Abstract
Background:
Approximately 2000 American men are diagnosed with breast cancer every year. Limited data are available evaluating toxicity of antihormonal treatments in male breast cancer patients.
Patients and methods:
We reviewed male breast cancer patients evaluated at our institution (1999–2009). Of 126 patients, 64 met the following inclusion criteria: stage I–III, treated with tamoxifen, at least one follow-up visit after starting tamoxifen. A descriptive analysis of toxic effects was carried out on these 64 patients.
Results:
Median follow-up from start of tamoxifen therapy was 3.9 years (range 0.3–19.4 years). Median age at diagnosis was 61 years (range 30–79 years). Breakdown by stage: 29.7% (n = 19) stage I, 54.7% (n = 35) stage II, and 15.6% (n = 10) stage III. Thirty-four (53%) patients experienced one or more toxicity while taking tamoxifen. Most common toxic effects are weight gain (14 patients, 22%) and sexual dysfunction (14 patients, 22%). Thirteen (20.3%) patients discontinued tamoxifen due to toxicity: one ocular, one leg cramps, two neurocognitive deficits, two bone pain, three sexual dysfunction, and four thromboembolic events.
Conclusions:
To our knowledge, this is the largest study examining tamoxifen-related toxic effects among male breast cancer patients. Among male patients, there is a high rate of discontinuation of tamoxifen. Prospective studies of antihormonal agents in male breast cancer are warranted.
Keywords: male breast cancer, tamoxifen, toxic effects
introduction
Male breast cancer is a rare malignant disease, making up <1% of all breast cancer diagnoses worldwide [1]. In the United States, it was estimated that ∼1970 new cases of male breast cancer were diagnosed in 2010 and 390 deaths occurred [2]. The median age of onset for male breast cancer in a recent large Veterans Affairs study was 67, which is ∼5 to 10 years older than female breast cancer patients [3]. (The median age at diagnosis of breast cancer is 64 years for women.) Although a rare disease, the incidence of male breast cancer appears to be rising [4].
Previous studies have shown that breast cancer in men is more often hormone receptor-positive than in female breast cancer patients, suggesting that the majority of patients with male breast cancer will be sensitive to antihormonal therapies such as tamoxifen [5]. However, because of the rarity of this disease, large prospective randomized trials have been difficult to perform, and therefore the optimal treatment strategies have not yet been defined [5]. In addition, few studies have evaluated side-effects of hormonal agents or rates of discontinuation in male breast cancer patients.
One of the largest retrospective studies specifically examining tamoxifen-related side-effects among male breast cancer patients treated with tamoxifen in the adjuvant setting revealed a 20.8% discontinuation rate in 24 patients [6]. Another study of male breast cancer patients treated with antihormonal therapy (either tamoxifen or aromatase inhibitors), treated in either the adjuvant or metastatic settings, demonstrated a 23.7% discontinuation rate in 59 male patients [7]. By comparison, in the literature, it appears that the corresponding discontinuation rate related to tamoxifen and antihormonal breast cancer treatments among female breast cancer patients shows a wide range from 30% to 50% [8]. In one study of female breast cancer patients undergoing treatment in the adjuvant setting, after 1 year of commencing treatment, the cumulative tamoxifen nonpersistence rate was 22.1%, and by the end of follow-up at 3.5 years, the cumulative nonpersistence rate had increased to 35.2% [9].
Clinical decision making in male breast cancer patients has historically been made on the basis of extrapolation from female breast cancer studies in the absence of direct evidence. Little is known with regard to selection of treatment regimens in this unique population, or the attendant side-effects of these treatments. In this study, we aimed to characterize, in a large population of male breast cancer patients, the specific effects of the most commonly utilized antihormonal therapy, tamoxifen, and to ascertain the reasons for discontinuation from tamoxifen-based treatment in order to better understand this specific population's experience with tamoxifen.
methods
After obtaining approval from our Institutional Review Board, we carried out a retrospective review of all male breast cancer patients evaluated at our institution from 1999 to 2009. Patients were identified through a list from the tumor registry. Charts were reviewed through our institution's electronic medical record. All pathology slides, including those slides originally reviewed at an outside institution, were reviewed by an MD Anderson breast pathologist. A total of 126 male breast cancer patients were evaluated during this time period. Patients were deemed eligible for inclusion in this analysis if they met each of the following criteria: male, aged ≥18 years, diagnosis of breast cancer of any histological subtype, stages I–III at diagnosis, treated with tamoxifen, and at least one documented follow-up visit after the initiation of tamoxifen with history and physical at our institution. Sixty-four patients met the inclusion criteria for this study. Descriptive statistics were used for patient characteristics, side-effects, and discontinuation of tamoxifen. A Kaplan–Meier curve was used to estimate time to discontinuation of tamoxifen. At our institution, the definition of human epidermal growth factor receptor 2 (HER-2) positivity was defined as HER-2 positive to be Immunohistochemistry 3+ or FISH positive, or a FISH ratio of >2.2.
results
A total of 126 male breast cancer patients were evaluated at MD Anderson from 1999 to 2009, with 64 of these patients meeting the inclusion criteria for this analysis.
Median follow-up time at our institution was 4.2 years (range 0.3–10.9 years). Median follow-up from the start of tamoxifen therapy was 3.9 years (range 0.3–19.4 years). The median age at diagnosis was 61 years (range 30–79 years). Breakdown by breast cancer stage included 29.7% (n = 19) stage I, 54.7% (n = 35) stage II, and 15.6% (n = 10) stage III. Median initial tumor size was 2.0 cm (range 0.5–6.5 cm). Examination of hormone receptor status revealed 97% (n = 62) of the patients had hormone receptor-positive tumors (one patient with unknown tumor status due to insufficient material for analysis) and seven tumors (11%) were HER-2/neu positive. Three patients had history of prior therapeutic radiation, and 11 patients (17%) had a prior or concomitant cancer diagnosis, including 6 patients with history of prostate cancer and 2 patients with history of renal cell carcinoma.
With regards to BRCA mutations, two patients in the cohort were definitively identified with BRCA2 mutations. There were no patients identified in the cohort with Klinefelter’s syndrome. Sixteen (25%) patients developed metastatic disease or recurrence during the study time period.
Thirty-four of the 64 (53%) patients experienced at least one or more side-effect while taking tamoxifen. There were a total of 62 documented side-effects observed during the study period (more than one side-effect in some patients). All side-effects were self-reported by patients during follow-up clinic visits and documented in clinic records. The most common toxicity was weight gain (14 out of 64 patients, 22%) and sexual dysfunction/loss of libido (14 patients, 22%), followed by hot flashes (8 patients, 13%), neurocognitive deficits (6 patients, 9%), thromboembolic events (6 patients, 9%), ocular events (3%), mood alterations (2%), depression (2%), gastrointestinal (GI) disturbance (2%), bone pain (2%), leg cramps (2%), and insomnia (1%). Please see Table 1 for tamoxifen-related toxic effects.
Table 1.
Tamoxifen-related toxic effects
| Side-effect | No. of patients experiencing side-effect | % |
| Weight gain | 14 | 22 |
| Sexual dysfunction/loss of libido | 14 | 22 |
| Hot flashes | 8 | 13 |
| Neurocognitive deficits | 6 | 9 |
| Thromboembolic events | 6 | 9 |
| Ocular events | 3 | 5 |
| Mood alterations | 2 | 3 |
| Depression | 2 | 3 |
| GI disturbance | 2 | 3 |
| Bone pain | 2 | 3 |
| Leg cramps | 2 | 3 |
| Insomnia | 1 | 2 |
GI, gastrointestinal.
Of the six neurocognitive toxic effects documented, two of these led to treatment discontinuation (both were secondary to memory impairment) and the other four events not leading to treatment discontinuation were two patients with memory impairment, one with decreased concentration, and one with thinking impairment. Of the three ocular toxic effects documented, one of these led to treatment discontinuation (development of rapid decrease in visual acuity) and two of the events did not lead to treatment discontinuation (one patient with development of cataracts and one patient with development of eye strain/blurriness of vision).
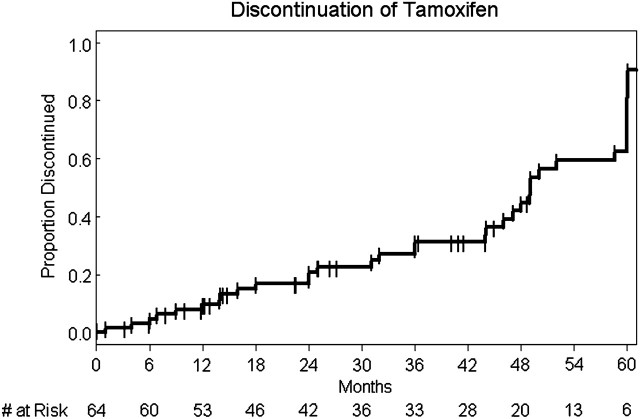
Importantly, 13 out of the 64 (20%) patients discontinued tamoxifen due to toxicity: one for ocular side-effects, two for neurocognitive deficits, four for thromboembolic events, two for bone pain, three for loss of libido, and one for leg cramps. Four out of 13 discontinuation decisions (31%) were physician-directed (4 thromboembolic events directly felt to be related to tamoxifen) and 9 of 13 discontinuation decisions (69%) were patient-directed preferences based on intolerable side-effect profile. Please see Table 2 for reasons for tamoxifen-related drug discontinuation. Figure 1 demonstrates the time to tamoxifen discontinuation from start of tamoxifen therapy. The median time to discontinuation was 49 months (Q1 = 31 months, Q3 = 60 months).
Table 2.
Reasons for tamoxifen-related discontinuation
| Dose-limiting toxicity | No. of patients (n = 13) | % |
| Thromboembolic events | 4 | 31 |
| Loss of libido | 3 | 23 |
| Bone pain | 2 | 15 |
| Neurocognitive deficits | 2 | 15 |
| Leg cramps | 1 | 8 |
| Ocular event | 1 | 8 |
Figure 1.
Time to tamoxifen discontinuation.
Overall, nine patients died. One patient died 2 days after stopping tamoxifen, and one patient died 164 days after stopping tamoxifen. All other patients died at least 379 days after stopping tamoxifen. Of the nine patients who died, the median time from stopping tamoxifen to death was 795 days (range 2–5613 days). Eight of the nine deaths were breast cancer related.
discussion
There are little data in the published literature regarding tamoxifen-related side-effects in male breast cancer patients. Because poor adherence to tamoxifen has been associated with poorer outcomes, such as event-free time, in previous studies [10], we aimed to delineate the reasons why patients were discontinuing treatment in order to better understand tamoxifen discontinuation. Before the present study, the largest study examining this issue was published by Anelli et al. [6]. In the Anelli study, a retrospective analysis was carried out on 24 male breast cancer patients at the Memorial Sloan Kettering Cancer Center from 1990 to 1993 treated with tamoxifen in the adjuvant setting. These authors reported that 15 of the 24 patients (62.5%) experienced at least one tamoxifen-related side-effect, with the most common side-effect of loss of libido, which occurred in 7 (29.2%) of the patients. This was followed in decreasing incidence by weight gain (six patients, 25%), hot flashes (five patients, 20.8%), mood alterations (five patients, 20.8%), depression (four patients, 16.6%), insomnia (three patients, 12.5%), and deep vein thrombosis (one patient, 4.2%). This study demonstrated a 20.8% discontinuation rate among male breast cancer patients associated with tamoxifen-related side-effects [6].
Our study, which examined approximately three times the number of patients from the Anelli study, confirms the previously published rate, with 13 out of the 64 male breast cancer (20.3%) patients discontinuing tamoxifen in the setting of tamoxifen-related side-effects. The most common toxicity in our study was weight gain (14 out of 64 patients, 22%) and sexual dysfunction/loss of libido (14 patients, 22%). This was followed by hot flashes (eight patients, 13%), neurocognitive deficits (six patients, 9%), thromboembolic events (six patients, 9%), ocular events (three patients, 5%), mood alterations (two patients, 3%), depression (two patients, 3%), GI disturbance (two patients, 3%), leg cramps (two patients, 3%), and insomnia (one patient, 2%).
The exact mechanisms of action leading to this high rate of discontinuation among male breast cancer patients among anti-hormonal treatments such as tamoxifen are incompletely understood. With regards to sexual dysfunction among male patients, one recent study hypothesized that male sexual dysfunction with tamoxifen (ranging from impotence to loss of libido) may be in part due to decreased testosterone levels, with this and other hypotheses needing testing and validation in prospective trials [7]. Elevated risk of thromboembolic events, especially venous thromboembolism, have indeed been observed with other antihormonal treatments in male cancer. An example of this phenomenon is in the prostate cancer literature with another selective estrogen receptor modulator toremifene. In a large phase III trial of prostate cancer patients receiving antiandrogen therapy, toremifene demonstrated an increased rate of venous thromboembolism versus placebo group (17 versus 7 patients). According to the authors of the study, the relative risk of venous thromboembolism in patients receiving toremifene was 2.4, which was similar to the relative rate of venous thromboembolism in postmenopausal women taking raloxifene (∼3.0) [11].
To our knowledge, this is the largest retrospective study specifically examining tamoxifen-related side-effects among male breast cancer patients. Among male breast cancer patients, there is a high rate of discontinuation of tamoxifen for various reasons. Because almost all male breast cancer patients have hormone receptor-positive tumors, most of these patients will receive antihormonal treatment at some point in their treatment course. Having accurate information on side-effects is crucial when discussing risks and benefits of a particular therapy. Further prospective studies to develop a better understanding of antihormonal agents in male breast cancer are warranted.
funding
This work was supported in part by the Cancer Center Support Grant (NCI Grant P30 CA016672).
disclosure
The authors declare no conflicts of interest.
References
- 1.Korde LA, Zujewski JA, Kamin L, et al. Multidisciplinary meeting on male breast cancer: summary and research recommendations. J Clin Oncol. 2010;28:2114–2122. doi: 10.1200/JCO.2009.25.5729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 3.Nahleh ZA, Srikantiah R, Safa M, et al. Male breast cancer in the veterans affairs population: a comparative analysis. Cancer. 2007;109:1471–1477. doi: 10.1002/cncr.22589. [DOI] [PubMed] [Google Scholar]
- 4.Giordano SH, Cohen DS, Buzdar AU, et al. Breast carcinoma in men: a population-based study. Cancer. 2004;101:51–57. doi: 10.1002/cncr.20312. [DOI] [PubMed] [Google Scholar]
- 5.Giordano SH, Buzdar AU, Hortobagyi GN. Breast cancer in men. Ann Intern Med. 2002;137:678–687. doi: 10.7326/0003-4819-137-8-200210150-00013. [DOI] [PubMed] [Google Scholar]
- 6.Anelli TF, Anelli A, Tran KN, et al. Tamoxifen administration is associated with a high rate of treatment-limiting symptoms in male breast cancer patients. Cancer. 1994;74:74–77. doi: 10.1002/1097-0142(19940701)74:1<74::aid-cncr2820740113>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 7.Visram H, Kanji F, Dent SF. Endocrine therapy for male breast cancer: rates of toxicity and adherence. Curr Oncol. 2010;17:17–21. doi: 10.3747/co.v17i5.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hershman DL, Kushi LH, Shao T, et al. Early discontinuation and nonadherence to adjuvant hormonal therapy in a cohort of 8,769 early-stage breast cancer patients. J Clin Oncol. 2010;28:4120–4128. doi: 10.1200/JCO.2009.25.9655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barron TI, Connolly R, Bennett K, Feely J, Kennedy MJ. Early discontinuation of tamoxifen: a lesson for oncologists. Cancer. 2007;109:832–839. doi: 10.1002/cncr.22485. [DOI] [PubMed] [Google Scholar]
- 10.Dezentje VO, van Blijderveen NJ, Gelderblom H, et al. Effect of concomitant CYP2D6 inhibitor use and tamoxifen adherence on breast cancer recurrence in early-stage breast cancer. J Clin Oncol. 2010;28:2423–2429. doi: 10.1200/JCO.2009.25.0894. [DOI] [PubMed] [Google Scholar]
- 11.Smith MR, Morton RA, Barnette KG, et al. Toremifene to reduce fracture risk in men receiving androgen deprivation therapy for prostate cancer. J Urol. 2010;184:1316–1321. doi: 10.1016/j.juro.2010.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]