Abstract
Background
Data from studies in patients with nonalcoholic steatohepatitis (NASH) suggest an increased hepatic fatty acid oxidation. We have previously shown higher fasting plasma bile acid concentrations in patients with NASH. In-vivo and in-vitro studies suggest that bile acids by binding to peroxisome proliferator-activated receptor α activate fibroblast growth factor 21 (FGF21) and increase hepatic fatty acid oxidation.
Methods
Plasma bile acid levels were quantified in healthy controls (n = 38) and patients with biopsy-proven NASH (n = 36). Plasma concentration of fatty acids, β-hydroxybutyrate, insulin, glucose, leptin, alanine aminotransferase, FGF21, and 8-hydroxydeoxyguanosine, a measure of oxidative stress, were measured in 16 healthy controls and 10 patients with NASH in the fasted state and in response to 3 h of infusion of intralipid. In a subgroup of these patients (n = 6 each), plasma ceramide subspecies were quantified.
Results
Fasting plasma bile acids, FGF21, and leptin concentrations were significantly higher in patients with NASH. In response to intralipid infusion there was an increase in plasma β-hydroxybutyrate and free fatty acid levels in both controls and NASH; however, the ratio of β-hydroxybutyrate/free fatty acid was higher in NASH (P = 0.02). Plasma FGF21 concentration increased in response to intralipid in patients with NASH only (P < 0.01). Plasma leptin, insulin, glucose, and alanine transferase concentrations did not change in either group after infusion of intralipid. Increase in total ceramides in response to intralipid was greater in NASH.
Conclusion
Elevated bile acids and FGF21 may be responsible for the higher hepatic fatty acid oxidation in NASH.
Keywords: bile acids, fatty acid oxidation, fibroblast growth factor 21, nonalcoholic steatohepatitis
Introduction
The contribution of hepatic fatty acid oxidation to the development and progression of nonalcoholic fatty liver disease (NAFLD) is controversial. Both an increase and decrease in hepatic fatty acid oxidation have been reported in human patients and in animal models [1–3]. The activity of hepatic mitochondrial short-chain and long-chain acyl CoA hydroxylase enzymes involved in fatty acid oxidation have been reported to be lower in patients with NAFLD compared with controls. However, a decrease in activity does not necessarily translate into a decrease in flux through the pathway [4]. Patients with defects in enzymes involved in mitochondrial long-chain fatty acid oxidation develop hepatic steatosis [5]. In addition, mice with decreased fatty acid oxidation [peroxisome proliferator-activated receptor α (PPARα) knockout and fatty acyl CoA oxidase deficiency] develop hepatic steatosis [6,7]. In contrast, several studies in patients with nonalcoholic steatohepatitis (NASH) show higher concentration of plasma β-hydroxybutyrate (BHB) after an overnight fast. As the liver is the only source of plasma BHB, these data suggest a high rate of hepatic fatty acid oxidation [8–10]. In a previous study, we examined the correlation between plasma concentration of BHB and fatty acids before and after an infusion of intralipid in patients with NASH compared with controls. The higher slope of the correlation suggested a higher fatty acid oxidation in NASH [11]. The mechanism of the increased hepatic fatty acid oxidation in NASH is not known.
Using untargeted metabolomic analysis, we have previously reported that the plasma concentrations of bile acids, glycocholate, taurocholate, and glycochenodeoxycholate were significantly higher in patients with NASH compared with controls [12]. Bile acids, taurocholate, and chenodeoxycholate bind to farnesoid X receptor (FXR) and increase the expression of hepatic PPARα [13]. PPARα, in turn, induces fibroblast growth factor 21 (FGF21) that increases the transcription of enzymes responsible for fatty acid oxidation [14–17] (Fig. 1). Elevated plasma concentration of FGF21 has been reported in obesity, type 2 diabetes mellitus, and NAFLD [16,18–21].
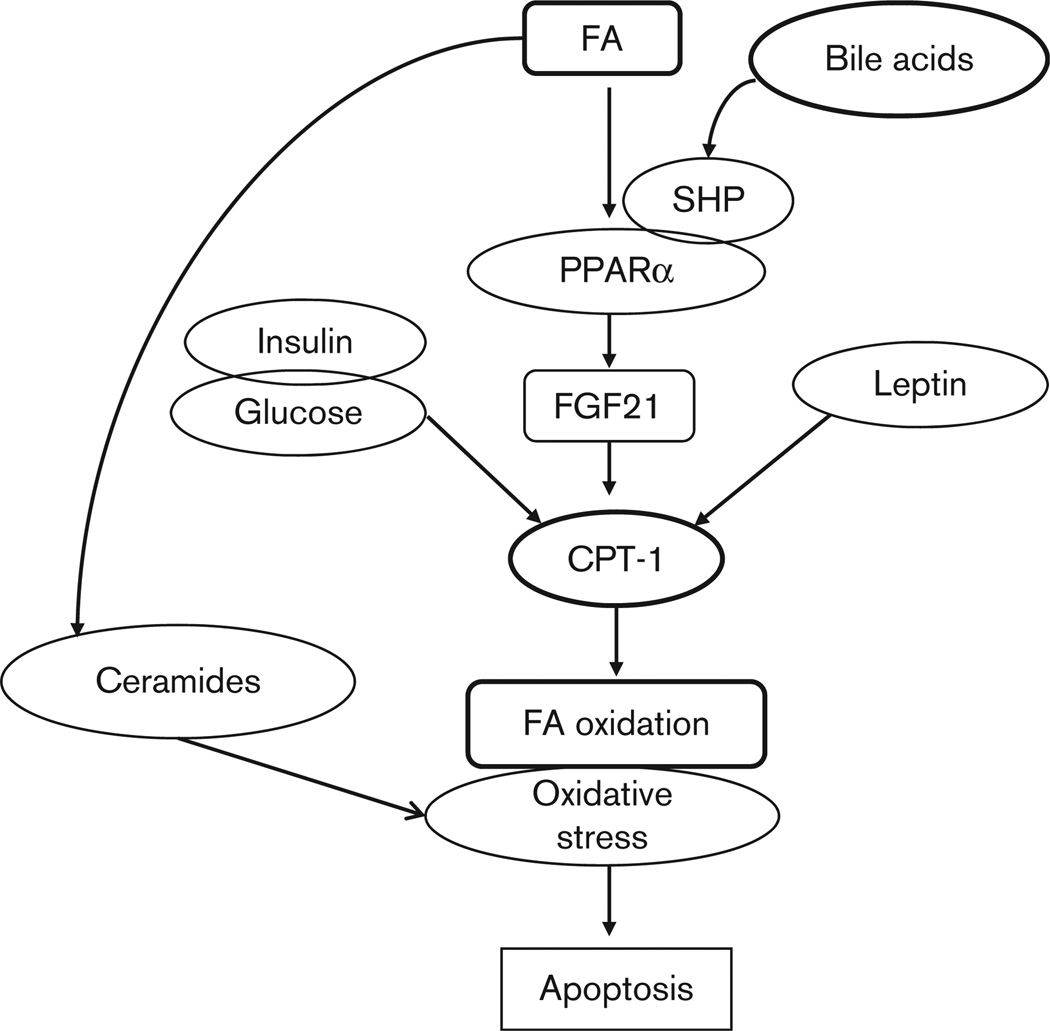
Fig. 1.
Schematic representation of putative sequence of metabolic abnormalities culminating in hepatocyte apoptosis in nonalcoholic steatohepatitis. The increased plasma bile acids have a permissive effect on peroxisome proliferator-activated receptor α (PPARα) whose downstream target fibroblast growth factor 21 (FGF21) is increased. FGF21 increases the expression of genes responsible for increased hepatic fatty acid (FA) oxidation that results in an increased oxidant stress and release of caspase-cleaved cytokeratin fragment 18 M30 (CK18 M30) into plasma. The high plasma fatty acid concentration additionally generates increased ceramides that aggravate the oxidant stress. CPT-1, carnitine palmitoyl transferase 1 (regulatory enzyme for mitochondrial fatty acid oxidation); SHP, short heterodimer partner.
Infusion of intralipid with heparin has been used to increase plasma fatty acid concentration in a reproducible manner [11,22,23]. As hepatic uptake of fatty acids depends on their plasma concentration, infusion of intralipid results in higher hepatic influx of fatty acids and consequently higher hepatic fatty acid oxidation and elevated plasma ketone body (BHB, acetoacetate, and acetone) levels [22,24]. In addition, an increase in the oxidation of fatty acids in the liver results in an increased generation of reactive oxygen species and oxidative stress [25,26]. We used fatty acid infusion to show an increase in hepatic fatty acid oxidation in NASH.
In this study, the response to fatty acid infusion was quantified in controls and patients with histologically proven NASH using the Kleiner et al. [27] criteria. We hypothesized that in patients with NASH, elevated plasma bile acids, by increasing the expression of PPARα and FGF21 would result in an increased oxidation of fatty acids and as a consequence, would result in greater oxidative stress evidenced by an increase in plasma 8-hydroxydeoxyguanosine (8 OHdG; Fig. 1). We also measured the plasma concentration of leptin, insulin, and glucose that may alter hepatic fatty acid oxidation [28]. During states of elevated plasma fatty acids, the excess fatty acids form the substrate for ceramides that have also been suggested to cause oxidative stress and hepatic injury [29]. Therefore, we measured the plasma concentrations of multiple ceramide subspecies in response to infusion of intralipid. Plasma concentration of cytokeratin 18 M30 has been used as a biomarker for the diagnosis of fatty liver [30]. Caspase cleavage of cytokeratin proteins at two distinct sites, Asp238 and Asp396, during apoptosis [31]. The M30 detection antibody recognizes a neoepitope mapped to positions 387–396 of CK18 and is believed to be a marker of apoptosis. The CK18 M65, on the other hand, detects a common epitope present in the full-length protein as well as in the caspase-cleaved product, and measures CK18 released intact from necrotic cells [32]. As CK18 M30 has been shown to reflect apoptosis, and plasma concentrations have been reported to be high in patients with NASH, we quantified this in the fasted state and to determine whether this is altered during the increased hepatic fatty acid oxidation during infusion of intralipid.
Methods
Studies were carried out in patients with NASH and healthy controls. The diagnosis of NASH was established in all patients by liver biopsy. Healthy controls were recruited by advertisement. A detailed clinical evaluation was done in each patient followed by a physical examination. Hepatic steatosis was excluded in controls by ultrasound examination (performed by the same investigator, Srinivasan Dasarathy) using previously established criteria [33]. The study protocol was approved by the Institutional Review Board of the Cleveland Clinic. Written informed consent was obtained from all patients after explaining the procedures completely.
Patients were studied after an overnight fast, after a weight-maintenance diet containing at least 75 g of protein per day for 7 days. On the study day, after 4 h of the basal period, intravenous infusion of lipid was administered for 4 h using 20% intralipid (linoleate 50%; oleate 26.5%, palmitate 10.5%, and stearate 3.5%) with heparin (0.2 U/L) at 40 ml/h (control, n = 16; NASH, n = 10). Some of these patients were part of another metabolic study reported previously [11]. We examined the blood samples obtained at 180 min before and after the start of infusion of intralipid. These time points were chosen because plasma fatty acids had reached a stable concentration and we were not determining the temporal changes in regulatory proteins in plasma. Plasma was separated immediately by centrifugation in cold and stored at −80°C for assays.
Analysis
Plasma free fatty acids (FFAs) were measured colorimetrically and BHB concentration was measured enzymatically using a spectrofluorometer [11]. Plasma leptin was quantified by radioimmunoassay (Linco Diagnostics, St Louis, Missouri, USA). Plasma insulin was measured in the Clinical Research Unit core laboratory of the Cleveland Clinic using a standard enzyme-linked immunosorbent assay (ELISA) method. Plasma FGF21 was quantified using the ELISA kits (Biovendor Laboratory Medicine, Czech Republic) using the manufacturer’s protocol. This assay has been reported to be highly specific to human FGF21 and does not cross-react with other members of the FGF family [21]. The reported intra-assay and interassay variations were 5.5 and 8.9%, respectively. 8 OHdG was quantified by a competitive ELISA using the manufacturer-recommended protocol. The M30 apoptosome ELISA assay (Peviva, Bromma, Sweden) was used to quantify the plasma concentration of apoptosis-associated CK18 Asp396 neoepitope. Changes in concentrations of ceramide species were measured in a subgroup of patients using methods reported earlier [34]. In brief, after Bligh and Dyer extraction and silica gel column isolation, ceramides were analyzed by liquid chromatography/electrospray spin ionization/mass spectrometry/mass spectrometry. Plasma levels of ceramides were measured in six patients with histologically confirmed NASH and six healthy controls before and after intralipid infusion. The separation and quantification of nine endogenous long-chain and very-long-chain ceramides using two nonphysiological odd-chain ceramide (C17 and C25) internal standards were achieved within a single 21-min chromatographic run. Plasma bile acids were quantified (n = 36 in NASH and n = 38 in controls) using liquid chromatography mass spectrometry/mass spectrometry [35].
Statistical methods
All data are reported as mean ± standard deviation unless specified. Quantitative variables were compared using Student’s t-test for data with a normal distribution. Skewed data were compared using the Mann–Whitney test. Comparison of the data in the same patient during the fasted state and in response to intralipid infusion was done using the paired t-test. A P value of less than 0.05 was considered significant.
Results
The clinical and biochemical characteristics of the patients are shown in Table 1. There were no age or sex differences between patients with NASH and healthy controls as they were matched for these criteria. Patients with NASH had higher BMI, higher concentration of plasma aspartate aminotransferase, alanine aminotransferase (ALT), triglycerides, and lower high-density lipoprotein compared with controls. They were also significantly (P < 0.01) more insulin resistant than controls as evidenced by their higher homeostatic model of assessment scores.
Table 1.
Clinical and biochemical characteristics of patients
| Control | NASH | |
|---|---|---|
| n | 16 | 10 |
| Age (years, mean ± SD) | 41.9 ± 16.0 | 43.3 ± 7.04 |
| Sex (male : female) | 8 : 8 | 5 : 5 |
| BMI (kg/m2) | 29.1 ± 5.5 | 34.0 ± 5.0** |
| AST (IU/l) | 20.8 ± 5.0 | 39.5 ± 19.7** |
| ALT (IU/l) | 16.1 ± 5.7 | 52.0 ± 28.8* |
| Triglycerides (mg/dl) | 93.1 ± 38.7 | 196.4 ± 119.4* |
| HDL (mg/dl) | 52.0 ± 12.7 | 41.0 ± 9.5* |
| HOMA | 0.76 ± 0.26 | 3.4 ± 2.03* |
ALT, alanine aminotransferase; AST, aspartate aminotransferase; HDL, high-density lipoproteins; HOMA, homeostatic model of assessment; NASH, nonalcoholic steatohepatitis; SD, standard deviation.
P<0.05.
P<0.01.
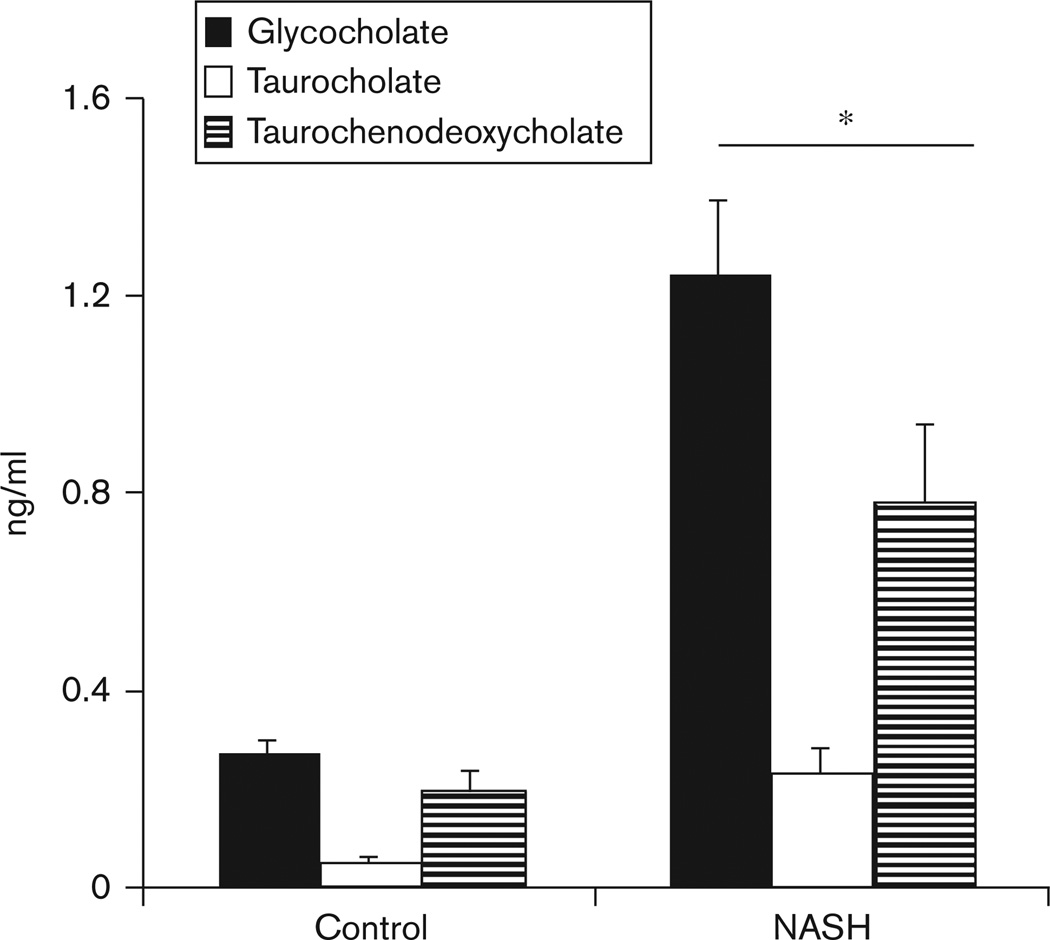
The plasma concentration of bile acids (glycocholate, taurocholate, and taurochenodeoxycholate) during fasting was significantly higher in patients with NASH (P < 0.01; Fig. 2).
Fig. 2.
Plasma concentrations of glycocholate, taurocholate, and taurochenodeoxycholate in patients with biopsy-proven nonalcoholic steatohepatitis (NASH; n = 36), were significantly higher than that in healthy controls (n = 38). *P < 0.01 for each of the bile acids between NASH and controls.
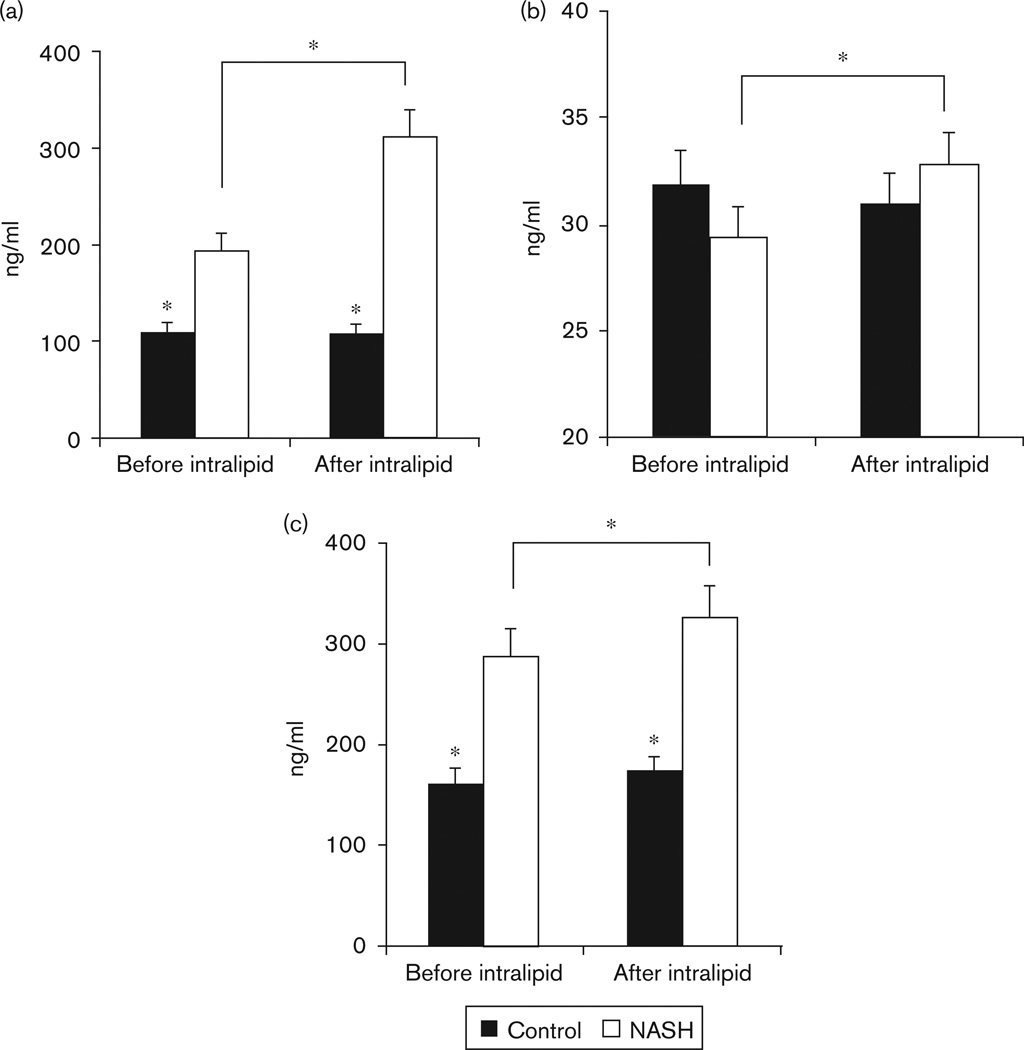
In the fasted state, plasma concentration of FGF21 (Fig. 3a) and leptin (Table 2) were significantly higher (P < 0.01) in patients with NASH compared with controls. Plasma concentration of 8 OHdG was not significantly different in the two groups. CK18 levels were significantly (P < 0.01) higher in patients with NASH compared with controls.
Fig. 3.
(a) Plasma concentration of fibroblast growth factor 21 (FGF21) in healthy controls and patients with nonalcoholic steatohepatitis (NASH) before and after infusion of intralipid and heparin. Plasma concentration of FGF21 was significantly higher in patients with NASH compared with controls and increased further only in patients with NASH (*P<0.01). (b) Plasma concentrations of 8 hydroxy deoxyguanosine (8 OHdG) in healthy controls and patients with NASH are shown. No significant difference was observed in the fasted state between controls and NASH. In response to infusion of intralipid, plasma concentration of 8 OHdG increased only in patients with NASH (*P < 0.05). (c) Plasma concentration of CK18 M30 fragment in healthy controls and patients with NASH is shown. In the fasting state, patients with NASH had significantly higher plasma concentration of CK18 M30 fragment compared with controls. In response to an infusion of intralipid with heparin, plasma concentration of CK18 increased further only in patients with NASH (*P<0.01).
Table 2.
Response to intralipid
| Control (n = 16) | NASH (n = 10) | |||
|---|---|---|---|---|
| Before | After | Before | After | |
| Fatty acid (mmol/l) | 0.6 ± 0.29 | 1.1 ± 0.3** | 0.5 ± 0.2 | 1.04 ± 0.4** |
| BHB (mmol/l) | 0.3 ± 0.2 | 0.6 ± 0.3** | 0.4 ± 0.2 | 0.9 ± 0.3** |
| BHB/FFA ratio | 0.64 ± 0.46 | 0.59 ± 0.37 | 0.89 ± 0.55 | 0.90 ± 0.25*** |
| Insulin (µU/l) | 11.98 ± 3.97 | 10.59 ± 2.46 | 29.6 ± 12.3* | 24.17 ± 12.16* |
| Glucose (mg/dl) | 87.8 ± 7.6 | 84.9 ± 5.8 | 94.5 ± 8.0 | 85.4 ± 8.0 |
| Leptin (ng/ml) | 15.9 ± 14.9 | 14.5 ± 14.5 | 30.1 ± 22.9* | 30.3 ± 21.8* |
| ALT (U/l) | 18.6 ± 12.6 | 17.9 ± 12.0 | 40.0 ± 26.5* | 41.8 ± 28.8* |
ALT, alanine amino transferase; BHB, β-hydroxybutyrate; FFA, free fatty acid; NASH, nonalcoholic steatohepatitis.
P < 0.01 between patients with NASH and controls.
P < 0.05 before and after intralipid infusion.
P < 0.05 between controls and NASH after intralipid infusion.
In response to infusion of intralipid with heparin, there was an expected increase (P < 0.001) in plasma concentration of fatty acids and BHB in both groups. The ratio of BHB to FFAs was significantly (P < 0.05) higher in NASH compared with controls. Plasma concentrations of leptin, insulin, and glucose did not change in either group after intralipid infusion (Table 2). During intralipid infusion, the plasma concentration of FGF21 increased significantly (P = 0.007) in patients with NASH but not in controls (Fig. 3a). Plasma concentration of various ceramide species is shown in Table 3. During fasting, the total ceramide concentration was similar in patients with NASH and controls. The concentrations of C22 and C24 : 1 ceramides were significantly higher (P < 0.05) in patients with NASH. In response to intralipid infusion, total ceramide concentration increased significantly (P < 0.05) only in patients with NASH. Plasma concentrations of 8 OHdG (Fig. 3b) and of CK18 M30 fragment (Fig. 3c) increased significantly in response to intralipid only in the patients with NASH. There was no change in plasma ALT concentration in response to intralipid infusion in either group (Table 2).
Table 3.
Plasma ceramide subspecies in response to intralipid infusion
| Control | NASH | |||
|---|---|---|---|---|
| Before | After | Before | After | |
| C12 | 9.1 ± 4.5 | 7.49 ± 2.8 | 9.59 ± 7.0 | 6.07 ± 3.0 |
| C14 | 0.19 ± 0.08 | 0.12 ± 0.03 | 0.22 ± 0.17 | 0.20 ± 0.19 |
| C16 | 1.42 ± 0.51 | 1.47 ± 0.60 | 1.40 ± 0.53 | 1.76 ± 1.22 |
| C18 | 0.44 ± 0.20 | 0.34 ± 0.20 | 0.56 ± 0.29 | 0.68 ± 0.09 |
| C18 : 1 | 0.10 ± 0.07 | 0.07 ± 0.07 | 0.08 ± 0.06 | 0.09 ± 0.05 |
| C20 | 0.09 ± 0.02 | 0.16 ± 0.06 | 0.10 ± 0.03 | 0.12 ± 0.04 |
| C22 | 1.68 ± 0.34 | 1.79 ± 0.36 | 2.67 ± 0.47 | 3.10 ± 0.59** |
| C24 | 1.65 ± 0.35 | 1.80 ± 0.27 | 2.14 ± 0.42 | 2.33 ± 0.54 |
| C24 : 1 | 1.05 ± 0.43 | 1.37 ± 0.51 | 1.29 ± 0.34 | 1.98 ± 0.66** |
| Total | 7.5 ± 1.5 | 8.1 ± 1.1 | 7.1 ± 1.7 | 10.0 ± 2.5* |
NASH, nonalcoholic steatohepatitis.
P < 0.05 between before and after intralipid infusion.
P < 0.01 between before and after intralipid infusion.
Discussion
In this study, we have confirmed our previous observation of higher plasma BHB and FFA ratio in patients with NASH suggesting greater hepatic fatty acid oxidation. We also confirmed our previous report that plasma concentrations of bile acids are elevated during fasting in patients with NASH [12]. Plasma concentrations of fasting FGF21, CK18 M30 fragment, and 8 OHdG were higher and increased further in response to intralipid only in patients with NASH. These data provide evidence that hepatic oxidation of fatty acids is higher in NASH, possibly mediated by bile acids through the FGF21 pathway (Fig. 1) and may contribute to the oxidative injury and progression of NASH.
These data suggest higher fatty acid oxidation in the liver of patients with NASH. As ketone bodies are released into the plasma from only the liver [36,37], the higher BHB/fatty acid ratio suggests that the site of increased oxidation of fatty acids is the liver. These data are also consistent with the recent data in obese patients with an increased hepatic fatty acid oxidation seen using 11C palmitate positron emission tomogram [38]. However, the mechanism of an increased hepatic fatty acid oxidation in NASH is unclear. Transcriptional regulation of the genes involved in the oxidation of fatty acids is mediated by the activation of PPARα. Bile acids are endogenous ligands of PPARα. Bile acids induce hepatic PPARα by binding to their receptor, FXR [13]. The increase in the expression of PPARα in human hepatocyte cell lines with both chenodeoxycholic acid and taurocholic acid was transcriptionally regulated by the binding of FXR to the promoter of the PPARα. Critical enzymes for hepatic fatty acid oxidation are regulated by PPARα activation [39]. We speculate that the elevated bile acids in patients with NASH result in an increase in PPARα expression. In response to fatty acid infusion, there is a further increase in PPARα activation, which results in an elevated plasma concentration of FGF21, the immediate downstream target of PPARα [17,40,41]. Elevated plasma FGF21 concentrations in patients with NASH may be responsible for the increased hepatic fatty acid oxidation [8,9,11].
FGF21 induces hepatic fatty acid oxidation in animal models and cell cultures by transcriptional regulation of key enzymes of fatty acid oxidation [42,43]. In this study, plasma FGF21 concentration was higher in NASH than in controls. Similar data have been reported by others recently [19,20]. Intralipid infusion was accompanied by an increase in plasma FGF21 only in patients with NASH. These data suggest that NASH may be an FGF21-resistant state, similar to that recently reported in obesity [19,44]. The elevated plasma bile acids in NASH may also have an additive or synergistic effect to PPARα activated by fatty acids with greater FGF21 response to fatty acid infusion. This also explains the greater hepatic fatty acid oxidation in NASH with higher plasma FGF21 compared with controls.
Leptin has also been shown to increase hepatic fatty acid oxidation by the activation of critical cellular energy sensor, AMP kinase [28,45]. Our studies showed that even though fasting leptin concentrations in patients with NASH were significantly higher than that in controls, there was no change in plasma leptin in response to infusion of intralipid in either group. These observations were similar to those reported earlier that plasma leptin concentrations are not altered in response to infusion of intralipid [46,47]. Higher plasma leptin concentration may contribute to the increased hepatic fatty acid oxidation in the fasted state. However, we did not observe significantly higher plasma BHB in patients with NASH compared with controls (P = 0.08), the dominant role of leptin may be on the skeletal muscle. Furthermore, plasma leptin concentration did not change after intralipid infusion, and this suggests that the increase in hepatic fatty acid oxidation after intralipid infusion is due to other mechanisms including an increase in FGF21.
Increased hepatic fatty acid oxidation during infusion of fatty acids generates more reducing equivalents (NADH) and greater oxidative stress, as evidenced by higher 8 OHdG in NASH in this study [48,49]. Plasma ceramides increased in patients with NASH compared with controls during intralipid infusion and these may also contribute to the oxidative injury [50]. Animal studies have suggested that high-fat diet increases hepatic ceramide content and this is reflected by elevated plasma ceramide concentration [50,51]. Our observations of an increase in plasma ceramide after intralipid infusion suggest that the increase in hepatic entry of fatty acids contributes to ceramide synthesis. In the fasted state, however, there was no difference in plasma ceramide concentration between NASH and controls that was similar to a previous report that patients with NAFLD did not have higher hepatic ceramide concentration [52].
Our observations showed that in the fasted state, plasma concentration of 8 OHdG was similar in NASH and controls that was similar to previous reports [11,25]. However, after infusion of fatty acids, plasma 8 OHdG was increased significantly only in NASH suggesting more oxidant stress and injury in this group. This is consistent with our previous report, which states that plasma glutathione concentration (that is also a measure of increased hepatic oxidant stress) increased after intralipid infusion only in NASH [11]. In contrast, plasma 8 OHdG did not change in controls that may be related to either a lower rate of hepatic fatty acid oxidation or better hepatic antioxidant defenses. These studies show that measures of oxidant injury were not different in the fasted state, but these differences became overt during an infusion of fatty acids with greater hepatic oxidation.
Increased oxidant injury was accompanied by greater increase in the plasma concentration of caspase-cleaved CK18 M30 fragment in NASH compared with controls during infusion of intralipid. This reflects hepatocyte apoptosis that results from an activation of the caspase system that cleaves the cytokeratin 18. Cytokeratin 18 is the major intermediate filament protein in the liver and the major substrate of caspases during apoptosis [31,53]. Bile acids can also activate caspases and hepatocyte apoptosis [54], and higher plasma bile acids in NASH may also contribute to the higher plasma CK18 M30 fragment in these patients. The increase in plasma CK18 M30 after an infusion of intralipid in NASH was not accompanied by an increase in plasma ALT, a measure of hepatocellular necrosis and death. There could be a number of potential reasons for this observation. The lack of increase of plasma ALT despite an increase in CK18, suggests that the caspase-cleavage system activated during apoptosis is reversible [54] or that the release of ALT requires necrotic cell death that is different from apoptosis [55,56]. Alternatively, in response to infusion of intralipid, plasma CK18 M30 concentration did not increase sufficiently to result in an elevation in plasma ALT concentration. This is supported by previous reports that plasma ALT is not a sensitive measure of hepatocellular injury on liver biopsy [57]. Another possible reason for the lack of concordance between the increase in CK18 M30 and ALT after intralipid infusion is that a measurement of ALT at a single time point may not be a sensitive measure of hepatocellular injury.
In this study, all our measurements were taken only at a single time point in the fasted state and in response to intralipid. As mentioned earlier, these time points were chosen because plasma concentrations of fatty acids and BHB were at a steady state at the times chosen. Furthermore, we were not examining the temporal changes in response to infusion of fatty acids and our aim was to determine whether bile acids and FGF21, both regulators of hepatic fatty acid metabolism could be related to the change in hepatic fatty acid oxidation.
We conclude that an infusion of fatty acids permits us to examine the metabolic disturbances in NASH that provide a potential mechanism for the progression of hepatic injury. Our observations suggest that the elevated fasting plasma bile acids may have a permissive effect on PPARα with an increase in FGF21, and greater hepatic fatty acid oxidation. A higher rate of fatty acid oxidation would result in oxidative stress and consequent oxidant injury and activation of the caspase-cleavage system (increase in CK18 M30 fragment). The lack of change in plasma ALT suggests a potentially reversible abnormality. Studies in animal models and in-vitro cell culture system are needed to establish that this sequence of events mediates the progression of injury in NASH.
Acknowledgement
The authors thank the nurses and laboratory staff of the Clinical Research Unit for their help with the studies and Joyce Nolan for secretarial assistance. This work was supported by NIH grants DK079937 to Satish C. Kalhan and CTSA 1UL1 RR024989 to Case Western Reserve University.
Footnotes
Conflict of interest: none declared.
References
- 1.Kotronen A, Seppala-Lindroos A, Vehkavaara S, Bergholm R, Frayn KN, Fielding BA, et al. Liver fat and lipid oxidation in humans. Liver Int. 2009;29:1439–1446. doi: 10.1111/j.1478-3231.2009.02076.x. [DOI] [PubMed] [Google Scholar]
- 2.Miele L, Grieco A, Armuzzi A, Candelli M, Forgione A, Gasbarrini A, et al. Hepatic mitochondrial beta-oxidation in patients with nonalcoholic steatohepatitis assessed by 13C-octanoate breath test. Am J Gastroenterol. 2003;98:2335–2336. doi: 10.1111/j.1572-0241.2003.07725.x. [DOI] [PubMed] [Google Scholar]
- 3.Nakamuta M, Kohjima M, Higuchi N, Kato M, Kotoh K, Yoshimoto T, et al. The significance of differences in fatty acid metabolism between obese and nonobese patients with non-alcoholic fatty liver disease. Int J Mol Med. 2008;22:663–667. [PubMed] [Google Scholar]
- 4.Perez-Carreras M, Del HP, Martin MA, Rubio JC, Martin A, Castellano G, et al. Defective hepatic mitochondrial respiratory chain in patients with nonalcoholic steatohepatitis. Hepatology. 2003;38:999–1007. doi: 10.1053/jhep.2003.50398. [DOI] [PubMed] [Google Scholar]
- 5.Boles RG, Martin SK, Blitzer MG, Rinaldo P. Biochemical diagnosis of fatty acid oxidation disorders by metabolite analysis of postmortem liver. Hum Pathol. 1994;25:735–741. doi: 10.1016/0046-8177(94)90240-2. [DOI] [PubMed] [Google Scholar]
- 6.Hashimoto T, Cook WS, Qi C, Yeldandi AV, Reddy JK, Rao MS. Defect in peroxisome proliferator-activated receptor alpha-inducible fatty acid oxidation determines the severity of hepatic steatosis in response to fasting. J Biol Chem. 2000;275:28918–28928. doi: 10.1074/jbc.M910350199. [DOI] [PubMed] [Google Scholar]
- 7.Fan CY, Pan J, Usuda N, Yeldandi AV, Rao MS, Reddy JK. Steatohepatitis, spontaneous peroxisome proliferation and liver tumors in mice lacking peroxisomal fatty acyl-CoA oxidase: implications for peroxisome proliferator-activated receptor alpha natural ligand metabolism. J Biol Chem. 1998;273:15639–15645. doi: 10.1074/jbc.273.25.15639. [DOI] [PubMed] [Google Scholar]
- 8.Sanyal AJ, Campbell-Sargent C, Mirshahi F, Rizzo WB, Contos MJ, Sterling RK, et al. Nonalcoholic steatohepatitis: association of insulin resistance and mitochondrial abnormalities. Gastroenterology. 2001;120:1183–1192. doi: 10.1053/gast.2001.23256. [DOI] [PubMed] [Google Scholar]
- 9.Chalasani N, Gorski JC, Asghar MS, Asghar A, Foresman B, Hall SD, et al. Hepatic cytochrome P450 2E1 activity in nondiabetic patients with nonalcoholic steatohepatitis. Hepatology. 2003;37:544–550. doi: 10.1053/jhep.2003.50095. [DOI] [PubMed] [Google Scholar]
- 10.Bugianesi E, Gastaldelli A, Vanni E, Gambino R, Cassader M, Baldi S, et al. Insulin resistance in non-diabetic patients with non-alcoholic fatty liver disease: sites and mechanisms. Diabetologia. 2005;48:634–642. doi: 10.1007/s00125-005-1682-x. [DOI] [PubMed] [Google Scholar]
- 11.Dasarathy S, Kasumov T, Edmison JM, Gruca LL, Bennett C, Duenas C, et al. Glycine and urea kinetics in non-alcoholic steatohepatitis in human: effect of intralipid infusion. Am J Physiol Gastrointest Liver Physiol. 2009;297:G567–G575. doi: 10.1152/ajpgi.00042.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kalhan SC, Guo L, Edmison J, Dasarathy S, McCullough AJ, Hanson RW, et al. Plasma metabolomic profile in nonalcoholic fatty liver disease. Metabolism. 2010;60:404–413. doi: 10.1016/j.metabol.2010.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pineda TI, Claudel T, Duval C, Kosykh V, Fruchart JC, Staels B. Bile acids induce the expression of the human peroxisome proliferator-activated receptor alpha gene via activation of the farnesoid X receptor. Mol Endocrinol. 2003;17:259–272. doi: 10.1210/me.2002-0120. [DOI] [PubMed] [Google Scholar]
- 14.Louet JF, Le MC, Pegorier JP, Decaux JF, Girard J. Regulation of liver carnitine palmitoyltransferase I gene expression by hormones and fatty acids. Biochem Soc Trans. 2001;29:310–316. doi: 10.1042/0300-5127:0290310. [DOI] [PubMed] [Google Scholar]
- 15.Oishi K, Uchida D, Ishida N. Circadian expression of FGF21 is induced by PPARalpha activation in the mouse liver. FEBS Lett. 2008;582:3639–3642. doi: 10.1016/j.febslet.2008.09.046. [DOI] [PubMed] [Google Scholar]
- 16.Cuevas-Ramos D, Almeda-Valdes P, Aguilar-Salinas CA, Cuevas-Ramos G, Cuevas-Sosa AA, Gomez-Perez FJ. The role of fibroblast growth factor 21 (FGF21) on energy balance, glucose and lipid metabolism. Curr Diabetes Rev. 2009;5:216–220. doi: 10.2174/157339909789804396. [DOI] [PubMed] [Google Scholar]
- 17.Inagaki T, Dutchak P, Zhao G, Ding X, Gautron L, Parameswara V, et al. Endocrine regulation of the fasting response by PPARalpha-mediated induction of fibroblast growth factor 21. Cell Metab. 2007;5:415–425. doi: 10.1016/j.cmet.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 18.Zhang X, Yeung DC, Karpisek M, Stejskal D, Zhou ZG, Liu F, et al. Serum FGF21 levels are increased in obesity and are independently associated with the metabolic syndrome in humans. Diabetes. 2008;57:1246–1253. doi: 10.2337/db07-1476. [DOI] [PubMed] [Google Scholar]
- 19.Dushay J, Chui PC, Gopalakrishnan GS, Varela-Rey M, Crawley M, Fisher FM, et al. Increased fibroblast growth factor 21 in obesity and nonalcoholic fatty liver disease. Gastroenterology. 2010;139:456–463. doi: 10.1053/j.gastro.2010.04.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yilmaz Y, Eren F, Yonal O, Kurt R, Aktas B, Celikel CA, et al. Increased serum FGF21 levels in patients with nonalcoholic fatty liver disease. Eur J Clin Invest. 2010;40:887–892. doi: 10.1111/j.1365-2362.2010.02338.x. [DOI] [PubMed] [Google Scholar]
- 21.Li H, Bao Y, Xu A, Pan X, Lu J, Wu H, et al. Serum fibroblast growth factor 21 is associated with adverse lipid profiles and gamma-glutamyltransferase but not insulin sensitivity in Chinese subjects. J Clin Endocrinol Metab. 2009;94:2151–2156. doi: 10.1210/jc.2008-2331. [DOI] [PubMed] [Google Scholar]
- 22.Gormsen LC, Gjedsted J, Gjedde S, Norrelund H, Christiansen JS, Schmitz O, et al. Dose-response effects of free fatty acids on amino acid metabolism and ureagenesis. Acta Physiol (Oxf) 2008;192:369–379. doi: 10.1111/j.1748-1716.2007.01771.x. [DOI] [PubMed] [Google Scholar]
- 23.Boden G, Chen X, Rosner J, Barton M. Effects of a 48-h fat infusion on insulin secretion and glucose utilization. Diabetes. 1995;44:1239–1242. doi: 10.2337/diab.44.10.1239. [DOI] [PubMed] [Google Scholar]
- 24.Keller U, Gerber PP, Stauffacher W. Fatty acid-independent inhibition of hepatic ketone body production by insulin in humans. Am J Physiol. 1988;254:E694–E699. doi: 10.1152/ajpendo.1988.254.6.E694. [DOI] [PubMed] [Google Scholar]
- 25.Machado MV, Ravasco P, Jesus L, Marques-Vidal P, Oliveira CR, Proenca T, et al. Blood oxidative stress markers in non-alcoholic steatohepatitis and how it correlates with diet. Scand J Gastroenterol. 2008;43:95–102. doi: 10.1080/00365520701559003. [DOI] [PubMed] [Google Scholar]
- 26.Haque M, Sanyal AJ. The metabolic abnormalities associated with non-alcoholic fatty liver disease. Best Pract Res Clin Gastroenterol. 2002;16:709–731. doi: 10.1053/bega.2002.0325. [DOI] [PubMed] [Google Scholar]
- 27.Kleiner DE, Brunt EM, Van NM, Behling C, Contos MJ, Cummings OW, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–1321. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 28.Wein S, Ukropec J, Gasperikova D, Klimes I, Sebokova E. Concerted action of leptin in regulation of fatty acid oxidation in skeletal muscle and liver. Exp Clin Endocrinol Diabetes. 2007;115:244–251. doi: 10.1055/s-2007-956166. [DOI] [PubMed] [Google Scholar]
- 29.Garcia-Ruiz C, Colell A, Mari M, Morales A, Fernandez-Checa JC. Direct effect of ceramide on the mitochondrial electron transport chain leads to generation of reactive oxygen species: role of mitochondrial glutathione. J Biol Chem. 1997;272:11369–11377. doi: 10.1074/jbc.272.17.11369. [DOI] [PubMed] [Google Scholar]
- 30.Wieckowska A, Zein NN, Yerian LM, Lopez AR, McCullough AJ, Feldstein AE. In-vivo assessment of liver cell apoptosis as a novel biomarker of disease severity in nonalcoholic fatty liver disease. Hepatology. 2006;44:27–33. doi: 10.1002/hep.21223. [DOI] [PubMed] [Google Scholar]
- 31.Yilmaz Y. Systematic review: caspase-cleaved fragments of cytokeratin 18, the promises and challenges of a biomarker for chronic liver disease. Aliment Pharmacol Ther. 2009;30:1103–1109. doi: 10.1111/j.1365-2036.2009.04148.x. [DOI] [PubMed] [Google Scholar]
- 32.Kramer G, Erdal H, Mertens HJ, Nap M, Mauermann J, Steiner G, et al. Differentiation between cell death modes using measurements of different soluble forms of extracellular cytokeratin 18. Cancer Res. 2004;64:1751–1756. doi: 10.1158/0008-5472.can-03-2455. [DOI] [PubMed] [Google Scholar]
- 33.Dasarathy S, Dasarathy J, Khiyami A, Joseph R, Lopez R, McCullough AJ. Validity of real time ultrasound in the diagnosis of hepatic steatosis: a prospective study. J Hepatol. 2009;51:1061–1067. doi: 10.1016/j.jhep.2009.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kasumov T, Huang H, Chung YM, Zhang R, McCullough AJ, Kirwan JP. Quantification of ceramide species in biological samples by liquid chromatography electrospray ionization tandem mass spectrometry. Anal Biochem. 2010;401:154–161. doi: 10.1016/j.ab.2010.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tagliacozzi D, Mozzi AF, Casetta B, Bertucci P, Bernardini S, Di IC, et al. Quantitative analysis of bile acids in human plasma by liquid chromatography-electrospray tandem mass spectrometry: a simple and rapid one-step method. Clin Chem Lab Med. 2003;41:1633–1641. doi: 10.1515/CCLM.2003.247. [DOI] [PubMed] [Google Scholar]
- 36.Foster DW, McGarry JD. The regulation of ketogenesis. Ciba Found Symp. 1982;87:120–131. doi: 10.1002/9780470720691.ch7. [DOI] [PubMed] [Google Scholar]
- 37.McGarry JD, Foster DW. Regulation of hepatic fatty acid oxidation and ketone body production. Annu Rev Biochem. 1980;49:395–420. doi: 10.1146/annurev.bi.49.070180.002143. [DOI] [PubMed] [Google Scholar]
- 38.Iozzo P, Bucci M, Roivainen A, Nagren K, Jarvisalo MJ, Kiss J, et al. Fatty acid metabolism in the liver, measured by positron emission tomography, is increased in obese individuals. Gastroenterology. 2010;139:846–856. doi: 10.1053/j.gastro.2010.05.039. [DOI] [PubMed] [Google Scholar]
- 39.Louet JF, Chatelain F, Decaux JF, Park EA, Kohl C, Pineau T, et al. Long-chain fatty acids regulate liver carnitine palmitoyltransferase I gene (L-CPT I) expression through a peroxisome-proliferator-activated receptor alpha (PPARalpha)-independent pathway. Biochem J. 2001;354:189–197. doi: 10.1042/0264-6021:3540189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reitman ML. FGF21: a missing link in the biology of fasting. Cell Metab. 2007;5:405–407. doi: 10.1016/j.cmet.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 41.Potthoff MJ, Inagaki T, Satapati S, Ding X, He T, Goetz R, et al. FGF21 induces PGC-1alpha and regulates carbohydrate and fatty acid metabolism during the adaptive starvation response. Proc Natl Acad Sci U S A. 2009;106:10853–10858. doi: 10.1073/pnas.0904187106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Badman MK, Pissios P, Kennedy AR, Koukos G, Flier JS, Maratos-Flier E. Hepatic fibroblast growth factor 21 is regulated by PPARalpha and is a key mediator of hepatic lipid metabolism in ketotic states. Cell Metab. 2007;5:426–437. doi: 10.1016/j.cmet.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 43.Badman MK, Koester A, Flier JS, Kharitonenkov A, Maratos-Flier E. Fibroblast growth factor 21-deficient mice demonstrate impaired adaptation to ketosis. Endocrinology. 2009;150:4931–4940. doi: 10.1210/en.2009-0532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fisher FM, Chui PC, Antonellis PJ, Bina HA, Kharitonenkov A, Flier JS, et al. Obesity is a fibroblast growth factor 21 (FGF21)-resistant state. Diabetes. 2010;59:2781–2789. doi: 10.2337/db10-0193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Huang W, Dedousis N, Bandi A, Lopaschuk GD, O’Doherty RM. Liver triglyceride secretion and lipid oxidative metabolism are rapidly altered by leptin in vivo. Endocrinology. 2006;147:1480–1487. doi: 10.1210/en.2005-0731. [DOI] [PubMed] [Google Scholar]
- 46.Stingl H, Raffesberg W, Nowotny P, Waldhausl W, Roden M. Reduction of plasma leptin concentrations by arginine but not lipid infusion in humans. Obes Res. 2002;10:1111–1119. doi: 10.1038/oby.2002.151. [DOI] [PubMed] [Google Scholar]
- 47.Peino R, Fernandez AJ, Penalva A, Considine RV, Rodriguez-Segade S, Rodriguez-Garcia J, et al. Acute changes in free-fatty acids (FFA) do not alter serum leptin levels. J Endocrinol Invest. 1998;21:526–530. doi: 10.1007/BF03347339. [DOI] [PubMed] [Google Scholar]
- 48.Scholz R, Schwabe U, Soboll S. Influence of fatty acids on energy metabolism. 1. Stimulation of oxygen consumption, ketogenesis and CO2 production following addition of octanoate and oleate in perfused rat liver. Eur J Biochem. 1984;141:223–230. doi: 10.1111/j.1432-1033.1984.tb08179.x. [DOI] [PubMed] [Google Scholar]
- 49.Soboll S, Grundel S, Schwabe U, Scholz R. Influence of fatty acids on energy metabolism. 2. Kinetics of changes in metabolic rates and changes in subcellular adenine nucleotide contents and pH gradients following addition of octanoate and oleate in perfused rat liver. Eur J Biochem. 1984;141:231–236. doi: 10.1111/j.1432-1033.1984.tb08180.x. [DOI] [PubMed] [Google Scholar]
- 50.Chocian G, Chabowski A, Zendzian-Piotrowska M, Harasim E, Lukaszuk B, Gorski J. High fat diet induces ceramide and sphingomyelin formation in rat’s liver nuclei. Mol Cell Biochem. 2010;340:125–131. doi: 10.1007/s11010-010-0409-6. [DOI] [PubMed] [Google Scholar]
- 51.Ichi I, Nakahara K, Kiso K, Kojo S. Effect of dietary cholesterol and high fat on ceramide concentration in rat tissues. Nutrition. 2007;23:570–574. doi: 10.1016/j.nut.2007.04.014. [DOI] [PubMed] [Google Scholar]
- 52.Kotronen A, Seppanen-Laakso T, Westerbacka J, Kiviluoto T, Arola J, Ruskeepaa AL, et al. Hepatic stearoyl-CoA desaturase (SCD)-1 activity and diacylglycerol but not ceramide concentrations are increased in the nonalcoholic human fatty liver. Diabetes. 2009;58:203–208. doi: 10.2337/db08-1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Linder S, Havelka AM, Ueno T, Shoshan MC. Determining tumor apoptosis and necrosis in patient serum using cytokeratin 18 as a biomarker. Cancer Lett. 2004;214:1–9. doi: 10.1016/j.canlet.2004.06.032. [DOI] [PubMed] [Google Scholar]
- 54.Wang K, Brems JJ, Gamelli RL, Ding J. Reversibility of caspase activation and its role during glycochenodeoxycholate-induced hepatocyte apoptosis. J Biol Chem. 2005;280:23490–23495. doi: 10.1074/jbc.M411607200. [DOI] [PubMed] [Google Scholar]
- 55.Kerr JF, Wyllie AH, Currie AR. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972;26:239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wyllie AH, Kerr JF, Currie AR. Cell death: the significance of apoptosis. Int Rev Cytol. 1980;68:251–306. doi: 10.1016/s0074-7696(08)62312-8. [DOI] [PubMed] [Google Scholar]
- 57.Luo JC, Hwang SJ, Lai CR, Lu CL, Li CP, Tsay SH, et al. Relationships between serum aminotransferase levels, liver histologies and virological status in patients with chronic hepatitis C in Taiwan. J Gastroenterol Hepatol. 1998;13:685–690. doi: 10.1111/j.1440-1746.1998.tb00714.x. [DOI] [PubMed] [Google Scholar]