Abstract
BACKGROUND & AIMS
Collagenous sprue (CS) is characterized by the presence of a distinctive band of subepithelial collagen deposition in the small bowel. We evaluated the clinical characteristics, treatments, and outcomes of patients with CS.
METHODS
Thirty patients with CS were identified at the 3 Mayo Clinic sites between 1993 and 2009. Clinical data from medical records were reviewed.
RESULTS
The study cohort was 70% female (age range, 53–91 years). Most patients had severe diarrhea and weight loss. Hospitalization to treat dehydration was necessary in 16 (53%) patients. Associated immune-mediated diseases were noted in 70% of the patients; celiac disease was the most frequent. Other associated diseases were microscopic colitis, hypothyroidism, and autoimmune enteropathy. The median thickness of the layer of subepithelial collagen deposition in the small bowel was 29 μm (20 –56.5 μm). Subepithelial collagen deposition in the colon or stomach was noted in 8 patients. A clinical response was observed in 24 (80%) patients after treatment with a combination of a gluten-free diet and immunosuppressive drugs. Histologic improvement was confirmed in 9 patients, with complete remission in 5. Two patients died (1 of complications of CS and 1 of another illness).
CONCLUSIONS
Most patients with CS are treated effectively with a combination of gluten-free diet and steroids. CS is often associated with collagen deposition or chronic inflammation in other segments of the gastrointestinal tract as well as other immune-mediated disorders.
Keywords: Sprue, Microscopic Colitis, Celiac Disease, Duodenum, Collagen, Fibrosis
Collagenous sprue (CS) is a rare enteropathy characterized by villous atrophy and a distinctive band of subepithelial collagen.1,2 The clinical manifestations include chronic diarrhea, malabsorption, and weight loss.1,3 CS has been regarded as a complication of celiac disease (CD); however, malabsorption is often unresponsive to a gluten-free diet (GFD) alone.1,4,5 Thus, although CS is rare, it is a frequent condition underlying refractory “unclassified” sprue.6,7
The pathophysiology of CS is not completely understood. Collagen deposition might result from increased expression of fibrogenic genes, especially fibrillar collagen type I, by myofibroblastic cells of the gut.8 Clinical characteristics and associated diseases suggest immune-mediated pathogenesis.3,5,9
Historically, the outcome of CS has been sinister, with significant morbidity and mortality despite treatment.1,3,6 Some patients improve clinically with a combination of immunosuppressive drugs and a GFD.5,8,10 The GFD alone might result in regression of collagen deposition5,6; however, it is not clear whether those patients were part of the spectrum of CS or just affected by active CD. One third of patients with untreated CD might have some nonspecific subepithelial collagen deposition that usually disappears with a GFD.11,12
Long-term outcome data on patients with CS are scarce, and reports are quite anecdotal. Thus, in the present study, we sought to describe the clinical features, treatment, and outcome of 30 patients with CS seen at the 3 sites of Mayo Clinic.
Methods
Patients
All patients seen at Mayo Clinic sites in Rochester (n = 22), Jacksonville (n = 7), and Scottsdale (n = 5) from 1993–2009 with CS were identified. Medical records were reviewed, and only patients who met histologic and clinical criteria for CS were included.
Pathology Material Evaluation
Original pathology material was re-reviewed by a single pathologist (T.T.W.). Subepithelial collagen thickness was measured by using a micrometer-equipped Olympus DP71 digital camera (Olympus, Center Valley, PA). Two well-oriented fields were chosen, representing the thickest and thinnest subepithelial collagen portions of each biopsy. When the collagen thickness did not vary within the specimen, one measurement was taken. Collagen distribution was graded as diffuse if each tissue fragment of the duodenum was similarly involved by subepithelial collagen deposition or patchy if collagen thickening either was not found in each tissue fragment or was not distributed throughout a particular fragment. The number of intraepithelial lymphocytes (IELs) per 100 epithelial cells was evaluated (normal, <40 IELs per 100 epithelial cells).13 The presence of aberrant (clonal) IELs determined by immunohistochemistry and polymerase chain reaction (PCR)– based T-cell clonality was reported (when available).14,15 Villous atrophy was graded with the modified Marsh classification.16 Surface injury, chronic inflammation, and acute inflammation were subjectively graded as follows: 0, none; 1, mild; 2, moderate; and 3, marked. Associated disorders (when present) were confirmed by using internationally accepted pathologic criteria.
Diagnostic Criteria for Collagenous Sprue
Collagenous sprue required the following: (1) Histologic criterion was the presence of a clear-cut layer of subepithelial collagen deposition extending into the lamina propria (in this series, the minimum single measure and average thickness of the collagen band were 18 μm and ≥20 μm, respectively) and villous atrophy. (2) Clinical criteria (at least 2 of 3) were (a) symptoms (diarrhea, abdominal pain, weight loss), (b) lack of clinical response to a GFD alone, and (c) need of alternative therapy (besides GFD) to control symptoms. No patient considered to have CS pathologically needed to be excluded in this series because of lack of clinical criteria. Four patients were excluded because of lack of either original histology for re-review (n = 3) or research authorization (n = 1).
A diagnosis of CD was supported by conventional criteria.7,17,18 Adult-onset autoimmune enteropathy was defined by clinical criteria and the presence of anti-enterocyte antibodies as previously described.19
Data Collection
Clinical, laboratory, and imaging data were collected from the medical record. We defined the categories of body weight according to level of body mass index.20 Only data that reflected conditions that existed before any alternative treatment besides GFD were included. The medical history was reviewed for associated disorders, travel history, and prescription drugs used at the time of CS diagnosis, especially medications known to be associated with intestinal injury or collagen deposition in the gastrointestinal tract (eg, collagenous colitis).21,22
Response to Treatment
Clinical response was defined by the resolution of diarrhea. Remission required both a clinical response and a normal intestinal biopsy. Reversal of fibrosis described the disappearance of the collagen band, with persistence of some villous atrophy.
Budesonide Instructions
We instructed our patients to open 2 of the 3 capsules of budesonide and mix with applesauce before being swallowed. The third capsule was swallowed whole. Budesonide was taken in 3 divided doses. The clinical rationale behind this strategy was to increase the chances of release budesonide in the proximal small bowel because of the predominantly topical anti-inflammatory effect of budesonide with extensive first-pass metabolism.23 We recognize that there are no validated experimental data to support this method of drug delivery, and it runs counter to the package insert. There is emerging evidence that suggests that budesonide might be effective in the treatment of both uncomplicated and complicated CD.24 –26 Also, we recommended that grapefruit and budesonide not be used concurrently.
Statistical Analysis
Data were summarized with frequencies, proportions, or medians (range). The χ2, Fisher exact test, and Mann–Whitney U test were used when appropriate to test clinical variables for significance in relation to collagen band thickness. A P value <.05 was considered statistically significant.
Ethical Issues
The study was approved by the Institutional Review Board of Mayo Foundation.
Results
Patients
We included 30 patients (21 [70%] female) with a median age (range) at diagnosis of 72.5 years (53–91 years). Twenty-nine patients were white, and 1 was black. Symptoms had been present for a median (range) of 9 months (1–120 months) before the CS diagnosis. At presentation, all patients had diarrhea, and 97% experienced marked loss of weight (median loss, 27 pounds). Abrupt onset of diarrhea was noted in 8 patients. Transient acute renal failure caused by diarrhea occurred in 6 patients; none required hemodialysis. Twenty-two (74%) patients had a normal weight, 4 (13%) were overweight, 3 (10%) were obese, and 1 (3%) was underweight at the time of CS diagnosis. Anemia and hypoalbuminemia occurred in 13 (43%) and 7 (23%) patients, respectively. Ten (33%) patients required total parenteral nutrition (Table 1).
Table 1.
Summary of Clinical Characteristics of Study Population
| Patient characteristics | CS (n = 30) |
|---|---|
| Age at diagnosis, ya | 72.5 (53–91) |
| Female, n (%) | 21 (70) |
| Diarrhea, n (%) | 30 (100) |
| Bowel movements per daya | 10 (4–20) |
| Stool weight (n = 11), g/daya | 790.5 (237–1902) |
| Stool fat content (n = 11), g/daya | 17 (11–76) |
| Need of antidiarrheal drug use, n (%) | 16 (53) |
| Hospitalization, n (%) | 16 (53) |
| Loss of weight, n (%) | 29 (97) |
| Weight loss, lbsa | 27 (8–65) |
| Body mass index at diagnosis, kg/m2a | 23 (18–38) |
| Nausea or vomit, n (%) | 10 (33) |
| Abdominal pain, n (%) | 6 (20) |
| Anorexia or fatigue, n (%) | 6 (20) |
| Total number of symptoms per patient | |
| ≤2 | 18 (60) |
| 3–4 | 9 (30) |
| ≥5 | 3 (10) |
| Hemoglobin, g/dLa | 12.5 (7.8–16.4) |
| Albumin, g/dLa | 3.2 (1.7–4.3) |
Data summarized as medians (range).
The medical history was significant for a diagnosis of CD in 11 (37%) patients. CD was diagnosed before CS in 10 of 11 patients with concurrent CD and CS. The median time (range) from CD diagnosis to CS diagnosis was 22 months (8 –300 months). One patient with total villous atrophy and diffuse collagen deposition (average collagen band, 28 μm) received both diagnoses at the same time because of the presence of endomysial antibodies (EMAs) in the serum. It is not possible to know whether this last patient had undiagnosed CD for years and then developed clinically severe CS that led to the diagnosis of the enteropathy. The diagnosis of CD was supported by a positive CD-specific serology sometime during clinical evolution (n = 6), presence of villous atrophy and either complete clinical response to GFD or HLAs DQ2 or DQ8 (n = 11), and the absence of other causes of villous atrophy (n = 11). Complete clinical response to GFD alone for ≥12 months was observed in 9 (82%) of the 11 patients with CD before the diagnosis of CS. The other 2 patients received additional therapies besides GFD because of either a simultaneous diagnosis of CS-CD or the absence of clinical response to 8 months of GFD alone, respectively.
No patient had a history of recent travel, infection, systemic sclerosis, amyloidosis, abdominal irradiation, or mesenteric ischemia. Low-dose aspirin was used by 8 patients and naproxen in 1 patient. Proton pump inhibitors were used in 9 (30%) patients. Other drugs were olmesartan (n = 8), simvastatin (n = 7), and ranitidine (n = 1).
Tissue transglutaminase antibodies (tTGAs) and EMAs were positive in 3 patients at the time of CS diagnosis (tTGA titers of 69.3, 72.0, and 250 U/mL, respectively). HLA alleles DQ2 or DQ8 were present in 17 (77%) of 22 patients tested (n = 14 DQ2+, n = 2 DQ2+/DQ8+, and n = 1 DQ2+homozygous).
Histologic Spectrum of Collagenous Sprue
The median thickness (of the average of the thickest and thinnest subepithelial collagen portions of each biopsy) of the layer of subepithelial collagen deposition was 29 μm (20 –56.5 μm). The minimal and maximal single measurements were 18 μm and 95 μm, respectively (Figure 1). The distribution of collagen deposition was diffuse in 23 (77%) and patchy in 7 (23%) patients. Total villous atrophy was observed in 20 (67%) and subtotal villous atrophy in 10 (33%) patients. Intraepithelial lymphocytosis was observed in 21 (70%) patients. No aberrant (clonal) IELs were detected in 17 (57%) patients tested. Other histologic findings are summarized in Supplementary Table 1.
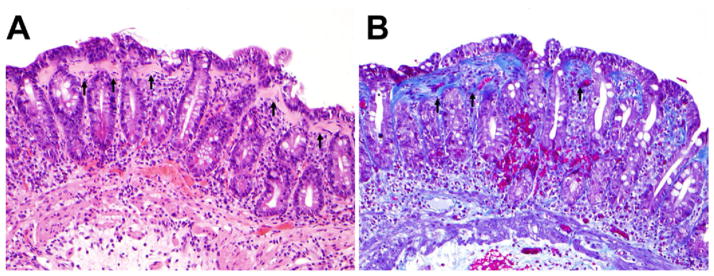
Figure 1.
Morphologic features of collagenous sprue. (A) Thickened subepithelial collagen layer (arrows). Note the absence of villi (total villous atrophy) and prominent surface epithelial injury (hematoxylin-eosin; original magnification, ×200). (B) Masson trichrome stain highlighted the thickened subepithelial collagen layer (arrows) (Masson trichrome; original magnification, ×200).
The thickness of the layer of subepithelial collagen deposition was not significantly different in patients who required hospitalization (clinically severe), had abnormal laboratory values (anemia or hypoalbuminemia), and those with CD or who did not achieve clinical response (data not shown).
Associated Diseases
Associated immune-mediated disorders were CD (n = 11), collagenous colitis (n = 6), autoimmune hypothyroidism (n = 4), lymphocytic colitis (n = 4), autoimmune enteropathy (n = 3), collagenous gastritis (n = 2), lymphocytic gastritis (n = 2), Raynaud’s phenomena, chronic interstitial pneumonitis, discoid lupus, antiphospholipid syndrome, lymphocytic myocarditis, and recurrent iritis. Other non–immune-mediated disorders were also documented. The clinical characteristics of the 30 patients with CS are summarized in Supplementary Table 2.
Patients with CD tended to be younger at CS diagnosis than those without CD (median age, 62 vs 75 years; P = .09) but had similar gender, history of hospitalization, body mass index, number of symptoms, hemoglobin, albumin, and median thickness of subepithelial collagen band (data not shown).
Medical Treatment
A GFD was recommended in all patients. Steroids were prescribed in 26 (87%) patients. Budesonide alone (dose, 9 mg/day) was used in 12 patients. A short course of prednisone (dose range, 20 –70 mg/day) followed by budesonide (dose, 9 mg/day) was used in 8 patients. Prednisone alone (dose, 40 – 60 mg/day) was used in 3 patients and in combination with azathioprine (dose, 50 mg) in another 2 patients. Dexamethasone (dose, 8 mg/day) was used in 1 patient. Octreotide alone was used in 1 patient. Two patients were treated with a combination of parenteral nutrition and a GFD alone. One patient with refractory iron-deficiency anemia was treated with a GFD and intravenous iron. Oral antibiotics were used to treat coexistent small-intestine bacterial overgrowth in 8 cases.
Maintenance therapy in the 26 patients who received steroids for initial treatment was a lower dose of steroids or immunosuppressive agents in 15 (58%) patients, including prednisone ≤10 mg/day (n = 3), budesonide 6 mg/day (n = 8), budesonide 3 mg/day (n = 3), and azathioprine 75 mg/day (n = 1). Steroids were successfully weaned off in 6 (23%) patients after a median of 22 months (12– 45 months), and 5 (17%) patients could not taper the steroid dose.
Clinical and Histologic Response
Clinical response was observed in 24 (80%) patients, and 15 (50%) patients had a follow-up biopsy of the duodenum after treatment for a median time (range) of 12.5 months (6 –72 months). Duodenal histology improved in 9 patients; complete remission was observed in 5 and reversal of fibrosis in the other 4 (Figure 2). Histology remained without change despite treatment in 6 patients.
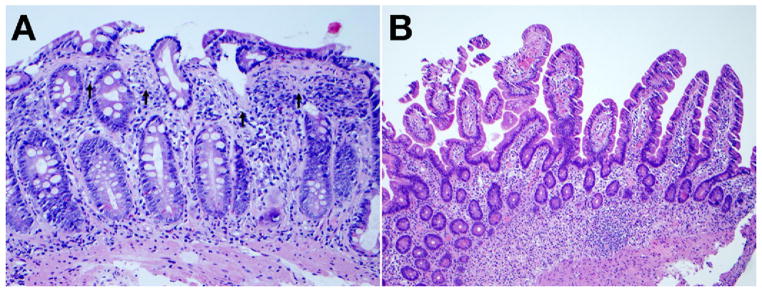
Figure 2.
Complete remission of CS after treatment with a combination of GFD and budesonide. (A) Duodenal biopsy before treatment with thickened subepithelial collagen layer (arrows) and total villous atrophy (hematoxylin-eosin; original magnification, ×200). (B) Duodenal biopsy 2 years after treatment, with recovery of villi and absence of subepithelial collagen deposition (hematoxylin-eosin; original magnification, ×100).
Outcome
The median follow-up was 18 months (1– 84 months). Follow-up data for 6 or more months were available in 24 (80%) patients. Two patients died after 6 and 65 months with emaciation and sepsis and ischemic stroke, respectively.
Discussion
Collagenous sprue was first described in 1947 by Schein.4 Since then, several more patients and small case series have been reported.1,5,6,8,10,27–31 Here we present the clinical characteristics, histologic findings, treatment, and prognosis of 30 patients with CS. This study represents the largest series of patients reported to date with the diagnosis of CS. Of clinical relevance, up to 80% of our patients had clinical response after treatment with a combination of immunosuppressive drugs (mainly steroids) and a GFD. Complete remission or reversal of fibrosis in the gut was documented in a minority of patients.
CD was the most common identifiable risk factor underlying CS. Although CS might have a wide clinical spectrum, in this series, CS presented with a severe malabsorptive syndrome most often refractory to GFD alone. This study and the historical observation of frequent collagen deposition in untreated CD suggest that the collagen deposition is the result of inflammation common to these 2 disorders. Ultimately, it is the successful treatment of the inflammation as in our series of CS or the effect of a GFD in untreated CD that is central to the reversal of this process.11 In this series, we re-reviewed pathology material of potential patients with an initial diagnosis of CS based on the subjective criteria of significantly increased collagen deposition in the context of refractory sprue syndrome. Systematic quantitative re-review of the band of collagen deposition by using the original pathology material revealed a minimal average thickness of 20 μm that correlated well with the severe clinical syndrome. It is clear that there is no consensus about the minimal thickness of the band of collagen deposition necessary to diagnose CS.5,6,9,31 Indeed, untreated CD might be associated with a somewhat less thickened collagen band that can resolve with a GFD, suggesting that lesser degrees of collagen deposition do not necessarily result in the substantial symptoms characteristic of refractory sprue syndrome associated with CS, originally described by Schein4 and Weinstein et al.1
The excellent premorbid nutritional state in this cohort might be a factor that partially explains the good clinical evolution, although the relatively limited follow-up time (median, 18 months) requires consideration.
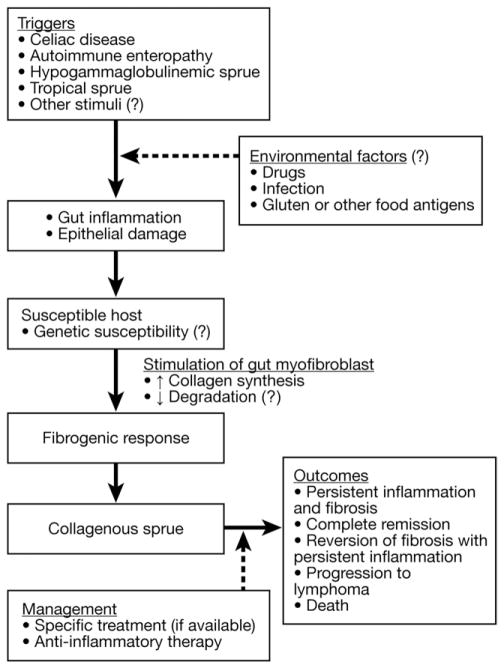
The etiology of CS in patients without evidence of CD remains enigmatic. Adult-onset autoimmune enteropathy (n = 3) and hypogammaglobulinemic sprue (n = 1) were the final diagnoses for their enteropathy in 4 of our patients; thus, it could be suggested that several enteropathies might precipitate fibrogenesis. Variable subepithelial collagen deposition has previously been reported in tropical sprue.12 CD can be excluded in the absence of human leukocyte antigens (HLA)-DQ2/DQ8,18 but it remains possible in others with those HLA genes, even in the absence of clinical or laboratory features of CD.7,18,26 The majority of our patients with CD who later developed CS had negative CD-specific antibodies at the time of CS diagnosis, suggesting strict adherence to GFD. Gluten contamination could be the trigger for CS in noncompliant CD patients. Other triggers of CS are unknown but might be environmental (Figure 3).
Figure 3.
Possible mechanisms of the pathogenesis of CS.
Acetylsalicylic acid or nonsteroidal anti-inflammatory drug (NSAID) consumption is another potential environmental factor that can cause a wide spectrum of damage to the small bowel and the colon (NSAIDs enteropathy) or worsen inflammation and epithelial injury induced by other intestinal disorders.21,32 One third of our patients were on acetylsalicylic acid or NSAIDs; however, the effect that these drugs might have in exacerbation and/or progression of intestinal injury is unknown but deserves consideration.32 Moreover, consumption of 1 or more drugs associated with microscopic colitis, including collagenous infiltration of the colon,22 was observed in 47% of our patients with CS, sometimes with associated microscopic colitis. Again, the clinical implications of the use of potentially pro-inflammatory and/or pro-fibrotic drugs in patients with CS are unknown because of the pure chronologic argument and absence of data on histologic improvement with drug withdrawal and deterioration with drug rechallenge.22 Indeed, olmesartan, a drug with antifibrotic properties outside the gastrointestinal tract,33,34 was used by one third of our patients.
Although open label and purely observational, our data suggest that a combination of a GFD and steroid treatment are effective therapy for most patients with CS. The response to budesonide is clinically relevant because of this drug’s excellent safety profile as a result of its predominantly topical effect on the intestine and extensive first-pass effect.23 Budesonide is clinically effective for treatment of refractory CD and other forms of refractory sprue.25,26,35 The most appropriate treatment for steroid-resistant or steroid-dependent CS is not known yet.
Interestingly, the abnormal collagen band disappeared in 9 patients with the addition of prednisone or budesonide to a GFD, consistent with prior experiences with the use of oral prednisone.10,36 A sampling artifact is possible but unlikely because of extensive biopsy sampling and the concurrent clinical response. Thus, fibrosis of the gut might be reversible in some patients with CS, despite initial dense collagen deposition.
In this series, CS was frequently associated with collagen deposition in other organs of the gastrointestinal tract (collagenous gastritis and collagenous colitis) and other immune-mediated disorders, consistent with previous reports.28,37,38 These findings suggest that collagen deposition could be a generalized disease affecting the entire gastrointestinal tract. Thus, active investigation of associated disorders may be advisable. The presence of collagen deposition in the colon and duodenum did not imply a worse prognosis, possibly because immunosuppressive drugs are clinically effective for either refractory sprue or microscopic colitis.25,39,40 Finally, the association between CS and lymphoma has been reported.6,27 None of the 17 patients tested in our cohort demonstrated the presence of clonal IELs in the intestine by PCR (a finding associated with a high risk of lymphomagenesis). However, clonal T-cell populations were found in 5 of 6 tested patients with CS from other recent series.31 Thus, lymphomagenesis might be a possible outcome in CS, especially in patients with prolonged survival and abnormal T-cell clones in the intestine.
Our data suggest that CS is heterogeneous and not exclusively a complication of CD. Lifelong GFD might be unnecessary in the absence of genetic predisposition to CD, and anti-inflammatory treatment alone might suffice in these patients. Negative celiac autoantibodies, which are gluten-dependent, do not rule out CD, because most CD-related patients were sero-negative at the time of CS diagnosis as observed in refractory CD or its complications.26,41,42
In conclusion, we report the largest case series of CS to date. Treatment with a combination of a GFD and immunosuppressive drugs (including budesonide) was effective for controlling symptoms in most patients, with healing or reversal of fibrosis in the small intestine documented in a minority of patients. CS was associated with CD, collagen deposition in other organs, and other immune-mediated conditions.
Supplementary Material
Acknowledgments
The authors thank Estela G. Staggs and Deanna Brogan for their assistance in collection of data and pathology material.
Funding
This article was supported by the National Institutes of Health (NIH), Ruth L. Kirschstein National Research Service Award/Training Grant in Gastrointestinal Allergy and Immunology (T32 AI-07047) (to A.R.T.), and NIH grant DK-57892 (to J.A.M.).
Abbreviations used in this paper
- CD
celiac disease
- CS
collagenous sprue
- EMA
endomysial antibody
- GFD
gluten-free diet
- HLA
human leukocyte antigens
- IELs
intraepithelial lymphocytes
- NSAID
nonsteroidal anti-inflammatory drug
- PCR
polymerase chain reaction
- tTGA
anti-tissue transglutaminase antibodies
Footnotes
Note: To access the supplementary material accompanying this article, visit the online version of Clinical Gastroenterology and Hepatology at www.cghjournal.org, and at doi:10.1016/ j.cgh.2009.12.023.
Conflicts of interest
The authors declare no conflicts.
References
- 1.Weinstein WM, Saunders DR, Tytgat GN, et al. Collagenous sprue: an unrecognized type of malabsorption. N Engl J Med. 1970;283:1297–1301. doi: 10.1056/NEJM197012102832401. [DOI] [PubMed] [Google Scholar]
- 2.Brandborg LL. Histologic diagnosis of diseases of malabsorption. Am J Med. 1979;67:999–1006. doi: 10.1016/0002-9343(79)90641-7. [DOI] [PubMed] [Google Scholar]
- 3.Collagenous sprue. Br Med J. 1971;2:65– 66. [PMC free article] [PubMed] [Google Scholar]
- 4.Schein J. Syndrome of non tropical sprue with hitherto undescribed lesions of the intestine. Gastroenterology. 1947;8:438–460. [PubMed] [Google Scholar]
- 5.Pepper HW, Brandborg LL, Shanser JD, et al. Collagenous sprue. Am J Roentgenol Radium Ther Nucl Med. 1974;121:275–282. doi: 10.2214/ajr.121.2.275. [DOI] [PubMed] [Google Scholar]
- 6.Robert ME, Ament ME, Weinstein WM. The histologic spectrum and clinical outcome of refractory and unclassified sprue. Am J Surg Pathol. 2000;24:676– 687. doi: 10.1097/00000478-200005000-00006. [DOI] [PubMed] [Google Scholar]
- 7.Green PH, Cellier C. Celiac disease. N Engl J Med. 2007;357:1731–1743. doi: 10.1056/NEJMra071600. [DOI] [PubMed] [Google Scholar]
- 8.Daum S, Foss HD, Schuppan D, et al. Synthesis of collagen I in collagenous sprue. Clin Gastroenterol Hepatol. 2006;4:1232–1236. doi: 10.1016/j.cgh.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 9.Freeman HJ. Collagenous mucosal inflammatory diseases of the gastrointestinal tract. Gastroenterology. 2005;129:338–350. doi: 10.1053/j.gastro.2005.05.020. [DOI] [PubMed] [Google Scholar]
- 10.Holdstock DJ, Oleesky S. Successful treatment of collagenous sprue with combination of prednisolone and gluten-free diet. Postgrad Med J. 1973;49:664– 667. doi: 10.1136/pgmj.49.575.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bossart R, Henry K, Doe WF, et al. Proceedings: Collagenous basement membrane thickening in jejunal biopsies from patients with adult coeliac disease. Gut. 1974;15:338. [PubMed] [Google Scholar]
- 12.Bossart R, Henry K, Booth CC, et al. Subepithelial collagen in intestinal malabsorption. Gut. 1975;16:18–22. doi: 10.1136/gut.16.1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marsh MN. Gluten, major histocompatibility complex, and the small intestine: a molecular and immunobiologic approach to the spectrum of gluten sensitivity (“celiac sprue”) Gastroenterology. 1992;102:330–354. [PubMed] [Google Scholar]
- 14.Patey-Mariaud De Serre N, Cellier C, Jabri B, et al. Distinction between coeliac disease and refractory sprue: a simple immunohistochemical method. Histopathology. 2000;37:70–77. doi: 10.1046/j.1365-2559.2000.00926.x. [DOI] [PubMed] [Google Scholar]
- 15.Ashton-Key M, Diss TC, Pan L, et al. Molecular analysis of T-cell clonality in ulcerative jejunitis and enteropathy-associated T-cell lymphoma. Am J Pathol. 1997;151:493– 498. [PMC free article] [PubMed] [Google Scholar]
- 16.Oberhuber G, Granditsch G, Vogelsang H. The histopathology of coeliac disease: time for a standardized report scheme for pathologists. Eur J Gastroenterol Hepatol. 1999;11:1185–1194. doi: 10.1097/00042737-199910000-00019. [DOI] [PubMed] [Google Scholar]
- 17.Rostom A, Murray JA, Kagnoff MF. American Gastroenterological Association (AGA) Institute technical review on the diagnosis and management of celiac disease. Gastroenterology. 2006;131:1981–2002. doi: 10.1053/j.gastro.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 18.Biagi F, Corazza GR. Defining gluten refractory enteropathy. Eur J Gastroenterol Hepatol. 2001;13:561–565. doi: 10.1097/00042737-200105000-00016. [DOI] [PubMed] [Google Scholar]
- 19.Akram S, Murray JA, Pardi DS, et al. Adult autoimmune enteropathy: Mayo Clinic Rochester experience. Clin Gastroenterol Hepatol. 2007;5:1282–1290. doi: 10.1016/j.cgh.2007.05.013. quiz 1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Calle EE, Rodriguez C, Walker-Thurmond K, et al. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348:1625–1638. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 21.Lanas A, Sopena F. Nonsteroidal anti-inflammatory drugs and lower gastrointestinal complications. Gastroenterol Clin North Am. 2009;38:333–352. doi: 10.1016/j.gtc.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 22.Beaugerie L, Pardi DS. Review article: drug-induced microscopic colitis - proposal for a scoring system and review of the literature. Aliment Pharmacol Ther. 2005;22:277–284. doi: 10.1111/j.1365-2036.2005.02561.x. [DOI] [PubMed] [Google Scholar]
- 23.Svoboda RP, Patel DH, Olden KW. Oral formulations of budesonide: a novel treatment for inflammatory bowel disease. Drugs Today (Barc) 2008;44:857– 863. doi: 10.1358/dot.2008.44.11.1297916. [DOI] [PubMed] [Google Scholar]
- 24.Ciacci C, Maiuri L, Russo I, et al. Efficacy of budesonide therapy in the early phase of treatment of adult celiac disease patients with malabsorption: an in vivo/in vitro pilot study. Clin Exp Pharmacol Physiol. 2009;35:1170–1176. doi: 10.1111/j.1440-1681.2009.05211.x. [DOI] [PubMed] [Google Scholar]
- 25.Brar P, Lee S, Lewis S, et al. Budesonide in the treatment of refractory celiac disease. Am J Gastroenterol. 2007;102:2265–2269. doi: 10.1111/j.1572-0241.2007.01380.x. [DOI] [PubMed] [Google Scholar]
- 26.Rubio-Tapia A, Kelly DG, Lahr BD, et al. Clinical staging and survival in refractory celiac disease: a single center experience. Gastroenterology. 2009;136:99–107. doi: 10.1053/j.gastro.2008.10.013. quiz 352–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Freeman HJ. Collagenous sprue associated with an extensive T-cell lymphoma. J Clin Gastroenterol. 2003;36:144–146. doi: 10.1097/00004836-200302000-00011. [DOI] [PubMed] [Google Scholar]
- 28.Eckstein RP, Dowsett JF, Riley JW. Collagenous enterocolitis: a case of collagenous colitis with involvement of the small intestine. Am J Gastroenterol. 1988;83:767–771. [PubMed] [Google Scholar]
- 29.McCashland TM, Donovan JP, Strobach RS, et al. Collagenous enterocolitis: a manifestation of gluten-sensitive enteropathy. J Clin Gastroenterol. 1992;15:45–51. [PubMed] [Google Scholar]
- 30.Barry RE, Morris JS, Read AE. A case of small-intestinal mucosal atrophy. Gut. 1970;11:743–747. doi: 10.1136/gut.11.9.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maguire AA, Greenson JK, Lauwers GY, et al. Collagenous sprue: a clinicopathologic study of 12 cases. Am J Surg Pathol. 2009;10:1440–1449. doi: 10.1097/PAS.0b013e3181ae2545. [DOI] [PubMed] [Google Scholar]
- 32.Smecuol E, Pinto Sanchez MI, Suarez A, et al. Low-dose aspirin affects the small bowel mucosa: results of a pilot study with a multidimensional assessment. Clin Gastroenterol Hepatol. 2009;7:524–529. doi: 10.1016/j.cgh.2008.12.019. [DOI] [PubMed] [Google Scholar]
- 33.Kurikawa N, Suga M, Kuroda S, et al. An angiotensin II type 1 receptor antagonist, olmesartan medoxomil, improves experimental liver fibrosis by suppression of proliferation and collagen synthesis in activated hepatic stellate cells. Br J Pharmacol. 2003;139:1085–1094. doi: 10.1038/sj.bjp.0705339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nakamoto H, Imai H, Fukushima R, et al. Role of the renin-angiotensin system in the pathogenesis of peritoneal fibrosis. Perit Dial Int. 2008;28(Suppl 3):S83–S87. [PubMed] [Google Scholar]
- 35.Daum S, Ipczynski R, Heine B, et al. Therapy with budesonide in patients with refractory sprue. Digestion. 2006;73:60– 68. doi: 10.1159/000092639. [DOI] [PubMed] [Google Scholar]
- 36.Freeman HJ, Davis JE, Myers DM. Complete histological resolution of collagenous sprue. Can J Gastroenterol. 2004;18:333–336. doi: 10.1155/2004/961380. [DOI] [PubMed] [Google Scholar]
- 37.Colletti RB, Trainer TD. Collagenous gastritis. Gastroenterology. 1989;97:1552–1555. doi: 10.1016/0016-5085(89)90403-4. [DOI] [PubMed] [Google Scholar]
- 38.Lagorce-Pages C, Fabiani B, Bouvier R, et al. Collagenous gastritis: a report of six cases. Am J Surg Pathol. 2001;25:1174–1179. doi: 10.1097/00000478-200109000-00008. [DOI] [PubMed] [Google Scholar]
- 39.Miehlke S, Heymer P, Bethke B, et al. Budesonide treatment for collagenous colitis: a randomized, double-blind, placebo-controlled, multicenter trial. Gastroenterology. 2002;123:978–984. doi: 10.1053/gast.2002.36042. [DOI] [PubMed] [Google Scholar]
- 40.Miehlke S, Madisch A, Bethke B, et al. Oral budesonide for maintenance treatment of collagenous colitis: a randomized, double-blind, placebo-controlled trial. Gastroenterology. 2008;135:1510–1516. doi: 10.1053/j.gastro.2008.07.081. [DOI] [PubMed] [Google Scholar]
- 41.Malamut G, Afchain P, Verkarre V, et al. Presentation and long-term follow-up of refractory celiac disease: comparison of type I with type II. Gastroenterology. 2009;136:81–90. doi: 10.1053/j.gastro.2008.09.069. [DOI] [PubMed] [Google Scholar]
- 42.Al-Toma A, Verbeek WH, Hadithi M, et al. Survival in refractory coeliac disease and enteropathy-associated T-cell lymphoma: retrospective evaluation of single-centre experience. Gut. 2007;56:1373–1378. doi: 10.1136/gut.2006.114512. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.