Abstract
Background: Epidermal growth factor receptor (EGFR) is overexpressed in a significant proportion of esophageal and gastric carcinomas. Although previous studies have examined tyrosine kinase inhibitors of EGFR, there remains limited data regarding the role of EGFR-directed monoclonal antibody therapy in these malignancies. We carried out a multi-institutional phase II study of cetuximab, a monoclonal antibody against EGFR, in patients with unresectable or metastatic esophageal or gastric adenocarcinoma.
Patients and Methods: Thirty-five patients with previously treated metastatic esophageal or gastric adenocarcinoma were treated with weekly cetuximab, at an initial dose of 400 mg/m2 followed by weekly infusions at 250 mg/m2. Patients were followed for toxicity, treatment response, and survival.
Results: Treatment with cetuximab was well tolerated; no patients were taken off study due to drug-related adverse events. One (3%) partial treatment response was noted. Two (6%) patients had stable disease after 2 months of treatment. Median progression-free survival and overall survival were 1.6 and 3.1 months, respectively.
Conclusion: Although well tolerated, cetuximab administered as a single agent had minimal clinical activity in patients with metastatic esophageal and gastric adenocarcinoma. Ongoing studies of EGFR inhibitors in combination with other agents may define a role for these agents in the treatment of esophageal and gastric cancer.
Keywords: cetuximab, esophageal adenocarcinoma, gastric adenocarcinoma
introduction
Gastric and esophageal cancers are among the most common cancers and leading causes of cancer-related death worldwide [1]. Over the past three decades in the United States, there has been an increase in the incidence of adenocarcinoma of the lower third of the esophagus and a decline in the incidence of squamous cell carcinoma of the esophagus. Additionally, there has been a shift in the incidence of gastric adenocarcinoma from distal to more proximal regions of the stomach and gastroesophageal (GE) junction [2]. The natural history, response to therapy, and overall prognosis of distal esophageal and proximal gastric cancers appear to be similar [3]. Given these similarities, treatments for advanced esophageal and gastric cancers have converged, and many clinical trials for patients with advanced esophagogastric cancer have included patients with carcinoma of the esophagus, GE junction, and stomach [4, 5].
Response rates to single-agent chemotherapy for advanced esophageal and gastric cancers range from 10% to 20% [6]. There remains no globally accepted standard regimen for first-line treatment of advanced esophagogastric cancer. Combination regimens containing two or three agents are associated with higher response rates and longer survival. In a recent phase III study of patients with advanced esophageal or gastric cancer randomized to receive triplet therapy with epirubicin and cisplatin plus either fluorouracil or capecitabine or triplet therapy with epirubicin and oxaliplatin plus either fluorouracil or capecitabine, progression-free survival (PFS) and response rates did not differ significantly among the regimens.[4] Similarly, in a phase III study of patients with advanced gastric cancer, there was no significant difference in PFS among patients treated with cisplatin plus capecitabine or cisplatin plus fluorouracil [7]. Although doublet and triplet regimens are associated with improved outcome, they typically are associated with significant toxicity, and median survival remains poor. Furthermore, there is no established second-line therapy for patients with advanced esophagogastric cancer.
There remains a great need for novel therapeutic agents and treatment strategies for advanced esophagogastric cancer. The epidermal growth factor receptor (EGFR) is a member of the ErbB receptor tyrosine kinase family and plays an important role in cell cycle progression, angiogenesis, metastasis, and protection from apoptosis [8]. Preclinical and clinical studies have demonstrated increased EGFR expression in both esophageal and gastric carcinomas. Overexpression of EGFR has been associated with poor clinical outcomes in patients with esophageal and gastric adenocarcinomas [9–11]. Modest activity has been observed when the small-molecule tyrosine kinase inhibitors (TKIs) of EGFR, erlotinib and gefitinib, have been studied in patients with advanced esophageal and gastric cancers [12–15]. Additionally, inactivation of EGFR through use of a monoclonal antibody in preclinical models has resulted in inhibition of tumor growth [16, 17]. Several phase II trials have examined the activity of cetuximab, a monoclonal antibody to EGFR, in combination with cytotoxic chemotherapy in patients with advanced gastric or GE junction cancer [18–21]. However, there remains limited data regarding the role of cetuximab as a single agent in this disease. To evaluate whether inhibition of EGFR through use of a monoclonal antibody is safe and effective in patients with esophagogastric cancer, we initiated a multi-institutional phase II study of cetuximab in patients with advanced esophageal or gastric adenocarcinoma who had experienced treatment failure after one to two prior chemotherapy regimens.
patients and methods
patient population
The study population consisted of patients with histologically confirmed unresectable or metastatic esophageal or gastric adenocarcinoma who had experienced treatment failure with one to two prior chemotherapy regimens. Tumors with squamous cell differentiation, including those with a mixture of squamous and adenomatous differentiation, were excluded. Tumor expression of EGFR was not required. Participating centers included Beth Israel Deaconess Medical Center, Brigham and Women’s Hospital, Dana-Farber Cancer Institute, and Massachusetts General Hospital (all in Boston, MA).
Patients were further required to have measurable disease (by RECIST), Eastern Cooperative Oncology Group (ECOG) performance status of two or better, life expectancy of at least 12 weeks, adequate renal function (serum creatinine ≤1.5 mg/dl), adequate hepatic function (serum bilirubin ≤2.0 mg/dl; aspartate aminotransferase/alanine aminotransferase ≤2.5 times upper limit of normal (ULN), or ≤5 times ULN if there was evidence of liver metastases), and adequate bone marrow function (absolute neutrophil count ≥1000 μl; platelets ≥75 000 μl). If the marker lesion was previously irradiated, evidence of progression after radiation was required. Patients were excluded if they had another malignancy (other than nonmelanoma skin cancer or in situ cervical carcinoma), uncontrolled central nervous system metastases or carcinomatous meningitis, uncontrolled concomitant medical illnesses (e.g. uncontrolled hypertension, unstable angina, congestive heart failure, myocardial infarction <6 months before registration, serious uncontrolled cardiac arrhythmia), or any of the following within 2 weeks of enrollment: major or minor surgery, radiotherapy, or systemic anticancer treatment. Patients may not have received prior cetuximab or other therapy targeting the EGFR pathway and must not have experienced a prior severe infusion reaction to a monoclonal antibody. Patients who were pregnant or lactating were excluded from study entry. All patients provided signed informed consent as required by the institutional review boards of their respective institutions.
treatment program
Cetuximab was administered on an outpatient basis. Patients received cetuximab at an initial dose of 400 mg/m2 administered i.v. over 120 min, followed by weekly infusions at 250 mg/m2 administered i.v. over 60 min. Four weeks of therapy was considered to be one cycle of treatment. All patients were premedicated with diphenhydramine hydrochloride 50 mg (or an equivalent antihistamine) by i.v. given 30–60 min before the first dose of cetuximab. Premedication was administered before subsequent doses, but at the investigator’s discretion, the dose of diphenhydramine (or a similar agent) could be reduced. Toxicity was graded using the NCI Common Terminology Criteria for Adverse Events (CTCAE) version 3. Grade 3 or 4 hypersensitivity reactions required cetuximab discontinuation; for grade 1 or 2 hypersensitivity reactions, the infusion was slowed to one half of the initial rate. If a patient experienced grade 3 skin toxicity, the next dose of cetuximab was delayed for up to 2 consecutive weeks with no change in dose level. If the skin toxicity resolved to grade 2 or less within 2 weeks, treatment resumed. Dose reduction of cetuximab was required for a second or third occurrence of grade 3 skin toxicity. Other toxic effects warranting cetuximab dose reduction included grade 3 or 4 neutropenia, thrombocytopenia, neutropenic fever, diarrhea, nausea, or vomiting.
On-study evaluations included toxicity assessments and measurement of peripheral blood counts and a full chemistry panel every other week. Patients were evaluated with computed tomography every 8 weeks; response and progression were evaluated using RECIST by independent radiological review.
statistical methods
The primary objective of this study was to determine the response rate of cetuximab in patients with previously treated unresectable or metastatic gastric or esophageal adenocarcinoma. Secondary objectives included assessment of PFS and overall survival (OS), as well as characterization of toxic effects.
Responses were determined by RECIST criteria with an intention-to-treat analysis. PFS was defined as the time between study enrollment and progression of disease or death while on protocol. In the analysis of PFS, patients who withdrew from the study for reasons other than progression or death were censored at the time of discontinuation of study therapy. OS was defined as the time between study enrollment and death. Both PFS and OS were estimated by the Kaplan–Meier method.
Power calculations were based on a phase II two-stage design. The proposed regimen was to be considered worthy for further investigation if a true response rate was ≥15% and not worthy if it was ≤5%. A total of 35 eligible patients (defined as receiving at least one dose of therapy) were entered into the study in a two-stage design. Twenty patients were entered in the first stage; one response was required to enroll an additional 15 patients in the second stage of the study. The probability of concluding the regimen effective after accruing 35 patients was 78% if the true response rate was 15% and 9% if it was 5%.
results
patient characteristics
Between September 2005 and November 2008, 43 patients were screened for study entry. Six patients were ineligible for participation and two withdrew from the study before receiving therapy and were excluded from analysis. The remaining 35 patients received at least 1 week of study drug and are included in our toxicity and efficacy analyses. Baseline characteristics of this patient population are listed in Table 1. The median age of the patient population was 61 years, and 89% were male. As anticipated among patients with metastatic esophageal and gastric cancers, most patients were symptomatic from their disease. At study baseline, the majority of patients (83%) had an ECOG performance status of zero or one, whereas 17% had a performance status of two. All patients had metastatic disease at study entry. The majority of patients (86%) had visceral, bone, or peritoneal metastases; in five patients (14%), metastatic disease was limited to abdominal lymph nodes. Most patients (71%) had two or more sites of metastatic disease. Twenty-six patients (74%) had received two prior lines of therapy, and 9 patients (26%) had received only one prior therapy. Patients received a median of 6 weeks of treatment with cetuximab (range 1–23). Three patients received treatment for <4 weeks due to decline in performance status and suspected continued progression of disease.
Table 1.
Baseline patient characteristics (N = 35)
| Characteristic | n | % |
| Age (years) | ||
| Median | 61 | |
| Range | 42–81 | |
| Gender | ||
| Female | 4 | 11 |
| Male | 31 | 89 |
| ECOG performance status | ||
| 0 | 6 | 17 |
| 1 | 23 | 66 |
| 2 | 6 | 17 |
| Location of primary tumor | ||
| Esophageal | 12 | 34 |
| GE junction | 8 | 23 |
| Gastric | 15 | 43 |
| Location of metastasesa | ||
| Lymph nodes | 24 | 69 |
| Liver | 20 | 57 |
| Bone | 9 | 26 |
| Lung | 7 | 20 |
| Peritoneum | 8 | 23 |
| No. of prior chemotherapy regimens | ||
| 1 | 9 | 26 |
| 2 | 26 | 74 |
| Prior treatment | ||
| Cisplatin/irinotecan | 14 | 40 |
| 5-fluorouracil/leuocovorin/oxaliplatin | 6 | 17 |
| Docetaxel/cisplatin/irinotecan/bevacizumab | 6 | 17 |
| Docetaxel/cisplatin/irinotecan | 4 | 11 |
| Epirubicin/cisplatin/fluorouracil | 4 | 11 |
| Docetaxel/bevacizumab | 4 | 11 |
| Docetaxel | 3 | 9 |
| Otherb |
Does not sum to 100%, as patients may have metastases to more than one location.
Other prior chemotherapy regimens include the following: epirubicin–cisplatin–capecitabine (1), epirubicin–oxaliplatin–capecitabine (2), cisplatin–docetaxel (1), cisplatin–5-fluorouracil (1), capecitabine–oxaliplatin (2), cisplatin–irinotecan–capecitabine (2), irinotecan (1), 5-fluorouracil–leucovorin–irinotecan (1), capecitabine (1), capecitabine–docetaxel (1), carboplatin–paclitaxel (1), XL880 (1), cyclophosphamide–methotrexate–celecoxib (1).
ECOG, Eastern Cooperative Oncology Group; GE, gastroesophageal.
toxicity
Treatment-related adverse events are listed in Table 2. Overall, treatment with cetuximab was well tolerated. Across all grades of toxicity, the most commonly reported adverse events of all grades were acne-like rash (77%), fatigue (63%), anemia (49%), hypomagnesemia (40%), anorexia (40%), and nausea (40%). Grade 3 toxicity was uncommon, and no grade 4 adverse events were noted. Fatigue was the most common grade 3 treatment-related toxicity, observed in 6% of patients. No patients required dose reduction of cetuximab for treatment-related toxicity. One patient experienced a delay in treatment due to grade 3 acneiform skin rash but was able to continue treatment without dose modification.
Table 2.
Treatment-related toxicity
| Toxicity | Maximum toxicity grade |
|||||||
| 1 |
2 |
3 |
4 |
|||||
| n | % | n | % | n | % | n | % | |
| Hematologic | ||||||||
| Hemoglobin | 9 | 26 | 7 | 20 | 1 | 3 | – | |
| Leukocytes | 4 | 11 | – | – | – | |||
| Lymphocytes | 3 | 9 | 4 | 11 | 1 | 3 | – | |
| Platelets | 1 | 3 | – | – | – | |||
| Biochemical parameters | ||||||||
| Hypomagnesemia | 14 | 40 | – | – | – | |||
| Hyperglycemia | 5 | 14 | 3 | 9 | – | – | ||
| Aspartate aminotransferase | 4 | 11 | 1 | 3 | – | – | ||
| Alanine aminotransferase | 3 | 9 | 1 | 3 | ||||
| Alkaline phosphatase | 2 | 6 | 3 | 9 | – | – | ||
| Hyponatremia | 4 | 11 | – | – | – | |||
| Hypokalemia | 1 | 3 | – | – | – | |||
| Hypoalbuminemia | 2 | 6 | 2 | 6 | – | – | ||
| Bilirubin | – | 1 | 3 | – | – | |||
| Other toxic effects | ||||||||
| Abdominal pain | 4 | 11 | 2 | 6 | – | – | ||
| Chest pain | 1 | 3 | 1 | 3 | – | – | ||
| Hypersensitivity reaction | 1 | 3 | – | – | – | |||
| Alopecia | 4 | 11 | 1 | 3 | – | – | ||
| Anorexia | 6 | 17 | 8 | 23 | – | – | ||
| Constipation | 5 | 14 | 4 | 11 | – | – | ||
| Cough | – | 2 | 6 | – | ||||
| Dehydration | – | 4 | 11 | – | – | |||
| Diarrhea | 5 | 14 | 2 | 6 | – | – | ||
| Dry skin | 5 | 14 | 1 | 3 | – | – | ||
| Dyspnea | 2 | 6 | – | – | – | |||
| Fatigue | 7 | 20 | 13 | 37 | 2 | 6 | – | |
| Fever | 6 | 17 | – | – | – | |||
| Headache | 2 | 6 | 2 | 6 | 1 | 3 | – | |
| Mucositis | 4 | 11 | – | – | – | |||
| Nail changes | 2 | 6 | – | – | – | |||
| Nausea | 10 | 29 | 2 | 6 | 1 | 3 | ||
| Sensory neuropathy | 1 | 3 | 1 | 3 | – | – | ||
| Acneiform rash | 16 | 46 | 10 | 29 | 1 | 3 | ||
| Vomiting | 5 | 14 | 3 | 9 | – | – | ||
| Weight loss | 4 | 11 | – | – | – | |||
| Rigors/chills | 1 | 3 | 1 | 3 | – | – | ||
| Pruritis/itching | 1 | 3 | – | 1 | 3 | – | ||
treatment efficacy
The majority of patients (77%) discontinued treatment due to progressive disease documented by imaging studies carried out at or before the 2-month evaluation (Table 3).
Table 3.
Reason for treatment discontinuation
| Reason off study | Patients |
|
| n | %a | |
| Disease progression | 29 | 83 |
| ≤2 months of treatment | 27 | 77 |
| >2 months of treatment | 2 | 6 |
| Clinical deterioration | 4 | 11 |
| Death | 1 | 3 |
| Patient withdrew consent | 1 | 3 |
Figures do not sum to 100% due to rounding.
Four patients (11%) discontinued therapy due to clinical deterioration or clinical progression of disease. One patient (3%) died due to complications from his disease while receiving treatment and one patient (3%) withdrew consent for treatment on protocol.
Treatment response was assessable in 30 of the 35 total enrolled patients (Table 4). One patient (3%) experienced a confirmed partial response by RECIST criteria. This response occurred in a patient with a primary GE junction tumor receiving third-line treatment. The patient subsequently withdrew consent for participation after 4 months of treatment due to logistical concerns related to travel required for treatment. Two patients (6%) had stable disease at the 2-month follow-up imaging. Of these two patients with stable disease, one with a primary GE junction tumor had received two prior chemotherapy regimens and experienced stable disease for 4 months, and the second patient with a primary gastric tumor had received one prior regimen and experienced stable disease for 6 months.
Table 4.
Tumor response among patients receiving cetuximab
| Disease response | n | % |
| Complete or partial response | 1 | 3 |
| Stable disease, months | 2 | 6 |
| >2 | 1 | 3 |
| >4 | 1 | 3 |
| Progressive disease | 27 | 77 |
| Not assessablea | 5 | 14 |
Four patients experienced clinical deterioration before the first restaging computed tomography (CT) scan. One patient died due to complications related to his disease before the first restaging CT scan.
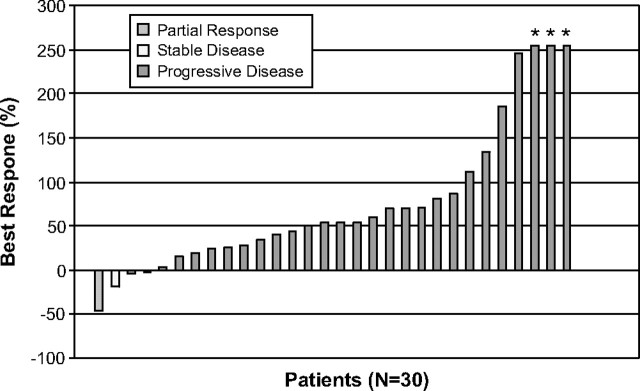
Best overall percentage change in target lesion measurement from baseline was available for 30 patients (Figure 1). Two patients (6%) had evidence of material tumor regression (46% and 19% reduction from baseline, respectively). Among all 35 patients, the median PFS time was 1.6 months. To date, 32 patients (91%) have died, and median OS time for the entire study population was 3.1 months.
Figure 1.
Best overall percentage change from baseline target lesion measurement by RECIST guidelines.
Note: Three patients had progressive disease as a result of the development of new lesions, rather than growth of the target lesions by 20%. Three additional patients (noted by *) also had progressive disease as a result of the development of new lesions; growth of previously noted lesions in these patients was difficult to quantify.
discussion
Identifying new, more effective agents for advanced gastric and esophageal cancers remains a critical challenge. We therefore evaluated the safety and efficacy of single-agent cetuximab among patients with previously treated gastric and esophageal adenocarcinomas. Among the 35 patients in the current study, cetuximab proved to be well tolerated. As anticipated, 77% of patients experienced acneiform skin rash, although few grade 3 or higher toxic effects were reported. The incidence of grade 3 or higher skin rash that we observed (3%) was lower compared with what has been observed in phase II studies of cetuximab in combination with chemotherapy in patients with advanced gastric cancer (17%–24%) [18–21]. However, the incidence of skin rash of any grade that we observed was similar. This may possibly reflect synergy and increased skin toxicity with cetuximab in combination with chemotherapy. Alternatively, precise grading of the severity of skin rash can be somewhat subjective such that distinction between grades 2 and 3 may be blurred. We do not have evidence to suggest that dosing influenced the incidence of grade 3 skin rash that we observed. In studies using single-agent cetuximab for metastatic colorectal cancer at the same doses used in our study, grade 3 skin rash was observed in 5.2% and 11.8% of patients [22, 23].
Although we did not formally assess pharmacodynamic end points, the presence of rash in the majority of patients suggests a biological effect of the drug on its intended target. Nonetheless, cetuximab as a single agent failed to demonstrate meaningful clinical activity in this patient population, with only one (3%) objective treatment response. Among all patients, median PFS and OS were 1.6 and 3.1 months, respectively. Recent clinical trials examining various cytotoxic chemotherapy regimens in previously treated patients have demonstrated response rates in the range of 10%–20%, with a PFS of 2–4 months [24, 25].
Our results are consistent with a report in abstract form of a phase II study of cetuximab in patients with metastatic esophageal adenocarcinoma conducted by the Southwest Oncology Group [26]. In their preliminary analysis of 55 assessable patients treated with cetuximab as second-line therapy, there was one confirmed partial response (2%). Median PFS was 1.8 months; the trial did not meet its primary objective of 40% PFS at 6 months.
The EGFR signal transduction pathway plays an important role in cell cycle progression, angiogenesis, metastasis, and resistance against apoptosis [8, 27]. EGFR is overexpressed in a significant proportion of esophageal and gastric cancers and is associated with poor clinical outcomes [9–11]. In a preclinical model, inactivation of EGFR with the monoclonal antibody MoAb 528 inhibited the growth of cultured human gastric cancer [17]. In another study, treatment of a human gastric carcinoma cell line with cetuximab inhibited formation of EGFR–EGFR homodimers and EGFR–human epidermal growth factor receptor 2 heterodimers and also inhibited downstream signaling molecules [28]. The growth of tumors derived from this cell line in athymic mice was also significantly inhibited by cetuximab compared with control groups. In a phase I trial with ABX-EGF, a high-affinity, fully human monoclonal antibody against EGFR, one of three patients with esophageal cancer had stable disease for 7 months [29]. These preclinical and early clinical studies suggest potential activity and provide rationale to examine inhibitors of EGFR in patients with esophageal and gastric cancers.
Therapeutic interventions to inhibit EGFR principally have involved either monoclonal antibodies, including cetuximab, that bind to the extracellular domain of the receptor, thereby preventing its activation, or low-molecular weight TKIs, such as gefitinib and erlotinib, that inhibit ATP binding within the tyrosine kinase domain of the receptor, thereby inhibiting EGFR autophosphorylation and signal transduction. Although there have been limited data on the role of EGFR monoclonal antibodies in advanced GE cancers, several studies have examined EGFR-directed TKIs in this patient population (Table 5). In a phase II trial of erlotinib in previously untreated GE junction and gastric adenocarcinomas, a response rate of 12% was observed [12]. All responses occurred in patients with GE junction tumors, suggesting possible regional differences in sensitivity to EGFR blockade. In phase II trials assessing single-agent gefitinib, response rates of 11% and 3% were reported in patients with advanced esophageal cancer and 1% in patients with metastatic gastric adenocarcinoma [13, 14, 31].
Table 5.
Phase II studies of single-agent EGFR TKIs for advanced esophagogastric cancer
| Agent | n | Tumor site | Tumor pathology | No. of prior chemotherapy regimens | Response rate (%) | Stable disease (%) | PFS (months) | OS (months) | |
| Tew et al. [30] | Erlotinib | 20 | Esophageal | Adenocarcinoma, squamous cell carcinoma | 1 | 15 | 40 | NR | NR |
| Dragovich et al. [12] | Erlotinib | 25 | Gastric | Adenocarcinoma | 0 | 0 | 4 | 1.6 | 3.5 |
| 43 | GEJ | Adenocarcinoma | 0 | 12 | 12 | 2 | 6.7 | ||
| Doi et al. [31] | Gefitinib | 75 | Gastric | Adenocarcinoma | 1–2 | 1 | 16 | NR | NR |
| Ferry et al. [13] | Gefitinib | 27 | Esophageal | Adenocarcinoma | 0–1 | 11 | 26 | 1.9 | 4.5 |
| Janmaat et al. [14] | Gefitinib | 36 | Esophageal | Adenocarcinoma, squamous cell carcinoma | 1–2 | 3 | 28 | 59 days | 164 days |
EGFR, epidermal growth factor receptor; GEJ, gastroesophageal junction; NR, not reported; PFS, progression-free survival; OS, overall survival; TKI, tyrosine kinase inhibitor.
Several phase II trials have examined the activity of cetuximab in combination with cytotoxic chemotherapy including 5-fluorouracil (FU), leucovorin, irinotecan (FOLFIRI), 5-FU, leucovorin, oxaliplatin (FUFOX, FOLFOX), and docetaxel plus cisplatin in untreated patients with advanced gastric or GE junction cancer [18–21]. Objective response rates have varied from 40% to 65%. Compared with the historical response of the various chemotherapy regimens alone, the addition of cetuximab appears to increase response rates.
Conflicting data exist regarding the correlation between EGFR expression and response to EGFR-directed therapy in patients with esophageal and gastric cancers. In two studies of patients with gastric cancer treated with cetuximab-based chemotherapy, no association was found between EGFR expression and treatment response [19, 21]. However, a third reported that EGFR expression was an independent predictor of longer time to progression and that outcome was improved for patients with EGFR expression and low serum ligand levels of EGF and transforming growth factor-α [18]. Among patients treated with TKIs of EGFR, no correlation between EGFR expression and outcome was observed in patients with GE junction and gastric adenocarcinoma treated with erlotinib or in patients with esophageal adenocarcinoma treated with gefitinib [12, 13]. In contrast, in another study, disease control rate was significantly higher for patients with high-EGFR-expressing esophageal cancers treated with gefitinib [14].
Somatic mutations involving the EGFR tyrosine kinase domain have been associated with tumor response to gefitinib in patients with non-small-cell lung cancer, and increased EGFR gene copy number has also been identified as a possible predictor for TKI sensitivity in non-small-cell lung cancer [32, 33]. However, the specific EGFR mutations described in lung cancer are uncommon in esophagogastric cancer. In a phase II study of patients with gastric and GE junction adenocarcinomas treated with erlotinib, no somatic mutations involving exons 18, 19, or 21 were detected [12]. Similarly, EGFR mutations were not identified in a cohort of patients with esophageal cancer receiving gefitinib [14]. Although it is possible that esophageal and gastric cancers contain mutations in unexamined regions of the EGFR gene, mutations associated with response to TKIs of EGFR in lung cancer appear absent in esophageal and gastric cancers.
In patients with metastatic colorectal cancer, the presence of mutations in K-ras has been associated with resistance to anti-EGFR therapy. K-ras mutations occur in ∼40% of patients with colorectal cancer but are rare in patients with esophagogastric cancer and therefore unlikely to explain resistance to cetuximab observed in this study [14, 18, 34]. The low response rate to cetuximab (3%) in this study regrettably rendered efforts to pursue correlative studies characterizing the molecular differences between responders and nonresponders largely untenable.
Several limitations of our study deserve comment. First, our study included patients who had received one to two lines of prior chemotherapy. As expected, we observed a high percentage of patients with advanced disease and who had received more than one prior treatment regimen. It is possible that better disease control would have been achieved with cetuximab in a population of chemotherapy-naive patients. However, among patients treated with TKIs of EGFR, similar objective response rates were observed regardless of whether patients received treatment as first-line therapy or a subsequent line of therapy (Table 5). Moreover, in studies of patients with advanced colorectal cancer, a clear benefit for EGFR-directed monoclonal antibody therapy is apparent in heavily pretreated patient populations [23, 35]. Finally, although we included patients with one to two prior regimens, we excluded patients with an impaired performance status or those with impaired biochemical or hematologic function. Second, consistent with other studies of advanced esophagogastric cancer, our study included patients with esophageal, gastric, and GE junction adenocarcinomas [4, 5]. Although there may be heterogeneity in biology according to primary site of disease, the low response rates across all sites of tumor origin in our study suggest that a substantial benefit for single-agent cetuximab for any particular site of origin is unlikely.
In conclusion, cetuximab administered as a single agent has minimal clinical activity in patients with unresectable or metastatic esophageal or gastric adenocarcinoma. Trials examining cetuximab in combination with cytotoxic chemotherapy are ongoing and may provide further information about the role of anti-EGFR therapy in the treatment of patients with advanced esophageal and gastric adenocarcinomas.
funding
Bristol-Myers Squibb provided cetuximab and clinical trial funding support.
disclosure
TAA, LSB, and PCE have received honoraria from Imclone and Bristol Myers Squibb. CSF has served in a consultant or an advisory role for Bristol Myers Squibb and Imclone.
References
- 1.Kamangar F, Dores GM, Anderson WF. Patterns of cancer incidence, mortality, and prevalence across five continents: defining priorities to reduce cancer disparities in different geographic regions of the world. J Clin Oncol. 2006;24:2137–2150. doi: 10.1200/JCO.2005.05.2308. [DOI] [PubMed] [Google Scholar]
- 2.Blot WJ, Devesa SS, Kneller RW, Fraumeni JF., Jr Rising incidence of adenocarcinoma of the esophagus and gastric cardia. JAMA. 1991;265:1287–1289. [PubMed] [Google Scholar]
- 3.Wijnhoven BP, Siersema PD, Hop WC, et al. Adenocarcinomas of the distal oesophagus and gastric cardia are one clinical entity. Rotterdam Oesophageal Tumour Study Group. Br J Surg. 1999;86:529–535. doi: 10.1046/j.1365-2168.1999.01082.x. [DOI] [PubMed] [Google Scholar]
- 4.Cunningham D, Starling N, Rao S, et al. Capecitabine and oxaliplatin for advanced esophagogastric cancer. N Engl J Med. 2008;358:36–46. doi: 10.1056/NEJMoa073149. [DOI] [PubMed] [Google Scholar]
- 5.Webb A, Cunningham D, Scarffe JH, et al. Randomized trial comparing epirubicin, cisplatin, and fluorouracil versus fluorouracil, doxorubicin, and methotrexate in advanced esophagogastric cancer. J Clin Oncol. 1997;15:261–267. doi: 10.1200/JCO.1997.15.1.261. [DOI] [PubMed] [Google Scholar]
- 6.Shah MA, Schwartz GK. Treatment of metastatic esophagus and gastric cancer. Semin Oncol. 2004;31:574–587. doi: 10.1053/j.seminoncol.2004.04.013. [DOI] [PubMed] [Google Scholar]
- 7.Kang YK, Kang WK, Shin DB, et al. Capecitabine/cisplatin versus 5-fluorouracil/cisplatin as first-line therapy in patients with advanced gastric cancer: a randomised phase III noninferiority trial. Ann Oncol. 2009;20:666–673. doi: 10.1093/annonc/mdn717. [DOI] [PubMed] [Google Scholar]
- 8.El-Rayes BF, LoRusso PM. Targeting the epidermal growth factor receptor. Br J Cancer. 2004;91:418–424. doi: 10.1038/sj.bjc.6601921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Takehana T, Kunitomo K, Suzuki S, et al. Expression of epidermal growth factor receptor in gastric carcinomas. Clin Gastroenterol Hepatol. 2003;1:438–445. doi: 10.1016/s1542-3565(03)00219-2. [DOI] [PubMed] [Google Scholar]
- 10.Tokunaga A, Onda M, Okuda T, et al. Clinical significance of epidermal growth factor (EGF), EGF receptor, and c-erbB-2 in human gastric cancer. Cancer. 1995;75:1418–1425. doi: 10.1002/1097-0142(19950315)75:6+<1418::aid-cncr2820751505>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 11.Yacoub L, Goldman H, Odze RD. Transforming growth factor-alpha, epidermal growth factor receptor, and MiB-1 expression in Barrett’s-associated neoplasia: correlation with prognosis. Mod Pathol. 1997;10:105–112. [PubMed] [Google Scholar]
- 12.Dragovich T, McCoy S, Fenoglio-Preiser CM, et al. Phase II trial of erlotinib in gastroesophageal junction and gastric adenocarcinomas: SWOG 0127. J Clin Oncol. 2006;24:4922–4927. doi: 10.1200/JCO.2006.07.1316. [DOI] [PubMed] [Google Scholar]
- 13.Ferry DR, Anderson M, Beddard K, et al. A phase II study of gefitinib monotherapy in advanced esophageal adenocarcinoma: evidence of gene expression, cellular, and clinical response. Clin Cancer Res. 2007;13:5869–5875. doi: 10.1158/1078-0432.CCR-06-1970. [DOI] [PubMed] [Google Scholar]
- 14.Janmaat ML, Gallegos-Ruiz MI, Rodriguez JA, et al. Predictive factors for outcome in a phase II study of gefitinib in second-line treatment of advanced esophageal cancer patients. J Clin Oncol. 2006;24:1612–1619. doi: 10.1200/JCO.2005.03.4900. [DOI] [PubMed] [Google Scholar]
- 15.Rojo F, Tabernero J, Albanell J, et al. Pharmacodynamic studies of gefitinib in tumor biopsy specimens from patients with advanced gastric carcinoma. J Clin Oncol. 2006;24:4309–4316. doi: 10.1200/JCO.2005.04.2424. [DOI] [PubMed] [Google Scholar]
- 16.Jung YD, Mansfield PF, Akagi M, et al. Effects of combination anti-vascular endothelial growth factor receptor and anti-epidermal growth factor receptor therapies on the growth of gastric cancer in a nude mouse model. Eur J Cancer. 2002;38:1133–1140. doi: 10.1016/s0959-8049(02)00013-8. [DOI] [PubMed] [Google Scholar]
- 17.Teramoto T, Onda M, Tokunaga A, Asano G. Inhibitory effect of anti-epidermal growth factor receptor antibody on a human gastric cancer. Cancer. 1996;77:1639–1645. doi: 10.1002/(SICI)1097-0142(19960415)77:8<1639::AID-CNCR33>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 18.Han SW, Oh DY, Im SA, et al. Phase II study and biomarker analysis of cetuximab combined with modified FOLFOX6 in advanced gastric cancer. Br J Cancer. 2009;100:298–304. doi: 10.1038/sj.bjc.6604861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lordick F, Luber B, Lorenzen S, et al. Cetuximab plus oxaliplatin/leucovorin/5-fluorouracil in first-line metastatic gastric cancer: a phase II study of the Arbeitsgemeinschaft Internistische Onkologie (AIO) Br J Cancer. 2010;102:500–505. doi: 10.1038/sj.bjc.6605521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pinto C, Di Fabio F, Barone C, et al. Phase II study of cetuximab in combination with cisplatin and docetaxel in patients with untreated advanced gastric or gastro-oesophageal junction adenocarcinoma (DOCETUX study) Br J Cancer. 2009;101:1261–1268. doi: 10.1038/sj.bjc.6605319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pinto C, Di Fabio F, Siena S, et al. Phase II study of cetuximab in combination with FOLFIRI in patients with untreated advanced gastric or gastroesophageal junction adenocarcinoma (FOLCETUX study) Ann Oncol. 2007;18:510–517. doi: 10.1093/annonc/mdl459. [DOI] [PubMed] [Google Scholar]
- 22.Cunningham D, Humblet Y, Siena S, et al. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med. 2004;351:337–345. doi: 10.1056/NEJMoa033025. [DOI] [PubMed] [Google Scholar]
- 23.Jonker DJ, O’Callaghan CJ, Karapetis CS, et al. Cetuximab for the treatment of colorectal cancer. N Engl J Med. 2007;357:2040–2048. doi: 10.1056/NEJMoa071834. [DOI] [PubMed] [Google Scholar]
- 24.Lo SS, Khorana AA, Javle M, et al. A phase II study of weekly docetaxel in combination with capecitabine in advanced gastric and gastroesophageal adenocarcinomas. Oncology. 2010;78:125–129. doi: 10.1159/000312654. [DOI] [PubMed] [Google Scholar]
- 25.Sun Q, Liu C, Zhong H, et al. Multi-center phase II trial of weekly paclitaxel plus cisplatin combination chemotherapy in patients with advanced gastric and gastro-esophageal cancer. Jpn J Clin Oncol. 2009;39:237–243. doi: 10.1093/jjco/hyp008. [DOI] [PubMed] [Google Scholar]
- 26.Gold PJ, Goldman B, Iqbal S, et al. Cetuximab as second-line therapy in patients with metastatic esophageal cancer: a phase II Southwest Oncology Group Study. J Clin Oncol ASCO Annu Meet Proc. 2008:26. doi: 10.1097/JTO.0b013e3181e77a92. (Abstract 4536) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Karamouzis MV, Grandis JR, Argiris A. Therapies directed against epidermal growth factor receptor in aerodigestive carcinomas. JAMA. 2007;298:70–82. doi: 10.1001/jama.298.1.70. [DOI] [PubMed] [Google Scholar]
- 28.Patel D, Bassi R, Hooper A, et al. Anti-epidermal growth factor receptor monoclonal antibody cetuximab inhibits EGFR/HER-2 heterodimerization and activation. Int J Oncol. 2009;34:25–32. [PubMed] [Google Scholar]
- 29.Figlin R, Belldegrun A, Crawford J, et al. ABX-EGF, a fully human anti-epidermal growth factor receptor (EGFR) monoclonal antibody (mAb) in patients with advanced cancer: phase 1 clinical results. Proc Am Soc Clin Oncol. 2002:21. (Abstract 35) [Google Scholar]
- 30.Tew W, Shah M, Schwartz G, et al. Phase II trial of erlotinib for second-line treatment in advanced esophageal cancer. Proc Am Soc Clin Oncol Gastrointest Cancer Symp. 2005:23. (Abstract 5) [Google Scholar]
- 31.Doi T, Koizumi W, Siena S, et al. Efficacy, tolerability and pharmacokinetics of gefitinib (ZD1839) in pretreated patients with metastatic gastric cancer. Proc Am Soc Clin Oncol. 2003:22. (Abstract 1036) [Google Scholar]
- 32.Lynch TJ, Bell DW, Sordella R, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350:2129–2139. doi: 10.1056/NEJMoa040938. [DOI] [PubMed] [Google Scholar]
- 33.Paez JG, Janne PA, Lee JC, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004;304:1497–1500. doi: 10.1126/science.1099314. [DOI] [PubMed] [Google Scholar]
- 34.Sasao S, Hiyama T, Tanaka S, et al. Clinicopathologic and genetic characteristics of gastric cancer in young male and female patients. Oncol Rep. 2006;16:11–15. [PubMed] [Google Scholar]
- 35.Van Cutsem E, Peeters M, Siena S, et al. Open-label phase III trial of panitumumab plus best supportive care compared with best supportive care alone in patients with chemotherapy-refractory metastatic colorectal cancer. J Clin Oncol. 2007;25:1658–1664. doi: 10.1200/JCO.2006.08.1620. [DOI] [PubMed] [Google Scholar]