 found no correlation between gyrification and thickness in most regions and suggested that cortical thinning may be a late developmental phenomenon in patients with schizophrenia. Our results are largely consistent with those of Janssen and colleagues,45 though we did observe a correlation between these 2 metrics in the left insula that was specific to patients. It is likely that in healthy controls the degree of gyrification is not the principal determinant of cortical thickness. On the contrary, the association of hypogyria with sulcogyral thinning in patients with schizophrenia may suggest a developmental disturbance that possibly predates the dissociation between radial and tangential cortical expansions during early phases of cortical development.46 This is supported by the observation that the insula is one of the earliest brain structures to show gyrification and neuronal differentiation and thus forms a core zone of sulcal and gyral maturation during normal intrauterine growth.47
Most of the other regions showing hypogyria in patients with schizophrenia (inferior frontal, superior temporal and inferior parietal regions) belong to the multimodal (heteromodal) association cortex described by Mesulam.48 A pathological perturbation in the development of multimodal association areas has been previously suggested as the core deficit in patients with schizophrenia.49 In healthy individuals, the regions constituting the multimodal association cortex show a prolonged maturational trajectory, attaining peak grey matter density at a later stage of development than unimodal sensory regions.50 This slow maturation may be linked to the relatively higher degree of cortical folding normally observed in these regions.51 An abnormally premature, delayed or arrested growth peak in these regions could putatively account for the reduced gyrification observed in patients with schizophrenia. But our observation that reduced gyrification is not limited to multimodal regions but rather extends to paralimbic cortices supports the hypothesis that a developmental abnormality in the connectivity within and between paralimbic and multimodal association areas is a characteristic feature of schizophrenia. Alternatively, the combined paralimbic and multimodal hypogyria could be related to a shared defect in fetal thalamocortical connectivity in patients with schizophrenia.52
Despite the substantial sample size, the need for stringent correction for multiple comparisons in a whole-brain vertex-wise search creates a risk of failing to identify cerebral regions in which there are relatively small abnormalities. It is noteworthy that in our previous study using an ROI approach to test the specific hypothesis of abnormal frontal gyrification in patients with schizophrenia,23 we found that hypergyria was localized to a circumscribed part of the prefrontal cortex, and the normal laterality of the frontal gyrification was reversed in patients with schizophrenia. This effect did not survive the stringent correction for multiple comparisons in the present study, but bilateral frontomarginal hypergyria emerged when the statistical threshold was more lenient (Table 3). According to the tension-based morphogenesis model, if the axonal tension is the primary determinant of gyrification, then a weakening of connectivity affecting specific pathways may result in relatively greater tension in other pathways leading to local hypergyria.10 Despite the limited power to detect small effects, the findings indicate that the most extensive diminution of gyrification in patients with schizophrenia occurs in the multimodal association and paralimbic cortex, especially in the left frontoinsular region.
Limitations
Sex differences have been noted in some14,53 but not all15,37 studies investigating gyrification in patients with schizophrenia. Our sample was predominantly male, precluding a meaningful analysis of sex effects on gyrification in these patients. Hence the results presented here must be interpreted with caution for mixed samples. All patients in our study were taking antipsychotic medications. The effect of antipsychotics on brain structure is a matter of debate but, similar to Cachia and colleagues,37 we found no correlation between gyrification and antipsychotic dose in the 6 weeks preceding the study. It is possible that earlier treatments could have affected the brain structure. Our results should be interpreted cautiously until replicated in unmedicated samples.
Conclusion
We identified a significant abnormality in cortical gyrification in patients with schizophrenia. The localization of these changes to the insula and regions of the multimodal association cortex and the observed relationship of insular hypogyria with reduced cortical thickness in patients suggest a prominent role for a developmental abnormality of connectivity of the insula and the multimodal cortex in the patho-physiology of schizophrenia. Future studies focusing on the interaction between the insula and the multimodal association regions are required to test the functional consequences of these gyrification defects.
Acknowledgements
This work was supported by a New Investigator grant from the University of Nottingham and an Interdisciplinary Research Award from the Nottingham Institute of Neuroscience, University of Nottingham. We are grateful to the volunteers who participated in this study and would like to acknowledge Pavan Mallikarjun and Verghese Joseph for clinical recruitment. We would like to thank Thomas White, Kathrin Doege, Dawn-Marie Walker and Dorothee Auer for assisting the data acquisition.
Footnotes
Competing interests: L. Palaniyappan has received a Young Investigator Travel Fellowship from Eli Lilly. P.F. Liddle has received honoraria for academic presentations from GlaxoSmithKline, AstraZeneca, Janssen-Cilag, Bristol Myers Squibb and Eli Lilly; has taken part in advisory panels for Bristol Myers Squibb, Eli Lilly, Pfizer and GlaxoSmithKline; has received institutional grant support from the Medical Research Council (G0601442 and MR/J01186X/1) and the Dr. Hadwen Trust; and receives book royalties from the Royal College of Psychiatrists.
Contributors: Both authors contributed to study design, data acquisition and analysis, article writing and review and approved article publication.
References
- 1.Friston KJ. The disconnection hypothesis. Schizophr Res. 1998;30:115–25. doi: 10.1016/s0920-9964(97)00140-0. [DOI] [PubMed] [Google Scholar]
- 2.Pettersson-Yeo W, Allen P, Benetti S, et al. Dysconnectivity in schizophrenia: Where are we now? Neurosci Biobehav Rev. 2011;35:1110–24. doi: 10.1016/j.neubiorev.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 3.Casanova MF, Tillquist CR. Encephalization, emergent properties, and psychiatry: a minicolumnar perspective. Neuroscientist. 2008;14:101–18. doi: 10.1177/1073858407309091. [DOI] [PubMed] [Google Scholar]
- 4.Montagu A. Neonatal and infant immaturity in man. JAMA. 1961;178:56–7. doi: 10.1001/jama.1961.73040400014011. [DOI] [PubMed] [Google Scholar]
- 5.Deacon TW. Problems of ontogeny and phylogeny in brain-size evolution. Int J Primatol. 1990;11:237–82. [Google Scholar]
- 6.Laughlin SB, Sejnowski TJ. Communication in neuronal networks. Science. 2003;301:1870–4. doi: 10.1126/science.1089662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Toro R, Perron M, Pike B, et al. Brain size and folding of the human cerebral cortex. Cereb Cortex. 2008;18:2352–7. doi: 10.1093/cercor/bhm261. [DOI] [PubMed] [Google Scholar]
- 8.Hilgetag CC, Barbas H. Developmental mechanics of the primate cerebral cortex. Anat Embryol (Berl) 2005;210:411–7. doi: 10.1007/s00429-005-0041-5. [DOI] [PubMed] [Google Scholar]
- 9.Van Essen DC. A tension-based theory of morphogenesis and compact wiring in the central nervous system. Nature. 1997;385:313–8. doi: 10.1038/385313a0. [DOI] [PubMed] [Google Scholar]
- 10.White T, Hilgetag CC. Gyrification and neural connectivity in schizophrenia. Dev Psychopathol. 2011;23:339–52. doi: 10.1017/S0954579410000842. [DOI] [PubMed] [Google Scholar]
- 11.Yücel M, Stuart GW, Maruff P, et al. Paracingulate morphologic differences in males with established schizophrenia: a magnetic resonance imaging morphometric study. Biol Psychiatry. 2002;52:15–23. doi: 10.1016/s0006-3223(02)01312-4. [DOI] [PubMed] [Google Scholar]
- 12.Wheeler DG, Harper CG. Localised reductions in gyrification in the posterior cingulate: schizophrenia and controls. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:319–27. doi: 10.1016/j.pnpbp.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 13.Falkai P, Honer WG, Kamer T, et al. Disturbed frontal gyrification within families affected with schizophrenia. J Psychiatr Res. 2007;41:805–13. doi: 10.1016/j.jpsychires.2006.07.018. [DOI] [PubMed] [Google Scholar]
- 14.Vogeley K, Schneider-Axmann T, Pfeiffer U, et al. Disturbed gyrification of the prefrontal region in male schizophrenic patients: a morphometric postmortem study. Am J Psychiatry. 2000;157:34–9. doi: 10.1176/ajp.157.1.34. [DOI] [PubMed] [Google Scholar]
- 15.Bonnici HM, William T, Moorhead J, et al. Pre-frontal lobe gyrification index in schizophrenia, mental retardation and comorbid groups: an automated study. Neuroimage. 2007;35:648–54. doi: 10.1016/j.neuroimage.2006.11.031. [DOI] [PubMed] [Google Scholar]
- 16.Palaniyappan L, Liddle PF. Does the salience network play a cardinal role in psychosis? An emerging hypothesis of insular dys-function. J Psychiatry Neurosci. 2012;37:17–27. doi: 10.1503/jpn.100176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wai MS, Shi C, Kwong W, et al. Development of the human insular cortex: differentiation, proliferation, cell death, and appearance of 5HT-2A receptors. Histochem Cell Biol. 2008;130:1199–204. doi: 10.1007/s00418-008-0497-5. [DOI] [PubMed] [Google Scholar]
- 18.Kalani MYS, Kalani MA, Gwinn R, et al. Embryological development of the human insula and its implications for the spread and resection of insular gliomas. Neurosurg Focus. 2009;27:E2. doi: 10.3171/2009.5.FOCUS0997. [DOI] [PubMed] [Google Scholar]
- 19.Chi JG, Dooling EC, Gilles FH. Gyral development of the human brain. Ann Neurol. 1977;1:86–93. doi: 10.1002/ana.410010109. [DOI] [PubMed] [Google Scholar]
- 20.Rajagopalan V, Scott J, Habas PA, et al. Local tissue growth patterns underlying normal fetal human brain gyrification quantified in utero. J Neurosci. 2011;31:2878–87. doi: 10.1523/JNEUROSCI.5458-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mangin JF, Jouvent E, Cachia A. In-vivo measurement of cortical morphology: means and meanings. Curr Opin Neurol. 2010;23:359–67. doi: 10.1097/WCO.0b013e32833a0afc. [DOI] [PubMed] [Google Scholar]
- 22.Zilles K, Armstrong E, Schleicher A, et al. The human pattern of gyrification in the cerebral cortex. Anat Embryol (Berl) 1988;179:173–9. doi: 10.1007/BF00304699. [DOI] [PubMed] [Google Scholar]
- 23.Palaniyappan L, Mallikarjun P, Joseph V, et al. Folding of the pre-frontal cortex in schizophrenia: regional differences in gyrification. Biol Psychiatry. 2011;69:974–9. doi: 10.1016/j.biopsych.2010.12.012. [DOI] [PubMed] [Google Scholar]
- 24.Palaniyappan L, Mallikarjun P, Joseph V, et al. Regional contraction of brain surface area involves three large-scale networks in schizophrenia. Schizophr Res. 2011;129:163–8. doi: 10.1016/j.schres.2011.03.020. [DOI] [PubMed] [Google Scholar]
- 25.Leckman JF, Sholomskas D, Thompson D, et al. Best estimate of lifetime psychiatric diagnosis: a methodological study. Arch Gen Psychiatry. 1982;39:879–83. doi: 10.1001/archpsyc.1982.04290080001001. [DOI] [PubMed] [Google Scholar]
- 26.Liddle PF, Ngan ETC, Duffield G, et al. Signs and Symptoms of Psychotic Illness (SSPI): a rating scale. Br J Psychiatry. 2002;180:45–50. doi: 10.1192/bjp.180.1.45. [DOI] [PubMed] [Google Scholar]
- 27.Ammons RB, Ammons CH. Quick test. Missoula (MT): Psychological Test Specialists; 1962. [Google Scholar]
- 28.Rose D, Pevalin DJ. A researcher’s guide to thenational statistics socioeconomic classification. London: Sage Publications; 2003. [Google Scholar]
- 29.Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis: I. Segmentation and surface reconstruction. Neuroimage. 1999;9:179–94. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- 30.Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci U S A. 2000;97:11050–5. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schaer M, Cuadra MB, Tamarit L, et al. A surface-based approach to quantify local cortical gyrification. IEEE Trans Med Imaging. 2008;27:161–70. doi: 10.1109/TMI.2007.903576. [DOI] [PubMed] [Google Scholar]
- 32.Luders E, Thompson PM, Narr KL, et al. A curvature-based approach to estimate local gyrification on the cortical surface. Neuroimage. 2006;29:1224–30. doi: 10.1016/j.neuroimage.2005.08.049. [DOI] [PubMed] [Google Scholar]
- 33.Destrieux C, Fischl B, Dale A, et al. Automatic parcellation of human cortical gyri and sulci using standard anatomical nomenclature. Neuroimage. 2010;53:1–15. doi: 10.1016/j.neuroimage.2010.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Welker W. Why does cerebral cortex fissure and fold? A review of determinants of gyri and sulci. In: Jones EG, Peters A, editors. Cerebral cortex. 8b. New York: Plenum Press; 1990. pp. 3–136. [Google Scholar]
- 35.Seeley WW, Menon V, Schatzberg AF, et al. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007;27:2349–56. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Craig AD. How do you feel — now? The anterior insula and human awareness. Nat Rev Neurosci. 2009;10:59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- 37.Cachia A, Paillère-Martinot M-L, Galinowski A, et al. Cortical folding abnormalities in schizophrenia patients with resistant auditory hallucinations. Neuroimage. 2008;39:927–35. doi: 10.1016/j.neuroimage.2007.08.049. [DOI] [PubMed] [Google Scholar]
- 38.Narr K, Thompson PM, Sharma T, et al. Three-dimensional mapping of gyral shape and cortical surface asymmetries in schizophrenia: gender effects. Am J Psychiatry. 2001;158:244–55. doi: 10.1176/appi.ajp.158.2.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Finlay BL, Darlington RB, Nicastro N. Developmental structure in brain evolution. Behav Brain Sci. 2001;24:263–78. discussion 278–308. [PubMed] [Google Scholar]
- 40.Hill J, Inder T, Neil J, et al. Similar patterns of cortical expansion during human development and evolution. Proc Natl Acad Sci U S A. 2010;107:13135–40. doi: 10.1073/pnas.1001229107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Craig AD. Interoception: the sense of the physiological condition of the body. Curr Opin Neurobiol. 2003;13:500–5. doi: 10.1016/s0959-4388(03)00090-4. [DOI] [PubMed] [Google Scholar]
- 42.Buchanan KJ, Johnson JI. Diversity of spatial relationships of the claustrum and insula in branches of the mammalian radiation. Ann N Y Acad Sci. 2011;1225(Suppl 1):E30–63. doi: 10.1111/j.1749-6632.2011.06022.x. [DOI] [PubMed] [Google Scholar]
- 43.Hilgetag CC, Barbas H. Role of mechanical factors in the morphology of the primate cerebral cortex. PLOS Comput Biol. 2006;2:e22. doi: 10.1371/journal.pcbi.0020022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Goldman-Rakic P, Rakic P. Cerebral dominance: the biological foundations. Cambridge (MA): Harvard University Press; 1984. Experimental modification of gyral patterns. [Google Scholar]
- 45.Janssen J, Reig S, Alemán Y, et al. Gyral and sulcal cortical thinning in adolescents with first episode early-onset psychosis. Biol Psychiatry. 2009;66:1047–54. doi: 10.1016/j.biopsych.2009.07.021. [DOI] [PubMed] [Google Scholar]
- 46.Martínez-Cerdeño V, Noctor SC, Kriegstein AR. The role of inter-mediate progenitor cells in the evolutionary expansion of the cerebral cortex. Cereb Cortex. 2006;16(Suppl 1):i152–61. doi: 10.1093/cercor/bhk017. [DOI] [PubMed] [Google Scholar]
- 47.Afif A, Bouvier R, Buenerd A, et al. Development of the human fetal insular cortex: study of the gyration from 13 to 28 gestational weeks. Brain Struct Funct. 2007;212:335–46. doi: 10.1007/s00429-007-0161-1. [DOI] [PubMed] [Google Scholar]
- 48.Mesulam MM. Principles of behavioral and cognitive neurology. 2nd ed. New York: Oxford University Press; 2000. [Google Scholar]
- 49.Ross CA, Pearlson GD. Schizophrenia, the heteromodal association neocortex and development: potential for a neurogenetic approach. Trends Neurosci. 1996;19:171–6. doi: 10.1016/s0166-2236(96)10022-9. [DOI] [PubMed] [Google Scholar]
- 50.Gogtay N, Giedd JN, Lusk L, et al. Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci U S A. 2004;101:8174–9. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Finlay BL, Darlington RB. Linked regularities in the development and evolution of mammalian brains. Science. 1995;268:1578–84. doi: 10.1126/science.7777856. [DOI] [PubMed] [Google Scholar]
- 52.Lewis DA. Is there a neuropathology of schizophrenia? Recent findings converge on altered thalamic-prefrontal cortical connectivity. Neuroscientist. 2000;6:208–18. [Google Scholar]
- 53.Narr KL, Bilder RM, Kim S, et al. Abnormal gyral complexity in first-episode schizophrenia. Biol Psychiatry. 2004;55:859–67. doi: 10.1016/j.biopsych.2003.12.027. [DOI] [PubMed] [Google Scholar]