Abstract
Researchers have identified an association between baseline γ-glutamyltransferase (GGT) and prehypertension. However, data from China are limited. A cross-sectional study was performed among 2,205 subjects from Heilongjiang Province in China. Multiple logistic regression analyses revealed a significant association between baseline GGT and prehypertension [1.59, 95% confidence interval (CI), 1.18–2.16], comparing quartile 4 to quartile 1. Subgroup analyses showed a stronger association between GGT and prehypertension in Koreans; men, current alcohol drinkers and subjects with pre-diabetes. Receiver operating characteristics (ROC) analysis demonstrated that when GGT was higher than 20 U/l, the risk of developing prehypertension increased. Serum GGT is used as a biochemical liver test, but our findings suggest that baseline values may also predict prehypertension in Chinese.
Keywords: γ-glutamyltransferase, prehypertension
Introduction
γ-glutamyltransferase (GGT) is mostly used in clinical laboratories as a marker of hepatic inflammation. Recently, increasing evidence suggests that GGT plays an important role in the development of cardiovascular events. Longitudinal and cross-sectional investigations have associated GGT with an increase in all-cause mortality, as well as chronic heart diseases such as congestive heart failure (1–5).
The Seventh Report of the Joint National Committee on the Prevention, Detection, Evaluation and Treatment of High Blood Pressure (JNC7) (6) defines prehypertension as systolic blood pressure from 120–139 mm/Hg or diastolic pressure from 80–89 mm/Hg. Compared to normotension, prehypertension is associated with a higher risk of developing hypertension (7). Recent studies suggest that baseline GGT may also predict the development of hypertension independent of traditional risk factors. However, these conclusions are based on different study populations, designs and sampling procedures. In addition, the intensity of the association differs between countries and districts (8–14).
In China, hypertension is the leading preventable risk factor for death among adults (15). The aims of this study were to explore a more sensitive risk factor for prehypertension and investigate a new application for GGT. Blood pressure, age, gender and other established risk factors for hypertension were included in analyses and classified into subgroups to observe their interactions. We also defined the association between GGT and the risk of prehypertension.
Materials and methods
Study population
The data were part of the Expansion Investigation of Human Physiology Constant in China. This survey was conducted in Harbin and Hailin of Heilongjiang Province, which is located in the northeast of China. Subjects were selected from certain communities using cluster random sampling. The protocol was approved by the Institutional Review Board of the Institute of Basic Medical Science, Chinese Academy of Medical Sciences. All subjects signed written informed consent forms. Inclusion criteria were age between 25 and 80 years, no serious chronic diseases, and no high fever in the past 15 days. Exclusion criteria included hypertension (blood pressure 140/90 mm/Hg or antihypertensive treatment) at baseline and individuals with potential liver pathology.
Measurement
A precisely designed questionnaire was used to assess demographic data, including date of birth, gender, ethnicity, education level, health status, and health behavior (e.g., smoking, alcohol use and physical activity). All participants were asked to avoid smoking and heavy physical activity for at least 2 h before a physical examination, which included measures of resting blood pressure, height, weight, and waist and hip circumference. Two blood pressure measurements were taken with a mercury sphygmomanometer after participants had rested in a sitting position for at least 5 min. A blood sample was obtained after fasting for 8–12 h. For other biochemical analyses, we collected specimens in a vacuum tube with no additives. Samples were centrifuged at 3000 × g for 10 min at room temperature. After separation, they were measured using a chemistry analyzer (Hitachi 7020) (Hitachi, Tokyo, Japan). Reagents and calibrators were purchased from Hitachi. As part of a chemistry profile, GGT, total cholesterol (TC), glucose, triglycerides (TG), high density lipoprotein cholesterol (HDL-C), and low density lipoprotein cholesterol (LDL-C) were measured by kinetic enzyme assays.
Statistical methods
Summary statistics are expressed as numbers for categorical data, mean ± SD for approximately continuous variables. Comparison between groups was made using the Chi-square test for categorical data and the Mann-Whitney U test for continuous data.
Multivariable logistic regression analyses were performed to calculate the odds ratios (ORs) for prehypertension according to GGT quartile, adjusting for age, gender, smoking, alcohol consumption, BMI and waist circumference. The P-values quoted are two-sided, and those values with P<0.05 are regarded as statistically significant. All statistical analysis was performed with SPSS v.13.0 (IBM SPSS Statistics, Armonk, NY, USA).
Results
The baseline characteristics of the study population are summarized by blood pressure in Table I. A total of 789 males and 1416 females between the ages of 25.0 and 84.6 were included in the study. We excluded 110 individuals with GGT values above the normal reference range of the laboratory (GGT >67 U/l). The remaining 2,205 participants were included in this analysis. Compared with the normotensive group, those with prehypertension tended to be older and have higher GGT, systolic and diastolic blood pressure, fasting blood glucose, TG and TC. Whereas no significant difference between the two groups was found in mean values of physical activity.
Table I.
Baseline characteristics of participants grouped by blood pressure status.
| Normotension (n=1111) mean ± SD | Prehypertension (n=1094) mean ± SD | aP-value | bP-value for trend | |
|---|---|---|---|---|
| Age (years) | 43.3±12.2 | 51.2±13.5 | <0.0001 | <0.0001 |
| Body mass index (kg/m2) | 22.9±2.9 | 24.3±3.0 | <0.0001 | <0.0001 |
| Waist circumference (cm) | 77.5±8.8 | 82.8±9.1 | <0.0001 | <0.0001 |
| Hip circumference (cm) | 93.2±6.1 | 95.3±6.5 | <0.0001 | <0.0001 |
| Systolic blood pressure (mm/Hg) | 110.1±7.6 | 126.7±6.5 | <0.0001 | <0.0001 |
| Diastolic blood pressure (mm/Hg) | 71.2±6.1 | 80.4±6.0 | <0.0001 | <0.0001 |
| Fasting plasma glucose (mmol/l) | 5.4±1.0 | 5.7±1.1 | <0.0001 | <0.0001 |
| GGT (U/l) | 19.4±11.4 | 24.8±13.7 | <0.0001 | <0.0001 |
| Triglycerides (mmol/l) | 1.3±0.9 | 1.7±1.3 | <0.0001 | <0.0001 |
| Total cholesterol (mmol/l) | 4.5±0.9 | 4.8±0.9 | <0.0001 | <0.0001 |
| HDL (mmol/l) | 1.5±0.4 | 1.5±0.4 | <0.0001 | <0.0001 |
| LDL (mmol/l) | 2.5±0.8 | 2.7±0.8 | <0.0001 | <0.0001 |
| n | n | aP-value | bP-value for trend | |
|---|---|---|---|---|
| Gender | <0.0001 | <0.0001 | ||
| Male | 298 | 491 | ||
| Female | 813 | 603 | ||
| Nationality | 0.006 | 0.006 | ||
| Chinese | 857 | 801 | ||
| Korean | 209 | 262 | ||
| Drinking status | <0.0001 | <0.0001 | ||
| Never-drinkers | 854 | 741 | ||
| Current drinkers | 203 | 288 | ||
| Smoking status | <0.0001 | <0.0001 | ||
| Never-smokers | 913 | 802 | ||
| Current smokers | 160 | 23 | ||
| Physical exercise | 0.117 | 0.195 | ||
| Never | 248 | 283 | ||
| Occasionally | 616 | 566 | ||
| Often | 247 | 245 | ||
| Education status | <0.0001 | <0.001 | ||
| Less than high school | 233 | 364 | ||
| High school | 241 | 286 | ||
| More than high school | 637 | 444 |
P-value for comparison between normotensive and hypertensive participants;
P-value estimated from logistic regression models.
Table II shows the distribution of the continuous variables across the quartiles of GGT. Significant linear trends were noted across most quartiles with correlations between most variables and GGT (P<0.01). Notably, ORs of TG, TC, LDL-C, HDL-C were 4.12 (3.36–5.05), 2.00 (1.74–2.29), 2.15 (1.84–2.50) and 4.33 (3.17–5.93), respectively.
Table II.
The distribution of the continuous variables with GGT quartile.
| GGT baseline (U/l) | ||||
|---|---|---|---|---|
|
|
||||
| <13 (n=641) | 13–18 (n=487) | >18–28 (n=510) | >28 (n=567) | |
| Age (years) | 43.8 (13.3) | 48.5 (13.4) | 49.0 (13.3) | 48.5 (13.0) |
| OR (95% CI) | reference | 1.03 (1.02–1.04)b | 1.030 (1.02–1.04)b | 1.028 (1.02–1.04)b |
| Body mass index (kg/m2) | 22.4 (2.8) | 23.1 (2.7) | 24.1 (3.0) | 24.9 (3.1) |
| OR (95% CI) | reference | 1.10 (1.05–1.15)b | 1.24 (1.19–1.30)b | 1.355 (1.30–1.42)b |
| Waist circumference (cm) | 75.1 (7.9) | 78.5 (8.4) | 82.1 (8.6) | 85.5 (8.8) |
| OR (95% CI) | reference | 1.05 (1.05–1.15)b | 1.11 (1.05–1.15)b | 1.16 (1.05–1.15)b |
| Hip circumference (cm) | 92.4 (6.0) | 93.2 (5.9) | 95.2 (6.5) | 96.4 (6.3) |
| OR (95% CI) | reference | 1.02 (1.00–1.04)a | 1.08 (1.06–1.10)b | 1.11 (1.09–1.14)b |
| Systolic blood pressure (mm/Hg) | 114.5 (11.5) | 118.2 (10.7) | 119.8 (10.0) | 121.5 (9.9) |
| OR (95% CI) | reference | 1.03 (1.05–1.15)b | 1.05 (1.05–1.15)b | 1.06 (1.05–1.15)b |
| Diastolic blood pressure (mm/Hg) | 73.5 (8.0) | 75.3 (7.3) | 76.7 (7.0) | 77.7 (7.2) |
| OR (95% CI) | reference | 1.03 (1.02–1.05)b | 1.06 (1.04–1.08)b | 1.08 (1.06–1.10)b |
| Glucose (mmol/l) | 5.3 (0.9) | 5.4 (0.8) | 5.6 (1.0) | 5.8 (1.4) |
| OR (95% CI) | reference | 1.35 (1.13–1.61)a | 1.62 (1.37–1.91)b | 1.86 (1.58–2.18)b |
| Triglycerides (mmol/l) | 1.0 (0.8) | 1.3 (0.9) | 1.6 (1.2) | 1.9 (1.3) |
| OR (95% CI) | reference | 2.29 (1.84–2.84)b | 3.44 (2.81–4.22)b | 4.12 (3.36–5.05)b |
| Total cholesterol (mmol/l) | 4.3 (0.8) | 4.6 (0.9) | 4.8 (0.9) | 4.8 (0.9) |
| OR (95% CI) | reference | 1.46 (1.27–1.68)b | 2.00 (1.74–2.30)b | 2.00 (1.74–2.29)b |
| HDL (mmol/l) | 1.6 (0.4) | 1.4 (0.4) | 1.5 (0.4) | 1.5 (0.4) |
| OR (95% CI) | reference | 1.81 (1.30–2.52)b | 2.90 (2.08–4.03)b | 4.33 (3.17–5.93)b |
| LDL (mmol/l) | 2.3 (0.8) | 2.5 (0.8) | 2.7 (0.8) | 2.8 (0.8) |
| OR (95% CI) | reference | 1.56 (1.33–1.82)b | 2.02 (1.73–2.36)b | 2.15 (1.84–2.50)b |
| GGT (U/l) | 10.4 (2.2) | 15.9 (1.4) | 22.5 (2.5) | 40.2 (10.7) |
P<0.01;
P<0.001.
Table III and Fig. 1 present adjusted ORs for prehypertension according to GGT quartiles in a series of logistic models. Increased GGT quartiles were positively associated with prehypertension for age, gender, and nationality-adjusted and multivariable-adjusted models. Trends in these associations were also statistically significant. Significant factors in Table I were included in the models. We examined the soaring ORs of prehypertension associated with increasing levels of serum GGT.
Table III.
Adjusted ORs of prehypertension according to GGT quartiles.
| OR (95% CI) | |||||
|---|---|---|---|---|---|
|
|
|||||
| GGT baseline (U/l) | |||||
|
|
|||||
| <13 | 13–18 | >18–28 | >28 | P-value for trend | |
| Model 1 | 1.0 | 0.95 (0.75–1.21) | 1.21 (0.94–1.55) | 1.77 (1.37–2.29) | <0.001 |
| Model 2 | 1.0 | 1.09 (0.83–1.44) | 1.23 (0.93–1.63) | 1.66 (1.23–2.23) | 0.007 |
| Model 3 | 1.0 | 1.10 (0.83–1.44) | 1.21 (0.89–1.58) | 1.59 (1.18–2.16) | 0.020 |
Model 1, adjusted for age, gender and nationality; model 2, adjusted as above plus BMI, smoking status, drinking status, waist circumference, hip circumference, education status; model 3, adjusted as above plus glucose, TG, TC, HDL-C, LDL-C.
Figure 1.

Adjusted ORs of prehypertension according to GGT quartiles. Model 1, adjusted age, gender and nationality; model 2, adjusted as above plus BMI, smoking status, drinking status, waist circumference, hip circumference and education status; model 3, adjusted as above plus glucose, TG, TC, HDL-C and LDL-C.
To eliminate confoundings, we stratified the analyses according to drinking status, gender, nationality, glucose and lipid level. Fig. 2A shows that the risk of prehypertension increased more rapidly with drinking status, yet there was no linear relationship across GGT quartiles. Stratification by gender produced the same outcome (Fig. 2B). Compared with females, males showed a greater trend toward prehypertension (top vs. bottom quartile OR, 2.06; 95% CI, 1.08–3.93). However, the trend was inconsistent within quartiles in males and risk in quartile 2 was lower than that in the other quartiles. The association between GGT and prehypertension within subgroup of nationality was positive in a dose-response pattern, even after full adjustment (Table IV, Fig. 2C). Adjusted ORs across quartiles of serum GGT in those of Korean nationality were 1.41, 1.43 and 2.12 respectively. In comparison, OR of individuals of Chinese nationality was 0.96, 1.13 and 1.40.
Figure 2.



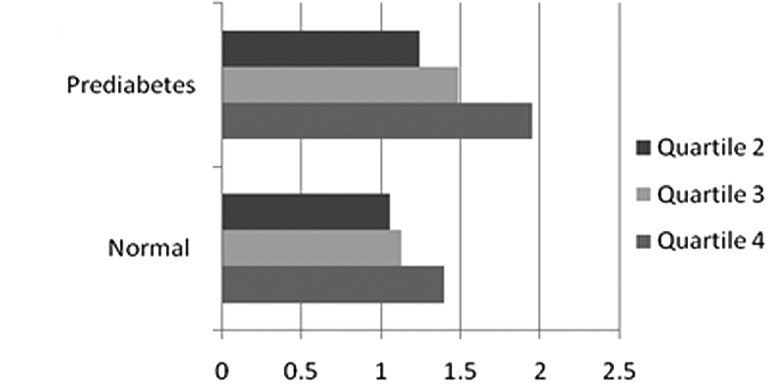

Comparative ORs of prehypertension according to GGT quartiles by stratification for (A) drinking status, (B) gender, (C) nationality, (D) glucose level and (E) lipid level in fully-adjusted model.
Table IV.
Adjusted ORs of prehypertension according to GGT quartiles by stratification for nationality, drinking status, gender, glucose and lipid level.
| OR (95% CI) | ||||||
|---|---|---|---|---|---|---|
|
|
||||||
| GGT baseline (U/l) | ||||||
|
|
||||||
| n | <13 | 13–18 | >18–28 | >28 | P-value | |
| Nationality | ||||||
| Chinese | 1658 | 1.0 | 0.96 (0.70–1.33) | 1.13 (0.80–1.53) | 1.40 (0.99–1.98) | 0.141 |
| Korean | 547 | 1.0 | 1.41 (0.83–2.40) | 1.43 (0.77–2.64) | 2.12 (1.05–4.26) | 0.209 |
| Drinking Status | ||||||
| Drinking | 610 | 1.0 | 2.10 (0.96–4.63) | 1.65 (0.76–3.59) | 2.48 (1.12–5.53) | 0.102 |
| Never drinking | 1595 | 1.0 | 1.03 (0.76–1.38) | 1.20 (0.88–1.64) | 1.48 (1.05–2.08) | 0.115 |
| Gender | ||||||
| Male | 791 | 1.0 | 1.60 (0.81–3.17) | 1.40 (0.74–2.65) | 2.06 (1.08–3.93) | 0.088 |
| Female | 1414 | 1.0 | 1.03 (0.76–1.39) | 1.22 (0.87–1.71) | 1.38 (0.94–2.02) | 0.326 |
| Glucose | ||||||
| Normal | 1607 | 1.0 | 1.06 (0.77–1.46) | 1.13 (0.80–1.59) | 1.40 (0.96–2.04) | 0.345 |
| Prediabetes | 598 | 1.0 | 1.24 (0.69–2.23) | 1.49 (0.83–2.67) | 1.95 (1.06–3.57) | 0.163 |
| Lipid level | ||||||
| Normal | 1697 | 1.0 | 1.02 (0.76–1.38) | 1.13 (0.82–1.56) | 1.60 (1.13–2.27) | 0.035 |
| Dyslipidemia | 508 | 1.0 | 1.53 (0.74–3.17) | 1.50 (0.74–3.07) | 1.63 (0.78–3.41) | 0.599 |
We also examined the impact of glucose on the development of prehypertension according to GGT quartiles. We classified glucose into two groups: normal (glucose <6.0) and prediabetic status (6.0< glucose <7.0). We found a significant linear relationship between GGT quartiles and prehypertension in both states. However, the association was stronger in the prediabetic group than in the normal one, which had an OR of 1.95 (1.06–3.57) in quartile 4 vs. an OR of 1.40 (0.96–2.04) in quartile 1 in the fully adjusted model (Table IV and Fig. 2D).
According to ‘Guide to China's Prevention and Treatment of Adult Dyslipidemia’, we sorted out two groups, normal level and dyslipidemia (TC >6.22 or TG >2.26 or HDL <1.55 or LDL >4.14), to highlight the relationship between the indices of lipid metabolism, GGT and prehypertension (16). Table IV shows that the association was significant only in the normal group (P<0.05).
Receiver operating characteristic (ROC) analysis was used to investigate GGT levels and other risk factors associated with prehypertension, and to try to identify the possible dividing line of GGT between healthy subjects and those with prehypertension (Fig. 3). The area under the curve (AUC) of GGT was 0.632, slightly higher than AUC of TG (0.630), glucose (0.626), LDL (0.601), HDL (0.559) and TC (0.581). The cutoff point of GGT was 20 IU/l, with a sensitivity of 0.52 and a specificity of 0.67.
Figure 3.

ROC curve of GGT and prehypertension.
Discussion
Several recent studies have identified baseline GGT as an independent biomarker for the development of cardiovascular diseases (1–5,8–14). In our study, we found that baseline GGT predicted prehypertension in a dose-response pattern. Adjusted relative risks were 1.13, 1.19 and 1.53 according to quartiles of baseline serum GGT (P<0.05).
This association was independent of the effects of alcohol consumption and was present in both nondrinkers and drinkers. As serum GGT is also a marker of alcohol intake, these findings are consistent with the hypothesis of an association with prehypertension independent of alcohol intake (2,17,18). Our outcomes, which show a stronger association among drinkers than nondrinkers, differ somewhat from those in other studies. However, the drinking group did not show a linear tendency across the GGT range. This might be due to our sample size, which precluded meaningful comparisons between lifetime abstainers and former drinkers.
The positive association between GGT and prehypertension was stronger in males. However, the female group showed a direct relation between the quartile of baseline GGT and the risk of prehypertension. Results classified by gender have varied among studies. In a prospective cohort study in Korea with 293 prehypertensive individuals, 5-year hypertension incidence suggested that baseline serum GGT within the normal range strongly predicted the future risk of hypertension, but only in females (8). Our study also differed by sample size and nationalities. The National Health and Nutrition Examination Survey (NHANES) found that among 5,827 US adults, higher serum GGT levels were associated with prehypertension without CVD and hypertension. This association persisted in separate analyses among males and females (19). However, NHANES did not exclude participants with abnormal GGT (GGT >55 U/l).
We observed a dose-response relationship among Hans and Koreans. Specifically, the association between GGT and prehypertension was stronger in Koreans compared with Hans. Similarly, a study showed that the adjusted relative risks of GGT and prehypertension in Koreans were 1.0, 3.7, 3.6 and 6.0 according to quartiles of baseline serum GGT (P<0.01) (7). Koreans appear to be at an increased risk for developing hypertension. The reasons are yet to be identified.
Based on our findings, participants with prediabetes and dyslipidemia had higher ORs for prehypertension than those in the normal group. After stratification, only the normal lipid group showed a significant association between GGT and the risk of prehypertension. In the separate logistic regression, the association between GGT and indices of lipids was notable (20,21). These outcomes suggest that in healthy subjects, GGT may give a warning for prehypertension before the occurrence of dyslipidemia. A British regional heart study by Wannamethee et al noted increasing GGT levels were strongly associated with all-cause mortality, and a strong positive correlation with body mass index, total cholesterol and diabetes mellitus (22). A study reported by Ruttmann et al in 2005 with the participation of 163,944 adults also provided similar results revealing that GGT was positively correlated with risk factors for cardiovascular disease including body mass index, serum triglycerides, total cholesterol, systolic and diastolic blood pressure, and glucose. Patients with higher GGT values had a more than 1.5-fold increased risk of total mortality from cardiovascular disease (23,24).
GGT is an enzyme that transfers γ-glutamyl functional groups. In relation to cardiovascular disease, GGT falls under a new classification of ‘oxidative stress’ in view of its role in the degradation of the antioxidant glutathione. Furthermore, GGT hydrolyzes glutathione into glutamate and a cysteinyl-glycine dipeptide, the latter acts as a strong reducing agent of iron, with the stepwise development of the super-oxide ion and hydrogen peroxide. Thus, GGT is involved directly in reactive oxygen species generation as a pro-oxidant (25). GGT could likewise be considered a proinflammatory marker.
We also tried to find the cutoff point for GGT as a tool to assess risk of prehypertension. ROC analysis showed an increased risk of hypertension when GGT exceeded 20 U/l. This is the first report to identify a parameter for discriminating healthy subjects from those at risk of prehypertension. According to our results, GGT was found to be a more valuable biomarker in the diagnosis of prehypertension than glucose, TC, TG, HDL and LDL.
One limitation of our study is its cross-sectional nature, which precludes inferences of causation. This study was conducted in several different districts, and samples were collected at nearly the same time. Although several studies have confirmed that GGT displays a polymorphism (26), we did not provide detection. The strengths of our study included its population-based nature, and stratification and ROC analyses.
In conclusion, GGT within the normal range is associated with prehypertension in China. Thus, GGT may be used to assess cardiovascular risk and plan appropriate treatment. Further study of the mechanisms of GGT as it relates to hypertension may provide a new understanding of how cardiovascular disease develops.
Acknowledgements
This study was supported by the Ministry of Science and Technology of P.R. China (The Basic Performance Key Project, no. 2006FY110300). We acknowledge the assistance of Dr Yazhuo Wang in the manuscript preparation.
Abbreviations
- GGT
γ-glutamyltransferase
- BMI
body mass index
- TC
total cholesterol
- TGs
triglycerides
- HDL
high density lipoprotein cholesterol
- LDL
low density lipoprotein cholesterol
References
- 1.Lee DS, Evans JC, Robins SJ, et al. Gamma glutamyl transferase and metabolic syndrome, cardiovascular disease, and mortality risk: the Framingham Heart Study. Arterioscler Thromb Vasc Biol. 2007;27:127–133. doi: 10.1161/01.ATV.0000251993.20372.40. [DOI] [PubMed] [Google Scholar]
- 2.Lee DH, Jacobs DR, Jr, Gross M, et al. Gamma-glutamyltransferase is a predictor of incident diabetes and hypertension: the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Clin Chem. 2003;49:1358–1366. doi: 10.1373/49.8.1358. [DOI] [PubMed] [Google Scholar]
- 3.Wannamethee G, Ebrahim S, Shaper AG. Gamma-glutamyltransferase: determinants and association with mortality from ischemic heart disease and all causes. Am J Epidemiol. 1995;142:699–708. doi: 10.1093/oxfordjournals.aje.a117699. [DOI] [PubMed] [Google Scholar]
- 4.Lee DH, Gross MD, Jacobs DR., Jr Association of serum carotenoids and tocopherols with gamma-glutamyltransferase: the Cardiovascular Risk Development in Young Adults (CARDIA) Study. Clin Chem. 2004;50:582–588. doi: 10.1373/clinchem.2003.028852. [DOI] [PubMed] [Google Scholar]
- 5.Ruttmann E, Brant LJ, Concin H, Diem G, Rapp K, Ulmer H. Gamma-glutamyltransferase as a risk factor for cardiovascular disease mortality: an epidemiological investigation in a cohort of 163, 944 Austrian adults. Circulation. 2005;112:2130–2137. doi: 10.1161/CIRCULATIONAHA.105.552547. [DOI] [PubMed] [Google Scholar]
- 6.Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289:2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 7.Vasan RS, Larson MG, Leip EP, Kannel WB, Levy D. Assessment of frequency of progression to hypertension in non-hypertensive participants in the Framingham Heart Study: A cohort study. Lancet. 2001;358:1682–1686. doi: 10.1016/S0140-6736(01)06710-1. [DOI] [PubMed] [Google Scholar]
- 8.Hwang JH, Shin JY, Chun B, et al. Association between gamma-glutamyltransferase and hypertension incidence in rural prehypertensive adults. J Prev Med Public Health. 2010;43:18–25. doi: 10.3961/jpmph.2010.43.1.18. [DOI] [PubMed] [Google Scholar]
- 9.Celik T, Yuksel UC, Kilic S, Yaman H, Iyisoy A, Karaeren H. The relationship of gamma-glutamyltransferase to aortic elastic properties in young patients with prehypertension. Clin Exp Hypertens. 2010;32:377–384. doi: 10.3109/10641961003628528. [DOI] [PubMed] [Google Scholar]
- 10.Stranges S, Trevisan M, Dorn JM, Dmochowski J, Donahue RP. Body fat distribution, liver enzymes, and risk of hypertension: evidence from the Western New York Study. Hypertension. 2005;46:1186–1193. doi: 10.1161/01.HYP.0000185688.81320.4d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miura K, Nakagawa H, Nakamura H, et al. Serum gamma-glutamyl transferase level in predicting hypertension among male drinkers. J Hum Hypertens. 1994;8:445–449. [PubMed] [Google Scholar]
- 12.Schutte AE, van Rooyen JM, Huisman HW, Kruger HS, de Ridder JH. Factor analysis of possible risks for hypertension in a black South African population. J Hum Hypertens. 2003;17:339–348. doi: 10.1038/sj.jhh.1001553. [DOI] [PubMed] [Google Scholar]
- 13.Kawamoto R, Kohara K, Tabara Y, Kusunoki T, Otsuka N, Miki T. Association between serum gamma-glutamyl transferase level and prehypertension among community-dwelling men. Tohoku J Exp Med. 2008;216:213–221. doi: 10.1620/tjem.216.213. [DOI] [PubMed] [Google Scholar]
- 14.Greenlund KJ, Croft JB, Mensah GA. Prevalence of heart disease and stroke risk factors in persons with prehypertension in the United States, 1999–2000. Arch Intern Med. 2004;164:2113–2118. doi: 10.1001/archinte.164.19.2113. [DOI] [PubMed] [Google Scholar]
- 15.Qin XJ, Shi HZ. Major causes of death during the past 25 years in China. Chin Med J (Engl) 2007;120:2317–2320. [PubMed] [Google Scholar]
- 16.Joint committee for developing Chinese guidelines on prevention and treatment of dyslipidemia in adults. Zhonghua Xin Xue Guan Bing Za Zhi. 2007;35:390–419. [PubMed] [Google Scholar]
- 17.Yamada Y, Ikai E, Tsuritani I, Ishizaki M, Honda R, Ishida M. The relationship between serum gamma-glutamyl transpeptidase levels and hypertension: common in drinkers and nondrinkers. Hypertens Res. 1995;18:295–301. doi: 10.1291/hypres.18.295. [DOI] [PubMed] [Google Scholar]
- 18.Ikai E, Honda R, Yamada Y. Serum gamma-glutamyl transpeptidase level and blood pressure in nondrinkers: a possible pathogenetic role of fatty liver in obesity-related hypertension. J Hum Hypertens. 1994;8:95–100. [PubMed] [Google Scholar]
- 19.Shankar A, Li J. Association between serum gamma-glutamyltransferase level and prehypertension among US adults. Circ J. 2007;71:1567–1572. doi: 10.1253/circj.71.1567. [DOI] [PubMed] [Google Scholar]
- 20.Paolicchi A, Emdin M, Passino C, et al. Beta-Lipoprotein- and LDL-associated serum gamma-glutamyltransferase in patients with coronary atherosclerosis. Atherosclerosis. 2006;186:80–85. doi: 10.1016/j.atherosclerosis.2005.07.012. [DOI] [PubMed] [Google Scholar]
- 21.Thamer C, Tschritter O, Haap M, et al. Elevated serum GGT concentrations predict reduced insulin sensitivity and increased intrahepatic lipids. Horm Metab Res. 2005;37:246–251. doi: 10.1055/s-2005-861411. [DOI] [PubMed] [Google Scholar]
- 22.Wannamethee G, Ebrahim S, Shaper AG. Gamma-glutamyltransferase: determinants and association with mortality from ischemic heart disease and all causes. Am J Epidemiol. 1995;142:699–708. doi: 10.1093/oxfordjournals.aje.a117699. [DOI] [PubMed] [Google Scholar]
- 23.Ruttmann E, Brant LJ, Concin H, et al. Gamma-glutamyltransferase as a risk factor for cardiovascular disease mortality: an epidemiological investigation in a cohort of 163,944 Austrian adults. Circulation. 2005;112:2130–2137. doi: 10.1161/CIRCULATIONAHA.105.552547. [DOI] [PubMed] [Google Scholar]
- 24.Giral P, Ratziu V, Chapman JC. Letter regarding article by Ruttmann et al, ‘gamma-Glutamyltransferase as a risk factor for cardiovascular disease mortality: an epidemiological investigation in a cohort of 163,944 Austrian adults’. Circulation. 2006;113:e299–e300. doi: 10.1161/CIRCULATIONAHA.105.594176. [DOI] [PubMed] [Google Scholar]
- 25.Emdin M, Pompella A, Paolicchi A. Gamma-glutamyl-transferase, atherosclerosis, and cardiovascular disease: triggering oxidative stress within the plaque. Circulation. 2005;112:2078–2080. doi: 10.1161/CIRCULATIONAHA.105.571919. [DOI] [PubMed] [Google Scholar]
- 26.Rouleau GA, Bazanowski A, Cohen EH, Guellaen G, Gusella JF. Gamma-glutamyl transferase locus (GGT) displays a PvuII polymorphism. Nucleic Acids Res. 1988;16:11848. doi: 10.1093/nar/16.24.11848. [DOI] [PMC free article] [PubMed] [Google Scholar]


