Abstract
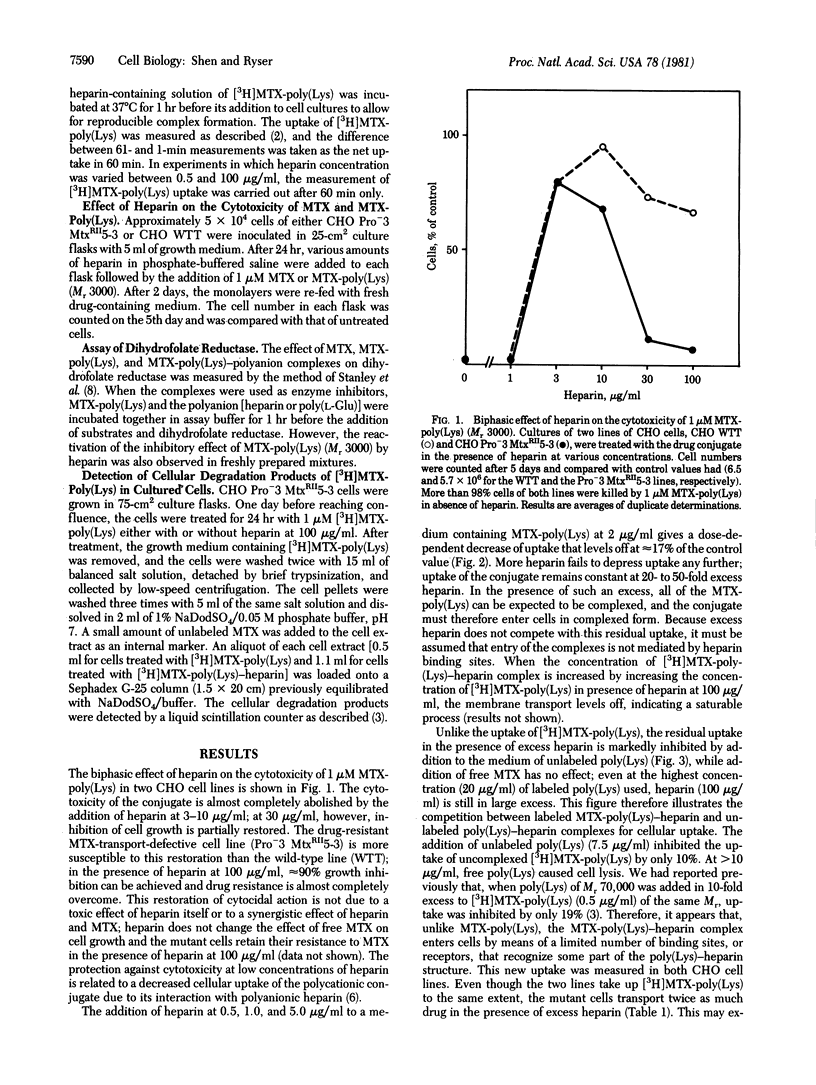
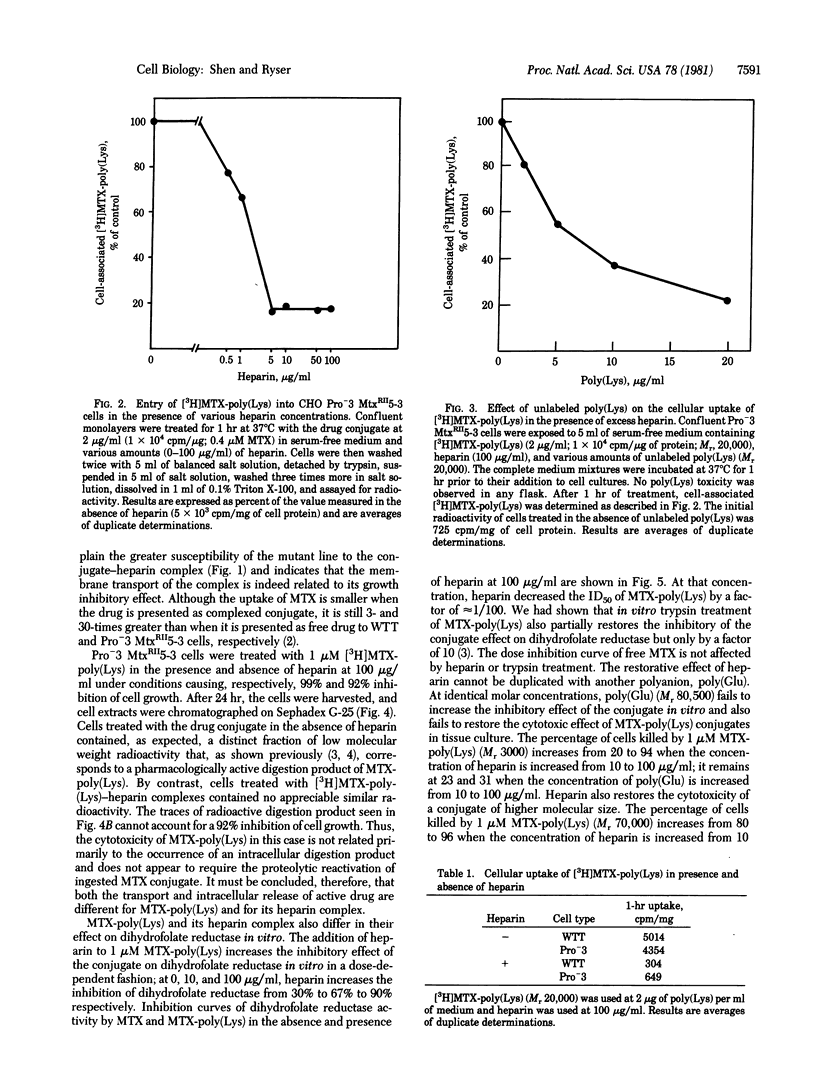
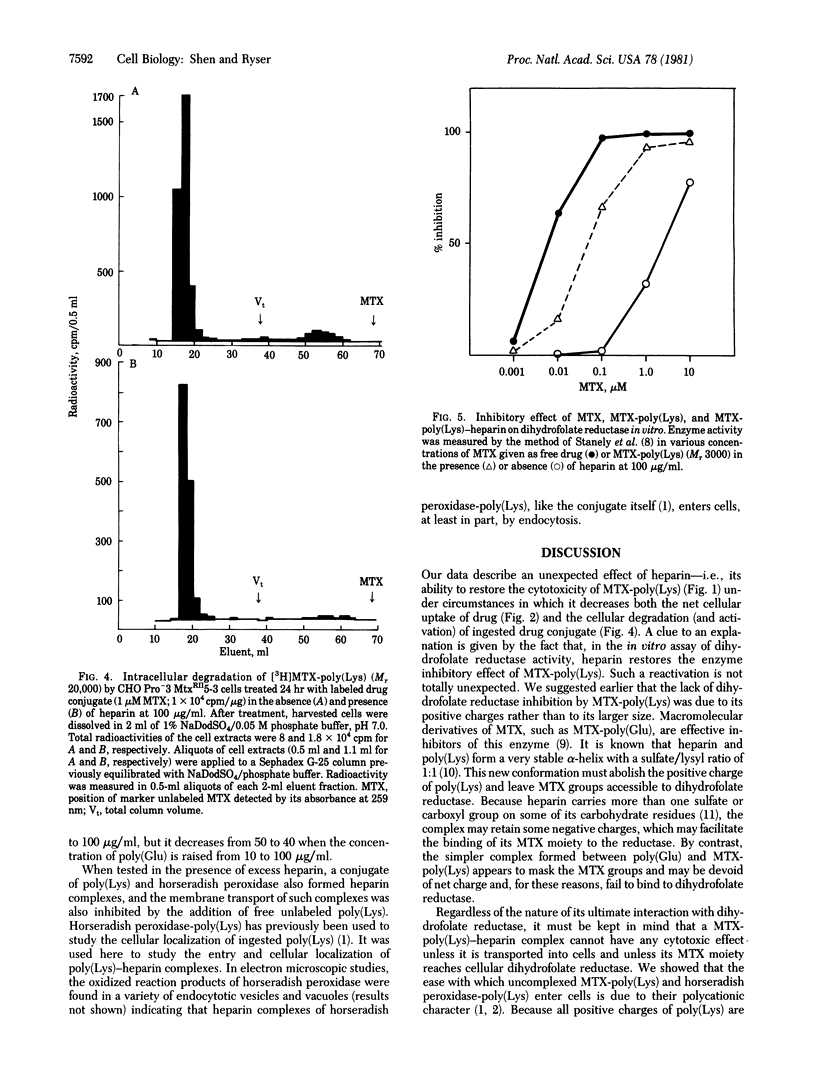
Methotrexate (MTX) conjugated to a Mr 3000 poly(L-Lys) markedly inhibits the growth of Pro-3 MtxRII5-3 Chinese hamster ovarian cells, a mutant line known to be drug resistant because of defective MTX transport. In these cells, membrane transport of [3H]MTX-poly(Lys) is sharply decreased by addition of 0.5- to 2.5-fold heparin but remains at 15-20% of control in 2.5- to 50-fold heparin excess. Heparin addition at first markedly inhibits but, at high concentration, restores the growth inhibitory effect of MTX-poly(Lys). In excess heparin, MTX-poly(Lys) is transported as a heparin complex. Because reduced transport (15-20%) is sufficient to cause a 90% inhibition of cell growth, MTX-poly(Lys) apparently gains pharmacologic potency when compared to heparin. This gain can be related to a greater inhibitory effect on dihydrofolate reductase and to a different mode of transport. The inhibitory effect of MTX-poly(Lys) on dihydrofolate reductase in vitro is increased nearly 100-fold in the presence of excess heparin but remains less than that of free MTX. Unlike that of MTX-poly(Lys), the transport of MTX-poly(Lys)-heparin has the characteristics and efficiency of a receptor-mediated process. It proceeds by endocytosis but is not, as in the case of uncomplexed conjugate, followed by the intracellular generation of pharmacologically active breakdown products that would account for cytotoxicity. These observations raise the possibility that at least part of the MTX-poly(Lys)-heparin reaches cellular dihydrofolate reductase in the form of macromolecular complexes that escape from entrapment in endocytotic structures. Our data illustrate a way to overcome drug resistance by taking advantage of the specific uptake of a macromolecular drug carrier. They offer a method of drug delivery in which heparin improves selectivity and decreases the unwanted toxicity inherent to polycationic carriers.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Azizkhan R. G., Azizkhan J. C., Zetter B. R., Folkman J. Mast cell heparin stimulates migration of capillary endothelial cells in vitro. J Exp Med. 1980 Oct 1;152(4):931–944. doi: 10.1084/jem.152.4.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu S. K., Brown M. S., Ho Y. K., Goldstein J. L. Degradation of low density lipoprotein . dextran sulfate complexes associated with deposition of cholesteryl esters in mouse macrophages. J Biol Chem. 1979 Aug 10;254(15):7141–7146. [PubMed] [Google Scholar]
- Bleiberg I., Fabian I., Aronson M. Mode of binding and internalization into mouse macrophages of heparin complexed with polycations. Biochim Biophys Acta. 1981 May 18;674(3):345–353. doi: 10.1016/0304-4165(81)90365-2. [DOI] [PubMed] [Google Scholar]
- Ehrlich J., Stivala S. S. Chemistry and pharmacology of heparin. J Pharm Sci. 1973 Apr;62(4):517–544. doi: 10.1002/jps.2600620402. [DOI] [PubMed] [Google Scholar]
- Flintoff W. F., Davidson S. V., Siminovitch L. Isolation and partial characterization of three methotrexate-resistant phenotypes from Chinese hamster ovary cells. Somatic Cell Genet. 1976 May;2(3):245–261. doi: 10.1007/BF01538963. [DOI] [PubMed] [Google Scholar]
- Geisow M. J., Beaven G. H., Hart P. D., Young M. R. Site of action of a polyanion inhibitor of phagosome-lysosome fusion in cultured macrophages. Exp Cell Res. 1980 Mar;126(1):159–165. doi: 10.1016/0014-4827(80)90481-4. [DOI] [PubMed] [Google Scholar]
- Gelman R. A., Blackwell J. Heparin-polypeptide interactions in aqueous solution. Arch Biochem Biophys. 1973 Nov;159(1):427–433. doi: 10.1016/0003-9861(73)90470-0. [DOI] [PubMed] [Google Scholar]
- Jaques L. B. Heparins--anionic polyelectrolyte drugs. Pharmacol Rev. 1979 Jun;31(2):99–166. [PubMed] [Google Scholar]
- Kjellén L., Oldberg A., Rubin K., Hök M. Binding of heparin and heparan sulphate to rat liver cells. Biochem Biophys Res Commun. 1977 Jan 10;74(1):126–133. doi: 10.1016/0006-291x(77)91384-5. [DOI] [PubMed] [Google Scholar]
- Longas M. O., Ferguson W. S., Finlay T. H. Studies on the interaction of heparin with thrombin, antithrombin, and other plasma proteins. Arch Biochem Biophys. 1980 Apr 1;200(2):505–602. doi: 10.1016/0003-9861(80)90392-6. [DOI] [PubMed] [Google Scholar]
- Nimrod A., Lindner H. R. Heparin facilitates the induction of LH receptors by FSH in granulosa cells cultured in serum-enriched medium. FEBS Lett. 1980 Sep 22;119(1):155–157. doi: 10.1016/0014-5793(80)81019-2. [DOI] [PubMed] [Google Scholar]
- Prem veer Reddy G., Pardee A. B. Multienzyme complex for metabolic channeling in mammalian DNA replication. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3312–3316. doi: 10.1073/pnas.77.6.3312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg R. D., Armand G., Lam L. Structure-function relationships of heparin species. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3065–3069. doi: 10.1073/pnas.75.7.3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryser H. J., Shen W. C. Conjugation of methotrexate to poly (L-lysine) as a potential way to overcome drug resistance. Cancer. 1980 Mar 15;45(5 Suppl):1207–1211. doi: 10.1002/1097-0142(19800315)45:5+<1207::aid-cncr2820451327>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Ryser H. J., Shen W. C. Conjugation of methotrexate to poly(L-lysine) increases drug transport and overcomes drug resistance in cultured cells. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3867–3870. doi: 10.1073/pnas.75.8.3867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen W. C., Ryser H. J. Conjugation of poly-L-lysine to albumin and horseradish peroxidase: a novel method of enhancing the cellular uptake of proteins. Proc Natl Acad Sci U S A. 1978 Apr;75(4):1872–1876. doi: 10.1073/pnas.75.4.1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen W. C., Ryser H. J. Poly (L-lysine) and poly (D-lysine) conjugates of methotrexate: different inhibitory effect on drug resistant cells. Mol Pharmacol. 1979 Sep;16(2):614–622. [PubMed] [Google Scholar]
- Shen W. C., Ryser H. J. Selective protection against the cytotoxicity of methotrexate and methotrexate-poly(lysine) by thiamine pyrophosphate, heparin and leucovorin. Life Sci. 1981 Mar 16;28(11):1209–1214. doi: 10.1016/0024-3205(81)90445-8. [DOI] [PubMed] [Google Scholar]
- Whitehead V. M. Synthesis of methotrexate polyglutamates in L1210 murine leukemia cells. Cancer Res. 1977 Feb;37(2):408–412. [PubMed] [Google Scholar]


