Abstract
Hypotheses regarding the developmental origins of health and disease postulate that developing fetuses–and potentially young children—undergo adaptive epigenetic changes with longstanding effects on metabolism and other processes. Ongoing research explores whether these adaptations occur during early life following malnutrition. In the developing world there remains a high degree of nutritional stunting—linear growth failure due to inadequate calories that may be exacerbated by inflammation from ongoing infections. In areas with poor sanitation children experience vicious cycles of enteric infections and malnutrition, resulting in poor nutrient absorption from intestinal mucosa changes now termed “environmental enteropathy.” Emerging evidence links early childhood diarrhea and/or growth failure with increased CVD risk factors in later life, including dyslipidemia, hypertension and glucose intolerance. The mechanisms for these associations remain poorly understood and may relate to epigenetic responses to poor nutrition, increased inflammation or both. Given increases in CVD in developing areas of the world, associations between childhood malnutrition, early life infections and increased CVD risk factors underscore further reasons to improve nutrition and infection-related outcomes for young children worldwide.
Keywords: Nutrition, diarrhea, stunting, cardiovascular disease, environmental enteropathy, Fetal Origins Hypothesis
Introduction
For over 20 years evidence has accumulated linking low birth weight (LBW) to an increased risk for both cardiovascular disease (CVD) and type 2 diabetes (T2DM).1, 2 These risks appear to be mediated at least in part by relationships between intrauterine growth restriction and an increase in individual risk factors related to the metabolic syndrome (MetS), such as insulin resistance,3, 4 dyslipidemia,5, 6 high blood pressure (BP),7, 8 and elevated cortisol9–11—findings that have been noted in children3, 4, 7–9 and adults.5–7, 12 Collectively this concept was originally referred to as the Fetal Origins Hypothesis or the Barker Hypothesis, postulating a causal link between nutrient restrictions in utero and subsequent epi-genetic changes in pathways related to metabolism, blood pressure (BP) and glucose regulation.13, 14 The exact mechanisms that account for these findings are still not fully understood and may depend in part on genes that increase susceptibility to intrauterine effects15, 16. Nevertheless, the consistency of findings continues to lend credibility to changes in programming in the developing fetus.
More recently, studies have also suggested that epi-genetic changes in metabolic pathways may not be limited to malnutrition in the prenatal period. “Stunting” refers to poor height gain due to malnutrition and infection, with height-for-age z-scores more than two standard deviations below normal. Stunting remains common in the developing world where contaminated water supplies lead to a cycle of enteric infections and malnutrition.17–20 Increasing sources of evidence suggest that poor weight gain in the first two years of life and subsequent stunting in children and adults are related to worsened metabolic findings, helping transform what was originally called the Fetal Origins Hypothesis to the developmental origins of health and disease.21, 22 These relationships between early stunting and later disease are significant given that such stunting affects up to 25% of children in impoverished areas, even as the obesity epidemic reaches into developing areas around the globe.17 Given this high prevalence of stunting, any relationship between stunting and future metabolic risk would be of extreme importance. This is particularly true as CVD increasingly becomes a leading cause of death in the developing world and as obesity and diabetes are increasingly recognized as diseases of poverty.23
In this review we will discuss the prevalence and etiologies of early childhood malnutrition, potential mechanisms behind the developmental origins of health and disease, evidence for links between stunting and metabolic risk, and potential implications.
Malnutrition as an enteric disease
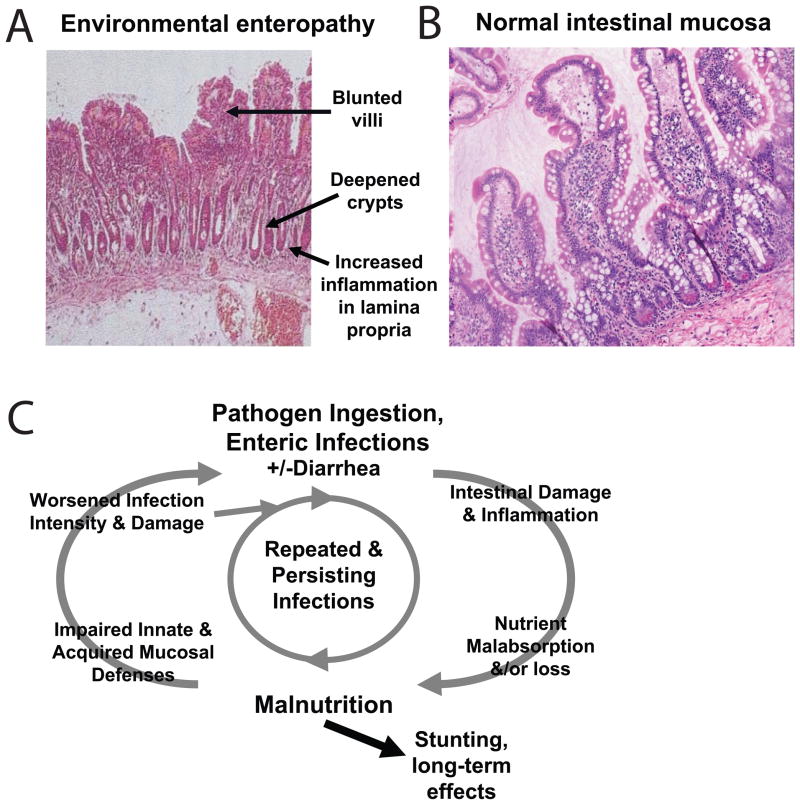
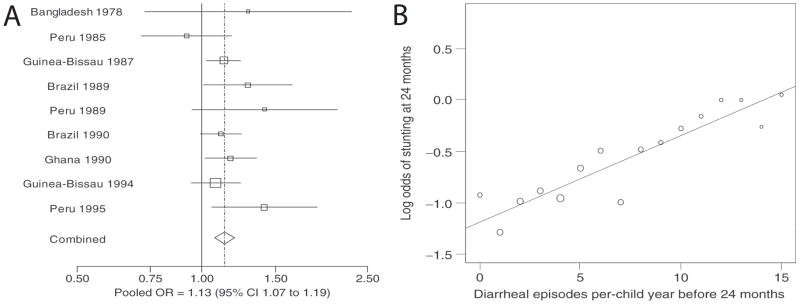
In developing regions of the world 1.1 billion people lack access to safe water, resulting in a vicious cycle of chronic enteric infections, leading to malabsorption of nutrients and malnutrition, which in turn increases susceptibility to more enteric infections.17 This cycle (reviewed in reference 17) typically begins soon after weaning and results in a chronic environmental enteropathy (Figure 1A–B24, 25), low childhood body mass index (BMI) and early stunting. The reciprocal nature of malnutrition and enteral infections are illustrated by the fact that malnourished children followed prospectively have both increased incidence and duration of diarrheal illnesses (Figure 1C).26–28 Similarly, children followed prospectively for the number of diarrheal illnesses have worsening growth failure with an increasing number of diarrheal episodes in the first two years of life (Figure 2).20, 21, 29, 30 In addition to episodes of overt diarrhea, longstanding subclinical enteral infections also affect linear growth over time.18
Figure 1. Environmental enteropathy: villus atrophy and a cycle of infections and malnutrition.
Children in developing areas in the world frequently exhibit abnormalities in their intestinal mucosa referred to as “environmental enteropathy.” Associated findings following on biopsy of the intestinal mucosa include villus blunting and deepened crypts (A) compared to normal mucosa (B). C. As shown in this model, these cases of environmental enteropathy are thought to be due to reciprocal relationships between malnutrition and recurrent enteric infections due in part to contaminated drinking water and poor sanitation. This cycle of undernutrition ultimately leads to poor weight gain and stunting. (From McKay24 International Health 2010; 2: 172–180, Levine25 BMC Biology 2010; 8:129 and Guerrant26 Nutr Rev 2008; 66:487–505, used by permission.)
Figure 2. Meta-analysis of the relationship between diarrheal illnesses and stunting in the developing world.
A. Point estimates and confidence intervals for the odds of stunting at 2 y.o. in children who had at least 5 episodes of diarrhea compared with those with less than 5 episodes. B. There was a linear relationship between the cumulative diarrheal incidence before 2 y.o. vs. the log odds of stunting at 2 y.o., suggesting a dosage effect of diarrhea on stunting. (From Checkley20 International J of Epidemiology 2008; 37:816–830, used by permission.)
These enteric infections in early childhood are associated with 1) a decrease in absorption of macronutrients,17 2) a decrease in absorption of micronutrients31, 32 and 3) increases in systemic inflammation, and each of these processes may contribute to growth delay 17, 33, 34. Interventions targeting increases in total calorie delivery have only improved a portion of the growth delay.35–37 Similarly, replacement of micronutrients including iron, vitamin A and zinc have had limited success in improving growth, potentially related to poor absorption from on-going infections.38–40 Thus, the exact causes of growth failure in the setting of enteric diseases may be multi-factorial.
Adding to the burden of poor absorption is an increase in intestinal and systemic inflammation in the setting of enteric disease. This has been demonstrated in a group of Gambian children whose growth and health status was prospectively followed from 2–15 months of age, during which time a high degree of diarrhea was noted (0.8 days/wk on average).33, 41 Levels of fecal neopterin correlated negatively with linear growth and weight gain,41 as did serum levels of antibodies to endotoxin.33 Growth and weight gain were also inversely related to a measure of intestinal permeability, but to a lesser degree, supporting the concept that inflammation itself may play a key role in suppression of growth in enteric disease.33
The burden of these issues in developing regions of the world remains significant, and in areas affected by these environmental enteropathies it is common to see 14–34% of young children with height-for-age z-scores of <-2.42–45 When children remain in the same living environment of malnutrition and enteric infections throughout childhood, this childhood stunting leads to a stunted adult height as well, shown in prospective studies.21 There are clear additional costs related to the care of these children during illness, the future cognitive development of affected children,46–48 and quality of life,17, 45 resulting in a high burden of disease in terms of disability-adjusted life years (DALYs), an estimate of the cost across the lifespan.49, 50
However, pertinent to this review—and potentially adding to the costs of enteric disease in terms of DALYs—there has been accumulating evidence that such nutritional stunting from enteric disease in early life has longstanding effects on risk factors for diabetes and cardiovascular disease. This evidence has accumulated from multiple study designs, as described below.
Evidence for long-term effects of nutritional stunting
Stunting and obesity frequently co-exist in the same setting
Studies from both urban and rural populations in developing areas over the past decade have revealed increases in rates of obesity throughout the lifespan and related chronic diseases in adults. Often there is the presence of both stunting in early childhood and later obesity in the same impoverished neighborhoods, often referred to as the “dual burden paradox.”51, 52 This has been seen in Pakistan, South Africa and Brazil (Table 1).42–44 One study in Brazil was notable for documenting the presence of both undernutrition and obesity together in 30% of households.44 This thus raises the possibility that poor early growth and later obesity may also exist among some individuals in communities in the developing world. Interestingly, in many of these studies it was the females who were much more affected by obesity among older children and adults (Table 1). As we shall see, this predominance of findings among females has also been shown among other studies of stunted individuals.
Table 1. Cross-sectional studies in developing parts of the world revealing co-incidence of stunting in early childhood and obesity in later childhood.
Adapted from Jafar Arch Dis. Child. 2008;93:373; Kimani-Murage BMC Public Health. 2010;10:158; and Florencio Br J Nutr. Aug 2001;86(2):277–284.
| Reference | Location, time | Stunting (%) | Obesity |
|---|---|---|---|
| Jafar (2008)42. | Urban Pakistan (’04-’05) | 5–6 y.o.’s: Girls 14.1% >Boys 30% |
13–14 y.o.’s: Girls 10.5% Boys 7.4% |
| Kimani-Murage (2010)43. | Rural South Africa (’07) | 2 y.o.’s: Girls 21% Boys 25% |
18 y.o.’s: Girls 25% Men 4.5% |
| Florencio (2001)44. | Maceió, Brazil (’99) | <10 y.o.: Boys & girls:17% |
Adults: Women 32% Men 16% |
Childhood growth of individuals who develop CVD and glucose intolerance
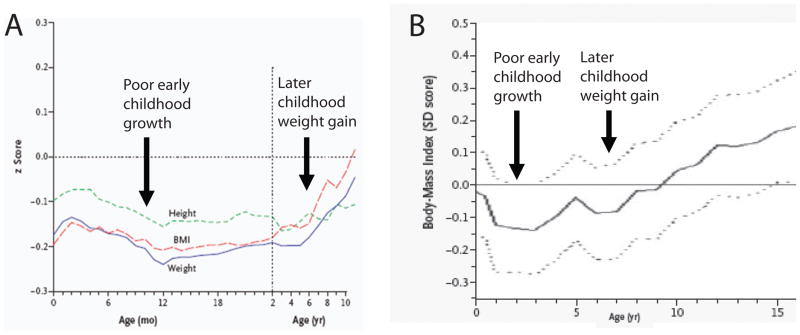
Large cohort studies have evaluated individuals who developed adult disease, looking back to childhood data to investigate for their pattern of growth. In Norway a group of investigators evaluated a long-term cohort of >8700 adults and selected those who had experienced cardiovascular events by 64 y.o.53 They then evaluated the average growth in childhood for the group, revealing that those who later developed CVD exhibited below-average height, weight and BMI in early life with rapid weight rebound in later childhood (Figure 3A).53
Figure 3. Individuals who develop CVD and glucose intolerance on average exhibit poor early childhood growth.
A. Childhood growth characteristics of 357 men who developed coronary heart disease as adults. Mean values for age of height, weight and BMI z-score for the overall sample (4630 boys) is set to a z-score of zero. On average, men who developed coronary heart disease exhibited below average growth parameters in early life followed by rapid weight gain in later childhood. B. Childhood BMI z-score of 156 individuals found to have glucose intolerance at age 25–34 years. The mean childhood BMI for the overall sample (1492 individuals evaluated as adults) is set to a z-score of zero. Individuals with glucose intolerance as adults exhibited a mean childhood BMI z-score (solid line, with confidence intervals as dotted lines) that was below average in early life followed by rapid weight gain later. (From Barker53 NEJM 2005; 353: 1802–1809 and Bhargava54 NEJM 2004; 350: 865–875; used by permission.)
Another group assessed a cohort of 1492 adults in New Delhi India by performing oral glucose tolerance tests (OGTT) on subjects when they were about 30 years old.54 They defined glucose intolerance as a blood sugar >140 mg/dL at the 2 hour point of the OGTT. In looking at the childhood growth of those with glucose intolerance, these researchers noted childhood growth patterns similar to the Norwegian cohort—below average height, weight and BMI in early life, followed by rapid weight gain thereafter (Figure 3B).54 In both of these studies the subjects overall exhibited below-normal growth prior to 3–4 years old, suggesting that poor growth in early childhood (along with later weight gain) may play a role in the development of these disease processes. The relatively early onset of rapid weight gain is termed “adiposity rebound” and is defined as the time when the physiologic nadir of BMI reverses and begins to increase with age.54 The early onset of this weight gain may indicate something different about these individuals. Potential explanations include genetic features or, alternatively, epigenetic changes conferring an increased predilection toward rapid weight gain.
Metabolic syndrome characteristics following poor childhood growth
The researchers investigating the cohort from New Delhi also looked for relationships between childhood growth and components of MetS among this group. They divided individuals into quintiles by childhood BMI at 2 years old and assessed correlations between low childhood BMI and MetS in adulthood, adjusting for adult BMI.55 With this adjustment, low childhood BMI was associated with less favorable measures of multiple findings related to MetS, including triglycerides, cholesterol, blood pressure, insulin resistance (as measured by HOMA), and glucose level during an OGTT (Table 4). The relationship with adult findings was particularly strong as related to the OGTT (p<0.001). Interestingly, when these relationships were evaluated prior to adjustment for adult BMI the relationships were less striking. Prior to adjustment, childhood BMI is only a significant predictor of abnormal glucose tolerance. This likely indicates that while low BMI at 2 years old is a contributor to risk for future MetS findings, adult BMI—potentially a marker for obesity-prone genetics or adult lifestyle—is a much stronger predictor of MetS.55
Table 4. Association of low BMI at 2 y.o. with MetS-related findings as adults 25.
–34 y.o. P values are given for the significance of association of the lowest quintile of BMI at 2 y.o. with the MetS finding listed, as compared to the other quintiles. Abbreviations: OGTT, oral glucose tolerance test; IGT, impaired glucose tolerance; HOMA, homeostasis model of insulin resistance; T2DM, type 2 diabetes mellitus. Adapted from Fall HD et al. Diabetes Care 2008; 31: 2349–2356.
| Adult MetS Finding | P value after adjustment for adult BMI | P value before adjustment for adult BMI |
|---|---|---|
| Adult MetS diagnosis | 0.01 | 0.1 |
| Triglycerides | 0.02 | 0.6 |
| Total cholesterol | 0.02 | 0.8 |
| Systolic BP | 0.05 | 0.1 |
| Insulin resistance (HOMA) | 0.002 | 0.1 |
| Glucose after OGTT | <0.001 | <0.001 |
| IGT/T2DM | <0.001 | 0.06 |
Similar findings was seen in a meta-analysis of cohort studies in which children in developing countries had growth followed in early childhood and were later evaluated as adults.21 Following adjustment for adult BMI, low childhood BMI at 2 years old was associated with higher adult BP (p<0.001) and blood glucose levels (p<0.05). These relationships were not apparent prior to adjustment for adult BMI, suggesting again that adult obesity is likely a stronger influence on these adult findings than low childhood BMI. Nevertheless, these prospective studies suggest an additive residual effect following low childhood weight, likely as a result of enteric disease.
The research team lead by Ana Sawaya from Sao Paulo, Brazil has reported multiple studies using stunting in adults from shanty towns as a surrogate for poor childhood growth due to enteric disease. As mentioned previously, the prevalence of stunting in these areas is high (Table 1), and while there may be other factors also associated with short stature (e.g. genetics), the association of stature with a history of impoverished living conditions and poor sanitation was felt to justify use of adult height as a surrogate measure for prior undernutrition/inflammation related to enteric disease in this context.56 In their investigations in a shanty town in Maceió, Brazil these researchers compared women with an adult height in the bottom quartile of the group (corresponding to a z-score of ≤-2, representing stunting) to women of normal stature from the same neighborhood. In this evaluation women with stunting had a higher odds ratio for having central obesity, high body fat, hypertension and insulin resistance (Table 2),56 and these higher odds remained significant, both before and after adjusting for age, current BMI and other MetS-related factors such as waist:hip ratio. Sawaya’s group also evaluated mean levels of MetS-related variables between women who were stunted vs. normal height and found that the women who were short had more extreme findings related to MetS, including insulin resistance and HDL cholesterol (Table 3).57
Table 2. Odds of MetS- related findings among women with stunting.
Women in Maceió, Brazil, aged 30.2 +/− 7.7 yrs (range 18–53) with adult height in the bottom quartile were compared with those in the top 3 quartiles in a multivariate analysis. P values are shown for significance of relationship both before and after adjusting for factors such as adult BMI, age and waist:hip ratio. Adapted from Ferreira HS et al. Br J Nutr. 2009;101(8):1239–1245 and Florencio TT et al. Eur J Cardiovasc Prev Rehabil. 2007;14(2):346–348.
| Adult MetS-related finding | Odds Ratio | P value before adjustment | P value after adjustment for adult BMI, age, other factors |
|---|---|---|---|
| Waist:Hip ratio ≥ 0.85 | 1.33 | 0.007 | <0.001 |
| Pecent body fat ≥ 30% | 2.13 | <0.001 | <0.001 |
| Insulin resistance | 3.24 | <0.05 | <0.05 |
| Systolic BP ≥ 140 | 3.07 | 0.006 | <0.01 |
Table 3. Differences in MetS-related variables among women with and without stunting.
Women were a random selection of a larger cohort from a shanty town in Maceió, Brazil. Adapted from Florencio TT et al. Eur J Cardiovasc Prev Rehabil. 2007;14(2):346–348.
| Adult MetS Finding | Stunted (n=20) | Average stature (n=20) | P value |
|---|---|---|---|
| Insulin (uU/mL) | 11.34 | 9.26 | 0.02 |
| HOMA-IR | 2.39 | 1.93 | 0.01 |
| LDL cholesterol (mg/dL) | 116 | 103.2 | 0.02 |
| HDL cholesterol (mg/dL) | 44.0 | 48.3 | 0.05 |
As alluded to earlier, these findings predominated among women. Men with stunting did exhibit a tendency toward increased overweight (24.5% in stunted men vs. 14% in non-stunted men)44 but did not exhibit the differences in hypertension that had been noted in women.58 The cause of these gender differences in the effect of stunting on MetS is not clear.
Sawaya’s group also used stunting as a marker for early childhood nutrition in evaluating children from impoverished areas in Sao Paulo, revealing that some of these findings were present as early as 4 years old. Notably, when compared to non-stunted children, stunted children have higher systolic and diastolic BP and a far greater rate of hypertension (71% in stunted children vs. 20% in non-stunted children from the same neighborhood).59 Additionally, stunted girls age 7–11 years old exhibited a greater waist:hip ratio than non-stunted girls.60
Nevertheless, most of the findings seen among adults with stunting (e.g. elevated levels of insulin and dyslipidemia) have not been noted during childhood, particularly related to insulin resistance in boys. Stunted boys also tended to be thinner than non-stunted boys and exhibited greater insulin sensitivity as measured by the homeostasis model of insulin resistance (HOMA) as may have been expected from their thinner body habitus.61
Metabolic syndrome characteristics following childhood illness
As mentioned previously, the changes in growth and weight gain in early childhood appear to be related to both systemic inflammation and poor absorption of nutrients. In addition to investigating the relationship between poor childhood growth and nutrition and future MetS, other researchers have examine the relationship between childhood illness and metabolic outcomes, including data from the Nutrition Institute of Central America (INCAP), a prospective study of nutrition and health in Guatemala.62, 63 This study followed the health and burden of illness in children <2 years old and reassessed the same individuals 25–35 years later. These data revealed that serious illness and anorexia during early childhood (as assessed by bi-weekly interviews with the mother during the first 2 years of life) were associated with an increased risk for a classification of MetS62 and high levels of triglycerides63 as adults. In a set of analyses of these data increasing days with serious illness or anorexia in the first 7 years of childhood (as determined by parent report) carried OR’s of 1.52 and 1.58, respectively, for developing MetS as adults. The relation persisted in with similar OR’s following adjustment for individual factors including birth weight, overall childhood morbidity and SES, and adult SES.62 Similarly, the number of days with fever had an OR of 1.15 for having elevated triglycerides as adults.63 Additionally, a higher burden of early childhood diarrhea was associated with modest increases in risk for elevated fasting glucose (OR 1.51),62 low HDL (OR 1.06),63 and abdominal obesity (OR 1.07)63 in adulthood. In general, these studies found higher associations of other markers of disease (anorexia, fever, serious illness) than diarrhea itself in predicting adult diseases and the associations were attenuated following adjustment for childhood stunting, underscoring the potential overlap between inflammation, poor growth and future metabolic disease.62
Sum of evidence
As seen above, data from multiple approaches including cross-sectional and cohort studies among children and adults begin to support a hypothesis that early childhood illness and malnutrition (resulting in low BMI at 2 years old and stunting of height) increases risk for findings related to MetS. The relationship of these issues to enteric infections is further supported by the independent association of frequent diarrhea illness in early childhood to elevations in MetS-related factors as adults.62, 63 Similarly, these associations involving low early childhood BMI in regions of the world in which enteric diseases are endemic suggests further links between environmental enteropathies and future risk. Many of the large-scale studies presented here include statistical adjustments for possible confounders such as parental education, degree of poverty and household sanitation.20, 21, 62, 63 Still, there may remain some residual confounders that contribute to the associations presented here.
Some of these findings regarding adult disease—in particular glucose intolerance—appear to have an especially tight link to poor childhood growth, while other adult findings appear to have a more modest link to early childhood processes. In most cases, known effects of adult obesity on these MetS factors appear to predominate over the effect of poor childhood growth. Nevertheless, even minor effects of childhood illness and poor growth on future disease may provide opportunities to learn mechanisms regarding the causes—and ultimately the prevention—of future disease.
Potential etiologies
Basic science data in models of LBW and inflammation
The etiology for these relationships between childhood nutrition and illness and adult metabolic outcomes is not known, including whether this process shares mechanisms with those connecting LBW and future disease. Also not known is whether potential epigenetic changes relate more strongly to nutritional deprivation or to inflammatory mechanisms. In the case of LBW, postulated mechanisms include methylation of genes and acetylation of histones, two epigenetic processes that can alter the accessibility of certain genes for transcription.64 Animal models models have been able to evaluate the effects of poor nutrition in the absence of inflammation via feeding restriction in neonatal mice in the first days of life (analogous to the late third trimester in humans).65 Such nutritional deprivation results in changes in acetylation of genes such as PDX-1, a transcription important for islet cell development and later insulin production.66 Another basic science approach gave pregnant mice 50% of their usual food intake and found that nutrient deprivation resulted in a decrease in expression of glucose transporters in muscle—a process linked to increase insulin resistance.67 This appeared to occur via alterations in the methylation of Glut4 and acetylation of histones associated with its promoter region. While it is difficult to evaluate for each of these processes in humans, investigators have demonstrated early evidence that similar mechanisms may be involved. For example, relative to unaffected control subjects, individuals exposed to prenatal famine during World War II with clear effects of nutritional deprivation exhibit differences in methylation in genes related to metabolism.68 As these types of epigenetic modifications become better delineated, it will be important to employ both basic and translational approaches to identify whether windows of vulnerability for these events persist after gestation and into early life. This could help to answer whether caloric deficits after birth could also result in life-long changes related to metabolism and glucose utilization.
While the exact effects of inflammation have not been as closely defined, basic science studies have shown changes in chromatin remodeling in response to inflammatory cytokines.69 These known effects are related to immunologic cells per se, but other epigenetic changes occur in the colonic mucosa through toll-like receptors in response to gut pathogens, suggesting a wide degree of complexity in cues for epigenetic changes and downstream tissue effects.70 Overall, the contributions of undernutrition and inflammation to MetS effects and the underlying mechanisms therein will require more detailed basic science investigation.
Clinical studies
Clinical studies investigating the connection between stunting and MetS have revealed interesting findings that address potential mechanisms. Florencio et al. assessed food intake in women living in poor urban areas of Maceió and reported that total calorie consumption per day was not increased among adult women with stunting compared to those without.71 Hoffman et al. assessed children from poor urban areas of Sao Paulo for their respiratory quotient, a measure of expired CO2 per calorie expended that serves as a marker of fat metabolism, for which lower respiratory quotient correlates with a lower degree of fat metabolism.72 They found that stunted children have a greater fasting respiratory quotient than those without stunting. During a re-feeding program, stunted girls had a more rapid rate of weight re-gain than girls without stunting, perhaps analogous to the “catch-up” growth seen in malnourished children who do not have heavy diarrhea burden.17, 73 Nevertheless, stunted girls did not exhibiting the concomitant increase in resting energy expenditure seen during weight gain among girls without stunting.60 Thus, while food intake did not appear to be affected among stunted individuals, long-term changes in energy utilization and metabolic rate may play a role in these long-term differences between stunted and non-stunted individuals.
One proposed mechanism for the connection between stunting and MetS relates to stress response, including the regulation of cortisol and epinephrine. Cortisol is released under control of the hypothalamic-pituitary-adrenal axis and epinephrine is released primarily by the adrenal medulla; both hormones produce an increase in hepatic release of glucose, an increase in insulin resistance and an increase in vascular tone leading to increased blood pressure.74 Indeed it has been noted that tonic increases in cortisol could produce findings related to MetS.75 Normally cortisol is released in a diurnal pattern but it is also released in response to physical and psychological stress. Long-term changes in cortisol regulation have also been proposed to occur following significant psychological stress.76 The mechanism behind such changes is not known, though animal models of psychological stress have demonstrated an increase in methylation of brain tissue, suggesting the possibility of alterations in hypothalamic-pituitary control of cortisol regulation.77–79
Cortisol levels have also been noted to be higher among individuals born with low birth weight as compared to those born with normal birth weight.9–11 Similarly, levels of cortisol have been found to be higher among stunted children in the developing world compared to non-stunted children. Fernald et al. measured random cortisol levels among children from a neighborhood in Haiti and reported higher levels of cortisol among stunted vs. non-stunted children.80 Stunted children also had higher levels of urinary epinephrine and norepinephrine.81 However, a follow-up study of similar size failed to reveal differences in cortisol levels and suggested a blunted response to psychological stress among stunted children in Nepal.82 Basic science approaches also support the potential for involvement of the hypothalamic-pituitary-adrenal axis following undernutrition.83, 84 Overall the role of stress response in mediating long term effects remains unclear.85
Hypertension in these settings could result from either effects at the levels of the kidney or the endothelium. The effect of childhood diarrhea on kidney function is unknown but there is potential that severe or recurrent cases may result in damage, as suggested by an increase in renal disease and CVD following accidental contamination of a Canadian city’s water supply with E.coli O157:H7 and Campylobacter.86 It is also important to note that infectious diarrhea can result in systemic inflammation which can contribute to chronic remodeling of the intima media of arteries, ultimately increasing BP.87 While this has not been demonstrated specifically in the case of recurrent diarrhea, it remains an additional potential mechanism for hypertension in later life. Clearly, more extensive investigations are necessary regarding this and other potential mechanisms of future disease risk.
It is likely that investigations into these mechanisms will reveal complex and overlying processes. This is particularly true regarding so-called “thrifty” genes that may decrease the weight loss incurred from malnutrition and enteric diseases but in the long-run increase risk for adult disease.88 An example of this is ApoE4, a gene involved in cholesterol regulation which in early childhood protects against recurrent diarrhea and the cognitive sequelae of diarrhea but in adulthood increases risk for CVD.89, 90 By protecting against early malnutrition but contributing to later CVD, such “thrifty” genes may mask the effect of stunting on future health. Hence a multitude of factors will have to be weighed in assessing mechanism of risk.
Markers of risk
With the mechanism of these associations unknown, questions persist regarding the optimal marker of risk to use in assessing the effects of nutrition on future disease risk. Potential markers of risk (along with their advantages and disadvantages) include the following:
Height-for-age z-score: Stunting (height-for-age z-score <2 standard deviations below the mean) is likely the most dramatic and noticeable of the markers of nutritional deficiency. It also has clinical implications in that the degree of stunting at 2 years old is associated with the number of diarrheal infections29 and with adult height.21 A single height measurement is likely the easiest of these markers to evaluate in settings in low income settings.91 However, in many ways height z-scores may not be the best predictor of risk. Some children who are genetically programmed to have tall stature may experience significant growth restriction but still not reach a height below the 3rd percentile. Alternatively, children with a height z-score below the 3 percentile may be genetically programmed to be short despite proper nutrition and a lower burden of intestinal disease. While not known, these confounding issues may have contributed to the lower associations of height with measures reported here.21
BMI-for-age z-score: Low BMI at 2 years old may offer benefits over evaluations based on stunting alone. BMI (though not a validated tool for use in children below 2 years old) offers the advantage that it takes into account both height and weight. Children with poor growth due to malnutrition or enteric infections are likely to have less lean and fat mass and thus have a lower weight for height. Thus, as a marker of risk, BMI-for-age is more likely to catch children with tall stature genes who are relatively undernourished but not technically stunted. BMI is also more likely to exclude children with short stature genes who are otherwise robust. However, using low BMI z-score as a marker of risk still may include children with a genetic tendency toward thinness, even in the setting of adequate nutrition. This drawback notwithstanding, of the childhood markers here, BMI z-score at 2 years old was the marker most predictive of future risk, though adult BMI was an even better marker.21, 55
Diarrheal illnesses: The presence of diarrheal illness represents a potentially powerful marker of future risk of adult disease because of the risk of both systemic inflammation and poor nutrient absorption. A meta-analysis of multiple studies suggested that the number of cases of diarrhea represents an important marker of risk for stunting, with an OR of stunting of 1.025 per episode of diarrhea.20, 62, 63 Similarly select enteric pathogens such as Giardia 34 and Cryptosporidium 27 or the duration of episodes (i.e. acute vs. prolonged vs. persistent) may prove markers of risk.27 Children with multiple cases of diarrhea are more likely to have poor gains in weight and height, presumably due to either an increase in intestinal or systemic inflammation 33, 41 or to a decrease in absorbed nutrients over time.27, 29 Systemic inflammatory responses to the infection may also play other roles in blood pressure remodeling.87 Nevertheless, counting the number of episodes of diarrhea could prove difficult in the developing world outside of research studies given limited family education and resources, though assessing duration of diarrheal episodes in early life may help identify at-risk children.27 Further, it has been well documented that a high proportion of children in the developing world have environmental enteropathy even in the absence of overt diarrhea, with measures of intestinal permeability correlating with poor growth while days with diarrhea did not.18, 33, 41 Lastly, diarrhea also identifies the undernourished child at risk of infectious comorbidities such as pneumonia, which in addition to its staggering toll on child survival, clearly contributes to growth faltering.92 Perhaps the greatest utility in using diarrheal burden as a marker of risk is that it may help us come closer than the other markers to understanding the mechanisms behind these effects.
Nutrition: A theoretical marker of increased risk could be some other marker of malnutrition. This could be based on something as simple as an accurate estimate of calories delivered or as complex as a biomarker of nutrition status. Estimates of calories delivered are still problematic given that not all of these calories are likely to be absorbed. Though there are currently no biomarkers of nutritional status in common use, there is great interest in the development of such predictive biomarkers as early warnings of nutritional shortfalls. The use of such a marker may help identify children before growth falters significantly and could also be evaluated in its relationship to the potential epigenetic processes noted above.
At this point, gain of weight and height over time remain the best markers of nutrition adequacy, even if these are more difficult to quantify in assessing long-term effects on adult health risk factors. Similar to questions about markers of risk, the timing of these potential epigenetic changes is also unknown. Many investigations have suggested that while early stunting <2 years old can exhibit “catch-up growth,” stunting at 2 years old is more likely to be persistent.18, 21 Determining the timing of potential effects on long-term health will require careful evaluation of long-term cohorts.
Conclusions
In conclusion, childhood infection and growth restriction as estimated by low BMI or stunting of height appears linked to risk factors for CVD and T2DM. These effects are similar to those seen following LBW and may compound the even greater overall risk posed by adult obesity. While the etiology remains unclear, it is possible that both calorie deficit and infection-related inflammation may play a role. Given the prevalence of enteropathy-related growth failure and worsening rates of obesity in the developing world, early childhood infections and growth failure may prove to be important contributors to future cardiovascular and metabolic diseases in addition to impacts on physical and cognitive development. If these relationships are confirmed through further research, this could bring important worldwide attention to yet another costly reason to prevent malnutrition and enteric disease in childhood.
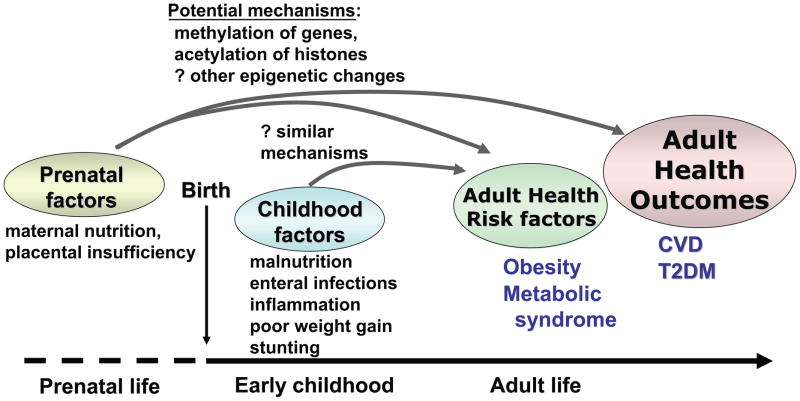
Figure 4. Model for the effects of early childhood nutrition on adult health.
According to hypothesies related to the early origins of adult disease, intrauterine growth restriction leads to epigenetic changes, affecting adult health outcomes. Data are beginning to accumulate that these changes may not be complete at birth but may be affected by further malnutrition and inflammation in early childhood. The mechanistic basis for this (and the ultimate effect on adult health outcomes) is not yet known.
Acknowledgments
This work was supported by NIH grants 5K08HD060739-03 (MDD)
Bibliography
- 1.Barker DJ. Fetal programming of coronary heart disease. Trends Endocrinol Metab. 2002 Nov;13(9):364–368. doi: 10.1016/s1043-2760(02)00689-6. [DOI] [PubMed] [Google Scholar]
- 2.Barker DJ. Adult consequences of fetal growth restriction. Clin Obstet Gynecol. 2006 Jun;49(2):270–283. doi: 10.1097/00003081-200606000-00009. [DOI] [PubMed] [Google Scholar]
- 3.Bavdekar A, Yajnik CS, Fall CH, et al. Insulin resistance syndrome in 8-year-old Indian children: small at birth, big at 8 years, or both? Diabetes. 1999 Dec;48(12):2422–2429. doi: 10.2337/diabetes.48.12.2422. [DOI] [PubMed] [Google Scholar]
- 4.Veening MA, Van Weissenbruch MM, Delemarre-Van De Waal HA. Glucose tolerance, insulin sensitivity, and insulin secretion in children born small for gestational age. J Clin Endocrinol Metab. 2002 Oct;87(10):4657–4661. doi: 10.1210/jc.2001-011940. [DOI] [PubMed] [Google Scholar]
- 5.Barker DJ, Hales CN, Fall CH, Osmond C, Phipps K, Clark PM. Type 2 (non-insulin-dependent) diabetes mellitus, hypertension and hyperlipidaemia (syndrome X): relation to reduced fetal growth. Diabetologia. 1993 Jan;36(1):62–67. doi: 10.1007/BF00399095. [DOI] [PubMed] [Google Scholar]
- 6.Barker DJ, Martyn CN, Osmond C, Hales CN, Fall CH. Growth in utero and serum cholesterol concentrations in adult life. BMJ. 1993 Dec 11;307(6918):1524–1527. doi: 10.1136/bmj.307.6918.1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barker DJ, Osmond C, Golding J, Kuh D, Wadsworth ME. Growth in utero, blood pressure in childhood and adult life, and mortality from cardiovascular disease. BMJ. 1989 Mar 4;298(6673):564–567. doi: 10.1136/bmj.298.6673.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Williams S, St George IM, Silva PA. Intrauterine growth retardation and blood pressure at age seven and eighteen. J Clin Epidemiol. 1992 Nov;45(11):1257–1263. doi: 10.1016/0895-4356(92)90167-l. [DOI] [PubMed] [Google Scholar]
- 9.Clark PM, Hindmarsh PC, Shiell AW, Law CM, Honour JW, Barker DJ. Size at birth and adrenocortical function in childhood. Clin Endocrinol (Oxf) 1996 Dec;45(6):721–726. doi: 10.1046/j.1365-2265.1996.8560864.x. [DOI] [PubMed] [Google Scholar]
- 10.Matthews SG. Early programming of the hypothalamo-pituitary-adrenal axis. Trends Endocrinol Metab. 2002 Nov;13(9):373–380. doi: 10.1016/s1043-2760(02)00690-2. [DOI] [PubMed] [Google Scholar]
- 11.Phillips DI, Jones A. Fetal programming of autonomic and HPA function: do people who were small babies have enhanced stress responses? J Physiol. 2006 Apr 1;572(Pt 1):45–50. doi: 10.1113/jphysiol.2005.104695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matthews SG, Challis JR. Regulation of the hypothalamo-pituitary-adrenocortical axis in fetal sheep. Trends Endocrinol Metab. 1996 Sep;7(7):239–246. doi: 10.1016/s1043-2760(96)00126-9. [DOI] [PubMed] [Google Scholar]
- 13.Kahn HS, Graff M, Stein AD, Lumey LH. A fingerprint marker from early gestation associated with diabetes in middle age: the Dutch Hunger Winter Families Study. Int J Epidemiol. 2009 Feb;38(1):101–109. doi: 10.1093/ije/dyn158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heijmans BT, Tobi EW, Stein AD, et al. Persistent epigenetic differences associated with prenatal exposure to famine in humans. Proc Natl Acad Sci U S A. 2008 Nov 4;105(44):17046–17049. doi: 10.1073/pnas.0806560105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Öberg S, Cnattingius S, Sandin S, Lichtenstein P, Iliadou AN. Birth Weight Predicts Risk of Cardiovascular Disease Within Dizygotic but Not Monozygotic Twin Pairs: A Large Population-Based Co-Twin Control Study. Circulation. 2011;123:2792–2798. doi: 10.1161/CIRCULATIONAHA.110.987339. [DOI] [PubMed] [Google Scholar]
- 16.Palinski W. It Takes Three to Tango: Genes Complicate the Association Between Birth Weight and Cardiovascular Disease. Circulation. 2011;123:2773–2775. doi: 10.1161/CIRCULATIONAHA.111.037432. [DOI] [PubMed] [Google Scholar]
- 17.Guerrant RL, Oria RB, Moore SR, Oria MO, Lima AA. Malnutrition as an enteric infectious disease with long-term effects on child development. Nutr Rev. 2008 Sep;66(9):487–505. doi: 10.1111/j.1753-4887.2008.00082.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lunn PG. The impact of infection and nutrition on gut function and growth in childhood. Proc Nutr Soc. 2000 Feb;59(1):147–154. doi: 10.1017/s0029665100000173. [DOI] [PubMed] [Google Scholar]
- 19.Sawaya AL, Martins PA, Baccin Martins VJ, et al. Malnutrition, long-term health and the effect of nutritional recovery. Nestle Nutr Workshop Ser Pediatr Program. 2009;63:95–105. 105–108, 259–168. doi: 10.1159/000209975. [DOI] [PubMed] [Google Scholar]
- 20.Checkley W, Buckley G, Gilman RH, et al. Multi-country analysis of the effects of diarrhoea on childhood stunting. Int J Epidemiol. 2008 Aug;37(4):816–830. doi: 10.1093/ije/dyn099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Victora CG, Adair L, Fall C, et al. Maternal and child undernutrition: consequences for adult health and human capital. Lancet. 2008 Jan 26;371(9609):340–357. doi: 10.1016/S0140-6736(07)61692-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Godfrey KM, Gluckman PD, Hanson MA. Developmental origins of metabolic disease: life course and intergenerational perspectives. Trends Endocrinol Metab. 2011 Apr;21(4):199–205. doi: 10.1016/j.tem.2009.12.008. [DOI] [PubMed] [Google Scholar]
- 23.Lieb DC, Snow RE, DeBoer MD. Socioeconomic factors in the development of childhood obesity and diabetes. Clin Sports Med. 2009 Jul;28(3):349–378. doi: 10.1016/j.csm.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McKay S, Gaudier E, Campbell DI, Prentice RA. Environmental enteropathy: new targets for nutritional interventions. International Health. 2010;2(3):172–180. doi: 10.1016/j.inhe.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 25.Levine MM. Immunogenicity and efficacy of oral vaccines in developing countries: lessons from a live cholera vaccine. BMC Biol. 2010;8:129. doi: 10.1186/1741-7007-8-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guerrant RL, Schorling JB, McAuliffe JF, de Souza MA. Diarrhea as a cause and an effect of malnutrition: diarrhea prevents catch-up growth and malnutrition increases diarrhea frequency and duration. Am J Trop Med Hyg. 1992 Jul;47(1 Pt 2):28–35. doi: 10.4269/ajtmh.1992.47.28. [DOI] [PubMed] [Google Scholar]
- 27.Moore SR, Lima NL, Soares AM, et al. Prolonged episodes of acute diarrhea reduce growth and increase risk of persistent diarrhea in children. Gastroenterology. 2010 Oct;139(4):1156–1164. doi: 10.1053/j.gastro.2010.05.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schorling JB, McAuliffe JF, de Souza MA, Guerrant RL. Malnutrition is associated with increased diarrhoea incidence and duration among children in an urban Brazilian slum. Int J Epidemiol. 1990 Sep;19(3):728–735. doi: 10.1093/ije/19.3.728. [DOI] [PubMed] [Google Scholar]
- 29.Moore SR, Lima AA, Conaway MR, Schorling JB, Soares AM, Guerrant RL. Early childhood diarrhoea and helminthiases associate with long-term linear growth faltering. Int J Epidemiol. 2001 Dec;30(6):1457–1464. doi: 10.1093/ije/30.6.1457. [DOI] [PubMed] [Google Scholar]
- 30.Ruel MT, Rivera J, Habicht JP, Martorell R. Differential response to early nutrition supplementation: long-term effects on height at adolescence. Int J Epidemiol. 1995 Apr;24(2):404–412. doi: 10.1093/ije/24.2.404. [DOI] [PubMed] [Google Scholar]
- 31.Quadro L, Gamble MV, Vogel S, et al. Retinol and retinol-binding protein: gut integrity and circulating immunoglobulins. J Infect Dis. 2000 Sep;182 (Suppl 1):S97–S102. doi: 10.1086/315920. [DOI] [PubMed] [Google Scholar]
- 32.Alam AN, Sarker SA, Wahed MA, Khatun M, Rahaman MM. Enteric protein loss and intestinal permeability changes in children during acute shigellosis and after recovery: effect of zinc supplementation. Gut. 1994 Dec;35(12):1707–1711. doi: 10.1136/gut.35.12.1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Campbell DI, Elia M, Lunn PG. Growth faltering in rural Gambian infants is associated with impaired small intestinal barrier function, leading to endotoxemia and systemic inflammation. J Nutr. 2003 May;133(5):1332–1338. doi: 10.1093/jn/133.5.1332. [DOI] [PubMed] [Google Scholar]
- 34.Goto R, Mascie-Taylor CG, Lunn PG. Impact of intestinal permeability, inflammation status and parasitic infections on infant growth faltering in rural Bangladesh. Br J Nutr. 2009 May;101(10):1509–1516. doi: 10.1017/S0007114508083554. [DOI] [PubMed] [Google Scholar]
- 35.Beaton GH, Ghassemi H. Supplementary feeding programs for young children in developing countries. Am J Clin Nutr. 1982 Apr;35(4 Suppl):863–916. doi: 10.1093/ajcn/35.4.864. [DOI] [PubMed] [Google Scholar]
- 36.Brown NK, Thompson DJ, Prentice RL. Nontreatment and aggressive narcotic therapy among hospitalized pancreatic cancer patients. J Am Geriatr Soc. 1998 Jul;46(7):839–848. doi: 10.1111/j.1532-5415.1998.tb02717.x. [DOI] [PubMed] [Google Scholar]
- 37.Martorell R. Results and implications of the INCAP follow-up study. J Nutr. 1995 Apr;125(4 Suppl):1127S–1138S. doi: 10.1093/jn/125.suppl_4.1127S. [DOI] [PubMed] [Google Scholar]
- 38.Chen P, Soares AM, Lima AA, et al. Association of vitamin A and zinc status with altered intestinal permeability: analyses of cohort data from northeastern Brazil. J Health Popul Nutr. 2003 Dec;21(4):309–315. [PubMed] [Google Scholar]
- 39.Lima AA, Soares AM, Lima NL, et al. Effects of vitamin A supplementation on intestinal barrier function, growth, total parasitic, and specific Giardia spp infections in Brazilian children: a prospective randomized, double-blind, placebo-controlled trial. J Pediatr Gastroenterol Nutr. 2010 Mar;50(3):309–315. doi: 10.1097/MPG.0b013e3181a96489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Solomons NW, Mazariegos M, Brown KH, Klasing K. The underprivileged, developing country child: environmental contamination and growth failure revisited. Nutr Rev. 1993 Nov;51(11):327–332. doi: 10.1111/j.1753-4887.1993.tb03758.x. [DOI] [PubMed] [Google Scholar]
- 41.Campbell DI, McPhail G, Lunn PG, Elia M, Jeffries DJ. Intestinal inflammation measured by fecal neopterin in Gambian children with enteropathy: association with growth failure, Giardia lamblia, and intestinal permeability. J Pediatr Gastroenterol Nutr. 2004 Aug;39(2):153–157. doi: 10.1097/00005176-200408000-00005. [DOI] [PubMed] [Google Scholar]
- 42.Jafar TH, Qadri Z, Islam M, Hatcher J, Bhutta ZA, Chaturvedi N. Rise in childhood obesity with persistently high rates of undernutrition among urban school-aged Indo-Asian children. Arch Dis Child. 2008 May;93(5):373–378. doi: 10.1136/adc.2007.125641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kimani-Murage EW, Kahn K, Pettifor JM, et al. The prevalence of stunting, overweight and obesity, and metabolic disease risk in rural South African children. BMC Public Health. 2010;10:158. doi: 10.1186/1471-2458-10-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Florencio TM, Ferreira HS, de Franca AP, Cavalcante JC, Sawaya AL. Obesity and undernutrition in a very-low-income population in the city of Maceio, northeastern Brazil. Br J Nutr. 2001 Aug;86(2):277–284. doi: 10.1079/bjn2001396. [DOI] [PubMed] [Google Scholar]
- 45.Walker SP, Wachs TD, Grantham-McGregor S, et al. Inequality in early childhood: risk and protective factors for early child development. Lancet. 2011 Oct 8;378(9799):1325–1338. doi: 10.1016/S0140-6736(11)60555-2. [DOI] [PubMed] [Google Scholar]
- 46.Walker SP, Wachs TD, Gardner JM, et al. Child development: risk factors for adverse outcomes in developing countries. Lancet. 2007 Jan 13;369(9556):145–157. doi: 10.1016/S0140-6736(07)60076-2. [DOI] [PubMed] [Google Scholar]
- 47.Mitchell J. The long term association of early childhood diarrhea with school success: A case study from Pakistan. Journal of Education for International Development. 2006;2(2):1–11. [Google Scholar]
- 48.Berkman DS, Lescano AG, Gilman RH, Lopez SL, Black MM. Effects of stunting, diarrhoeal disease, and parasitic infection during infancy on cognition in late childhood: a follow-up study. Lancet. 2002 Feb 16;359(9306):564–571. doi: 10.1016/S0140-6736(02)07744-9. [DOI] [PubMed] [Google Scholar]
- 49.Black RE, Allen LH, Bhutta ZA, et al. Maternal and child undernutrition: global and regional exposures and health consequences. Lancet. 2008 Jan 19;371(9608):243–260. doi: 10.1016/S0140-6736(07)61690-0. [DOI] [PubMed] [Google Scholar]
- 50.Guerrant RL, Kosek M, Lima AA, Lorntz B, Guyatt HL. Updating the DALYs for diarrhoeal disease. Trends Parasitol. 2002 May;18(5):191–193. doi: 10.1016/s1471-4922(02)02253-5. [DOI] [PubMed] [Google Scholar]
- 51.Doak CM, Adair LS, Bentley M, Monteiro C, Popkin BM. The dual burden household and the nutrition transition paradox. Int J Obes (Lond) 2005 Jan;29(1):129–136. doi: 10.1038/sj.ijo.0802824. [DOI] [PubMed] [Google Scholar]
- 52.Jehn M, Brewis A. Paradoxical malnutrition in mother-child pairs: untangling the phenomenon of over- and under-nutrition in underdeveloped economies. Econ Hum Biol. 2009 Mar;7(1):28–35. doi: 10.1016/j.ehb.2009.01.007. [DOI] [PubMed] [Google Scholar]
- 53.Barker DJ, Osmond C, Forsen TJ, Kajantie E, Eriksson JG. Trajectories of growth among children who have coronary events as adults. N Engl J Med. 2005 Oct 27;353(17):1802–1809. doi: 10.1056/NEJMoa044160. [DOI] [PubMed] [Google Scholar]
- 54.Bhargava SK, Sachdev HS, Fall CH, et al. Relation of serial changes in childhood body-mass index to impaired glucose tolerance in young adulthood. N Engl J Med. 2004 Feb 26;350(9):865–875. doi: 10.1056/NEJMoa035698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fall CH, Sachdev HS, Osmond C, et al. Adult metabolic syndrome and impaired glucose tolerance are associated with different patterns of BMI gain during infancy: Data from the New Delhi Birth Cohort. Diabetes Care. 2008 Dec;31(12):2349–2356. doi: 10.2337/dc08-0911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ferreira HS, Moura FA, Cabral CR, Jr, Florencio TM, Vieira RC, de Assuncao ML. Short stature of mothers from an area endemic for undernutrition is associated with obesity, hypertension and stunted children: a population-based study in the semi-arid region of Alagoas, Northeast Brazil. Br J Nutr. 2009 Apr;101(8):1239–1245. doi: 10.1017/S0007114508059357. [DOI] [PubMed] [Google Scholar]
- 57.Florencio TT, Ferreira HS, Cavalcante JC, Stux GR, Sawaya AL. Short stature, abdominal obesity, insulin resistance and alterations in lipid profile in very low-income women living in Maceio, north-eastern Brazil. Eur J Cardiovasc Prev Rehabil. 2007 Apr;14(2):346–348. doi: 10.1097/hjr.0b013e328010f24d. [DOI] [PubMed] [Google Scholar]
- 58.Florencio TT, Ferreira HS, Cavalcante JC, Sawaya AL. Short stature, obesity and arterial hypertension in a very low income population in North-eastern Brazil. Nutr Metab Cardiovasc Dis. 2004 Feb;14(1):26–33. doi: 10.1016/s0939-4753(04)80044-9. [DOI] [PubMed] [Google Scholar]
- 59.Sesso R, Barreto GP, Neves J, Sawaya AL. Malnutrition is associated with increased blood pressure in childhood. Nephron Clin Pract. 2004;97(2):c61–66. doi: 10.1159/000078402. [DOI] [PubMed] [Google Scholar]
- 60.Grillol LP, Siqueira AF, Silva AC, Martins PA, Verreschi IT, Sawaya AL. Lower resting metabolic rate and higher velocity of weight gain in a prospective study of stunted vs nonstunted girls living in the shantytowns of Sao Paulo, Brazil. Eur J Clin Nutr. 2005 Jul;59(7):835–842. doi: 10.1038/sj.ejcn.1602150. [DOI] [PubMed] [Google Scholar]
- 61.Martins PA, Sawaya AL. Evidence for impaired insulin production and higher sensitivity in stunted children living in slums. Br J Nutr. 2006 May;95(5):996–1001. doi: 10.1079/bjn20061754. [DOI] [PubMed] [Google Scholar]
- 62.Margolis R. The Effects of Early Childhood Diseases on Young Adult Health in Guatemala. PARC Working Paper Series. 2008:1–30. WPS 08–07. [Google Scholar]
- 63.Margolis R. Childhood Morbidity and Health in Early Adulthood: Life course linkages in a high morbidity context. Adv Life Course Res. 2010 Dec 1;15(4):132–146. doi: 10.1016/j.alcr.2010.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pinney SE, Simmons RA. Epigenetic mechanisms in the development of type 2 diabetes. Trends Endocrinol Metab. 2010 Apr;21(4):223–229. doi: 10.1016/j.tem.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kappeler L, De Magalhaes Filho C, Leneuve P, et al. Early postnatal nutrition determines somatotropic function in mice. Endocrinology. 2009 Jan;150(1):314–323. doi: 10.1210/en.2008-0981. [DOI] [PubMed] [Google Scholar]
- 66.Park JH, Stoffers DA, Nicholls RD, Simmons RA. Development of type 2 diabetes following intrauterine growth retardation in rats is associated with progressive epigenetic silencing of Pdx1. J Clin Invest. 2008 Jun;118(6):2316–2324. doi: 10.1172/JCI33655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Raychaudhuri N, Raychaudhuri S, Thamotharan M, Devaskar SU. Histone code modifications repress glucose transporter 4 expression in the intrauterine growth-restricted offspring. J Biol Chem. 2008 May 16;283(20):13611–13626. doi: 10.1074/jbc.M800128200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tobi EW, Lumey LH, Talens RP, et al. DNA methylation differences after exposure to prenatal famine are common and timing- and sex-specific. Hum Mol Genet. 2009 Nov 1;18(21):4046–4053. doi: 10.1093/hmg/ddp353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.McCall CE, El Gazzar M, Liu T, Vachharajani V, Yoza B. Epigenetics, bioenergetics, and microRNA coordinate gene-specific reprogramming during acute systemic inflammation. J Leukoc Biol. 2011 Sep;90(3):439–446. doi: 10.1189/jlb.0211075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kellermayer R, Dowd SE, Harris RA, et al. Colonic mucosal DNA methylation, immune response, and microbiome patterns in Toll-like receptor 2-knockout mice. Faseb J. 2011 May;25(5):1449–1460. doi: 10.1096/fj.10-172205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Florencio TT, Ferreira HS, Cavalcante JC, Luciano SM, Sawaya AL. Food consumed does not account for the higher prevalence of obesity among stunted adults in a very-low-income population in the Northeast of Brazil (Maceio, Alagoas) Eur J Clin Nutr. 2003 Nov;57(11):1437–1446. doi: 10.1038/sj.ejcn.1601708. [DOI] [PubMed] [Google Scholar]
- 72.Hoffman DJ, Martins PA, Roberts SB, Sawaya AL. Body fat distribution in stunted compared with normal-height children from the shantytowns of Sao Paulo, Brazil. Nutrition. 2007 Sep;23(9):640–646. doi: 10.1016/j.nut.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 73.Schorling JB, Guerrant RL. Diarrhoea and catch-up growth. Lancet. 1990;335(8689):599–600. doi: 10.1016/0140-6736(90)90378-i. [DOI] [PubMed] [Google Scholar]
- 74.Kronenberg HM, editor. Williams Textbook of Endocrinology. 11. Philadelphia: Sauders Elsevier; 2008. [Google Scholar]
- 75.Krikorian A, Khan M. Is metabolic syndrome a mild form of Cushing’s syndrome? Rev Endocr Metab Disord. 2010 Jun;11(2):141–145. doi: 10.1007/s11154-010-9142-4. [DOI] [PubMed] [Google Scholar]
- 76.McEwen BS, Gianaros PJ. Central role of the brain in stress and adaptation: links to socioeconomic status, health, and disease. Ann N Y Acad Sci. 2010 Feb;1186:190–222. doi: 10.1111/j.1749-6632.2009.05331.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.McGowan PO, Sasaki A, D’Alessio AC, et al. Epigenetic regulation of the glucocorticoid receptor in human brain associates with childhood abuse. Nat Neurosci. 2009 Mar;12(3):342–348. doi: 10.1038/nn.2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Meaney MJ, Szyf M, Seckl JR. Epigenetic mechanisms of perinatal programming of hypothalamic-pituitary-adrenal function and health. Trends Mol Med. 2007 Jul;13(7):269–277. doi: 10.1016/j.molmed.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 79.Zhang TY, Hellstrom IC, Bagot RC, Wen X, Diorio J, Meaney MJ. Maternal care and DNA methylation of a glutamic acid decarboxylase 1 promoter in rat hippocampus. J Neurosci. 2010 Sep 29;30(39):13130–13137. doi: 10.1523/JNEUROSCI.1039-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fernald LC, Grantham-McGregor SM. Stress response in school-age children who have been growth retarded since early childhood. Am J Clin Nutr. 1998 Sep;68(3):691–698. doi: 10.1093/ajcn/68.3.691. [DOI] [PubMed] [Google Scholar]
- 81.Fernald LC, Grantham-McGregor SM. Growth retardation is associated with changes in the stress response system and behavior in school-aged jamaican children. J Nutr. 2002 Dec;132(12):3674–3679. doi: 10.1093/jn/132.12.3674. [DOI] [PubMed] [Google Scholar]
- 82.Fernald LC, Grantham-McGregor SM, Manandhar DS, Costello A. Salivary cortisol and heart rate in stunted and nonstunted Nepalese school children. Eur J Clin Nutr. 2003 Nov;57(11):1458–1465. doi: 10.1038/sj.ejcn.1601710. [DOI] [PubMed] [Google Scholar]
- 83.Chaillou E, Baumont R, Tramu G, Tillet Y. Long-term undernutrition followed by short-term refeeding effects on the corticotropin-releasing hormone containing neurones in the paraventricular nucleus: an immunohistochemical study in sheep. J Neuroendocrinol. 2002 Apr;14(4):269–275. doi: 10.1046/j.1365-2826.2002.00768.x. [DOI] [PubMed] [Google Scholar]
- 84.Avraham Y, Hao S, Mendelson S, Berry EM. Hypothalamic-pituitary-adrenal responses to weight loss in mice following diet restriction, activity or separation stress: effects of tyrosine. Nutr Neurosci. 2002 Oct;5(5):327–335. doi: 10.1080/1028415021000033794. [DOI] [PubMed] [Google Scholar]
- 85.Bose M, Olivan B, Laferrere B. Stress and obesity: the role of the hypothalamic-pituitary-adrenal axis in metabolic disease. Curr Opin Endocrinol Diabetes Obes. 2009 Oct;16(5):340–346. doi: 10.1097/MED.0b013e32832fa137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Clark WF, Sontrop JM, Macnab JJ, et al. Long term risk for hypertension, renal impairment, and cardiovascular disease after gastroenteritis from drinking water contaminated with Escherichia coli O157:H7: a prospective cohort study. BMJ. 2010;341:c6020. doi: 10.1136/bmj.c6020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Nilsson PM. Elevated blood pressure predicts type 2 diabetes, but why? J Hypertens. 2008 Sep;26(9):1740–1741. doi: 10.1097/HJH.0b013e32830c6939. [DOI] [PubMed] [Google Scholar]
- 88.Prentice AM. Early influences on human energy regulation: thrifty genotypes and thrifty phenotypes. Physiol Behav. 2005 Dec 15;86(5):640–645. doi: 10.1016/j.physbeh.2005.08.055. [DOI] [PubMed] [Google Scholar]
- 89.Oria RB, Patrick PD, Blackman JA, Lima AA, Guerrant RL. Role of apolipoprotein E4 in protecting children against early childhood diarrhea outcomes and implications for later development. Med Hypotheses. 2007;68(5):1099–1107. doi: 10.1016/j.mehy.2006.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Oria RB, Patrick PD, Zhang H, et al. APOE4 protects the cognitive development in children with heavy diarrhea burdens in Northeast Brazil. Pediatr Res. 2005 Feb;57(2):310–316. doi: 10.1203/01.PDR.0000148719.82468.CA. [DOI] [PubMed] [Google Scholar]
- 91.Subramanian SV, Ozaltin E, Finlay JE. Height of Nations: A Socioeconomic Analysis of Cohort Differences and Patterns among Women in 54 Low- to Middle-Income Countries. PLoS One. 6(4):e18962. doi: 10.1371/journal.pone.0018962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Schlaudecker EP, Steinhoff MC, Moore SR. Interactions of diarrhea, pneumonia, and malnutrition in childhood: recent evidence from developing countries. Curr Opin Infect Dis. 2011 Oct;24(5):496–502. doi: 10.1097/QCO.0b013e328349287d. [DOI] [PMC free article] [PubMed] [Google Scholar]