Abstract
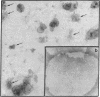
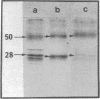
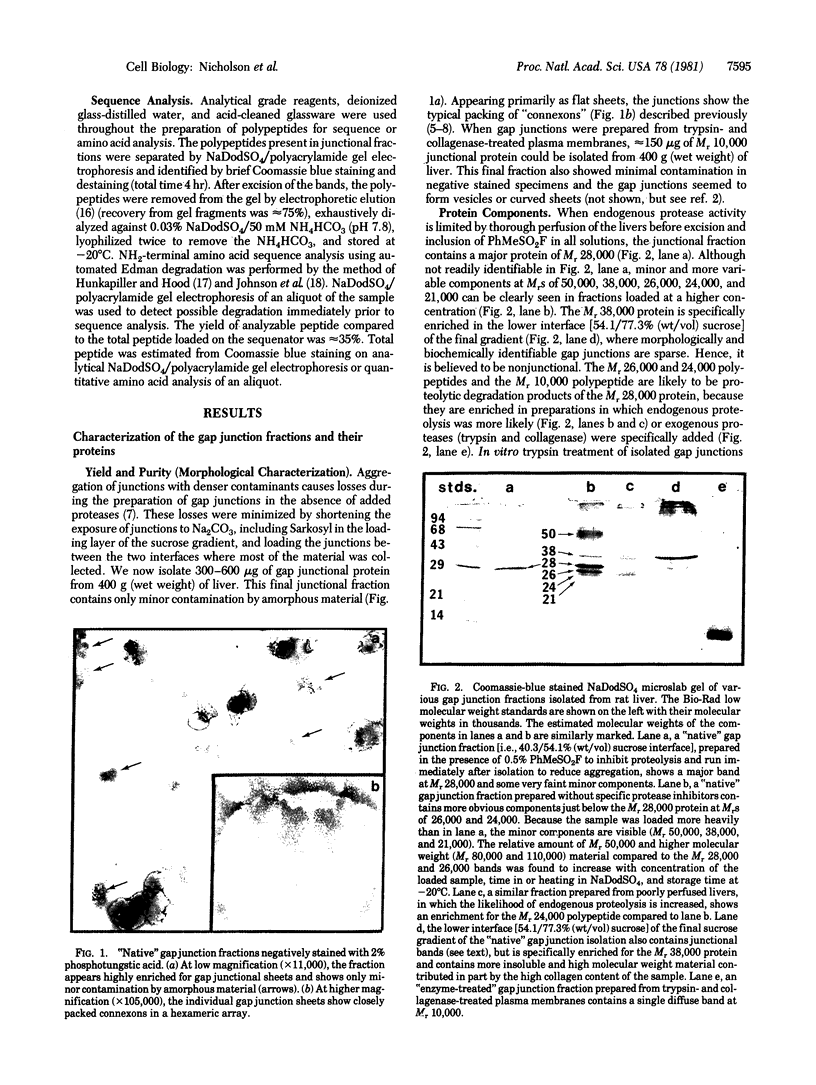
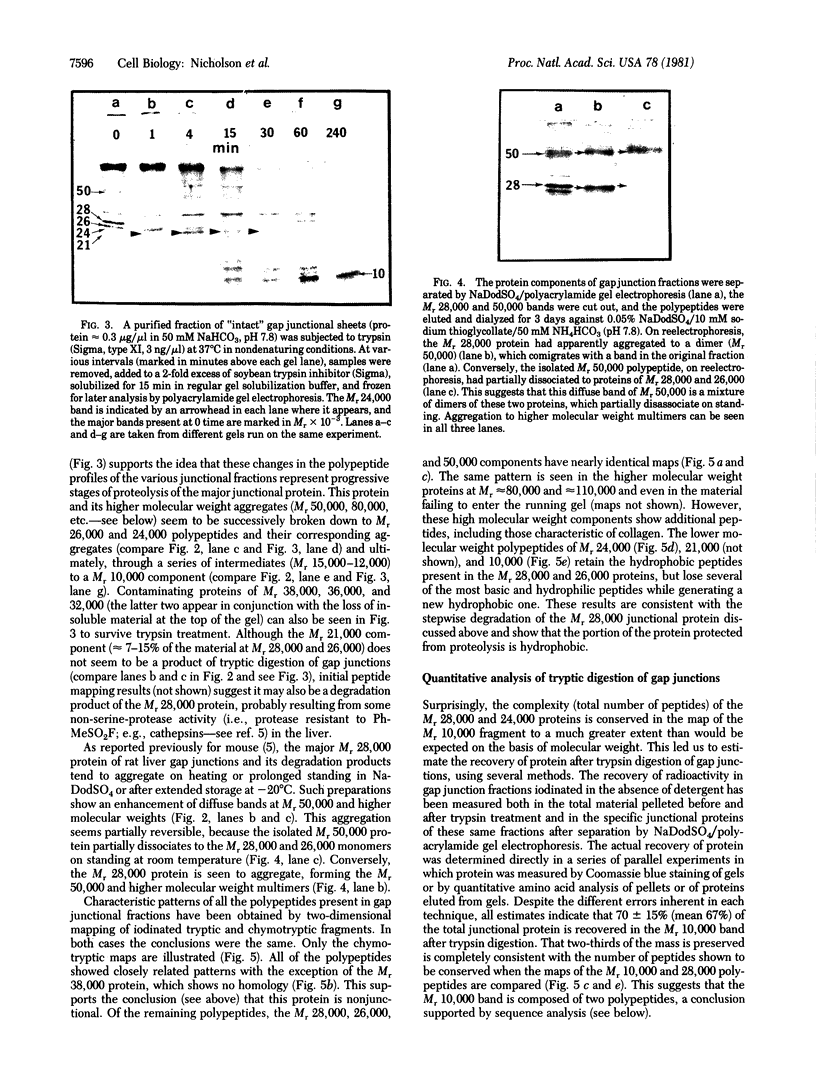
Gap junctions, strongly implicated as channels for direct cell-to-cell communication, have been isolated from rat liver in high yield and purity. These gap junction fractions contain few morphologically recognizable contaminants, but NaDodSO4/polyacrylamide gel electrophoresis reveals a number of polypeptides. With the exception of a nonjunctional component of Mr 38,000 and some poorly soluble material, including collagen, all the polypeptides have very similar or identical two-dimensional peptide maps and arise from proteolytic cleavage of the COOH-terminus or aggregation of a Mr 28,000 protein. We report the sequence of the NH2-terminal 52 amino acids of this protein. The polypeptide (Mr approximately equal to 10,000) characteristic of trypsin-treated gap junction preparations is shown to be two distinct polypeptides, both derived from the Mr 28,000 protein.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amos W. B. An apparatus for microelectrophoresis in polyacrylamide slab-gels. Anal Biochem. 1976 Feb;70(2):612–615. doi: 10.1016/0003-2697(76)90487-5. [DOI] [PubMed] [Google Scholar]
- Asbeck F., Beyreuther K., Köhler H., von Wettstein G., Braunitzer G. Virusproteine, IV. Die Konstitution des Hüllproteins des Phagen fd. Hoppe Seylers Z Physiol Chem. 1969 Sep;350(9):1047–1066. [PubMed] [Google Scholar]
- Bretscher M. S. C-terminal region of the major erythrocyte sialoglycoprotein is on the cytoplasmic side of the membrane. J Mol Biol. 1975 Nov 15;98(4):831–833. doi: 10.1016/s0022-2836(75)80014-3. [DOI] [PubMed] [Google Scholar]
- Chen R., Krämer C., Schmidmayr W., Henning U. Primary structure of major outer membrane protein I of Escherichia coli B/r. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5014–5017. doi: 10.1073/pnas.76.10.5014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Prediction of protein conformation. Biochemistry. 1974 Jan 15;13(2):222–245. doi: 10.1021/bi00699a002. [DOI] [PubMed] [Google Scholar]
- Coligan J. E., Kindt T. J., Uehara H., Martinko J., Nathenson S. G. Primary structure of a murine transplantation antigen. Nature. 1981 May 7;291(5810):35–39. doi: 10.1038/291035a0. [DOI] [PubMed] [Google Scholar]
- Duguid J. R., Revel J. P. The protein components of the gap junction. Cold Spring Harb Symp Quant Biol. 1976;40:45–47. doi: 10.1101/sqb.1976.040.01.007. [DOI] [PubMed] [Google Scholar]
- Elder J. H., Pickett R. A., 2nd, Hampton J., Lerner R. A. Radioiodination of proteins in single polyacrylamide gel slices. Tryptic peptide analysis of all the major members of complex multicomponent systems using microgram quantities of total protein. J Biol Chem. 1977 Sep 25;252(18):6510–6515. [PubMed] [Google Scholar]
- Engelman D. M., Henderson R., McLachlan A. D., Wallace B. A. Path of the polypeptide in bacteriorhodopsin. Proc Natl Acad Sci U S A. 1980 Apr;77(4):2023–2027. doi: 10.1073/pnas.77.4.2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finbow M., Yancey S. B., Johnson R., Revel J. P. Independent lines of evidence suggesting a major gap junctional protein with a molecular weight of 26,000. Proc Natl Acad Sci U S A. 1980 Feb;77(2):970–974. doi: 10.1073/pnas.77.2.970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREENWOOD F. C., HUNTER W. M., GLOVER J. S. THE PREPARATION OF I-131-LABELLED HUMAN GROWTH HORMONE OF HIGH SPECIFIC RADIOACTIVITY. Biochem J. 1963 Oct;89:114–123. doi: 10.1042/bj0890114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodenough D. A. Bulk isolation of mouse hepatocyte gap junctions. Characterization of the principal protein, connexin. J Cell Biol. 1974 May;61(2):557–563. doi: 10.1083/jcb.61.2.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodenough D. A. In vitro formation of gap junction vesicles. J Cell Biol. 1976 Feb;68(2):220–231. doi: 10.1083/jcb.68.2.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodenough D. A., Revel J. P. The permeability of isolated and in situ mouse hepatic gap junctions studied with enzymatic tracers. J Cell Biol. 1971 Jul;50(1):81–91. doi: 10.1083/jcb.50.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodenough D. A., Stoeckenius W. The isolation of mouse hepatocyte gap junctions. Preliminary chemical characterization and x-ray diffraction. J Cell Biol. 1972 Sep;54(3):646–656. doi: 10.1083/jcb.54.3.646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson D., Eibl H., Weber K. Structure and biochemistry of mouse hepatic gap junctions. J Mol Biol. 1979 Aug 5;132(2):193–218. doi: 10.1016/0022-2836(79)90391-7. [DOI] [PubMed] [Google Scholar]
- Hertzberg E. L. Biochemical and immunological approaches to the study of gap junctional communication. In Vitro. 1980 Dec;16(12):1057–1067. doi: 10.1007/BF02619256. [DOI] [PubMed] [Google Scholar]
- Hertzberg E. L., Gilula N. B. Isolation and characterization of gap junctions from rat liver. J Biol Chem. 1979 Mar 25;254(6):2138–2147. [PubMed] [Google Scholar]
- Hunkapiller M. W., Hood L. E. New protein sequenator with increased sensitivity. Science. 1980 Feb 1;207(4430):523–525. doi: 10.1126/science.7352258. [DOI] [PubMed] [Google Scholar]
- Johnson N. D., Hunkapiller M. W., Hood L. E. Analysis of phenylthiohydantoin amino acids by high-performance liquid chromatography on DuPont Zobax cyanopropylsilane columns. Anal Biochem. 1979 Dec;100(2):335–338. doi: 10.1016/0003-2697(79)90237-9. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lazarides E. Two general classes of cytoplasmic actin filaments in tissue culture cells: the role of tropomyosin. J Supramol Struct. 1976;5(4):531(383)–563(415). doi: 10.1002/jss.400050410. [DOI] [PubMed] [Google Scholar]
- Segrest J. P., Jackson R. L., Marchesi V. T., Guyer R. B., Terry W. Red cell membrane glycoprotein: amino acid sequence of an intramembranous region. Biochem Biophys Res Commun. 1972 Nov 15;49(4):964–969. doi: 10.1016/0006-291x(72)90306-3. [DOI] [PubMed] [Google Scholar]
- Stroud R. M., Kay L. M., Dickerson R. E. The structure of bovine trypsin: electron density maps of the inhibited enzyme at 5 Angstrom and at 2-7 Angstron resolution. J Mol Biol. 1974 Feb 25;83(2):185–208. doi: 10.1016/0022-2836(74)90387-8. [DOI] [PubMed] [Google Scholar]
- Wickner W. Asymmetric orientation of phage M13 coat protein in Escherichia coli cytoplasmic membranes and in synthetic lipid vesicles. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1159–1163. doi: 10.1073/pnas.73.4.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zampighi G., Unwin P. N. Two forms of isolated gap junctions. J Mol Biol. 1979 Dec 5;135(2):451–464. doi: 10.1016/0022-2836(79)90446-7. [DOI] [PubMed] [Google Scholar]