Abstract
Otoacoustic emissions (sound emitted from the ear) allow cochlear function to be probed noninvasively. The emissions evoked by pure tones, known as stimulus-frequency emissions (SFOAEs), have been shown to provide reliable estimates of peripheral frequency tuning in a variety of mammalian and non-mammalian species. Here, we apply the same methodology to explore peripheral auditory function in the largest member of the cat family, the tiger (Panthera tigris). We measured SFOAEs in 9 unique ears of 5 anesthetized tigers. The tigers, housed at the Henry Doorly Zoo (Omaha, NE), were of both sexes and ranged in age from 3 to 10 years. SFOAE phase-gradient delays are significantly longer in tigers—by approximately a factor of two above 2 kHz and even more at lower frequencies—than in domestic cats (Felis catus), a species commonly used in auditory studies. Based on correlations between tuning and delay established in other species, our results imply that cochlear tuning in the tiger is significantly sharper than in domestic cat and appears comparable to that of humans. Furthermore, the SFOAE data indicate that tigers have a larger tonotopic mapping constant (mm/octave) than domestic cats. A larger mapping constant in tiger is consistent both with auditory brainstem response thresholds (that suggest a lower upper frequency limit of hearing for the tiger than domestic cat) and with measurements of basilar-membrane length (about 1.5 times longer in the tiger than domestic cat).
Introduction
Body mass within the family Felidae varies by more than two orders of magnitude from the smallest to the largest member species. These size differences provide a fertile ground for the comparative and ethological exploration of peripheral auditory function. Studies across a large number of felid species (Huang et al. 1997, 2000, 2002) have shown that middle-ear morphology and hearing performance are related to body size, but much less is known about the variation of inner ear function. As the largest member of the felid family, the tiger is particularly attractive for further investigation. Anatomical studies have provided quantitative estimates of relevant features of the tiger cochlea, such as the length of the basilar membrane (BM) and the number of sensory hair cells (Ứlehlová et al. 1984; Walsh et al. 2004). Other experiments have examined auditory evoked potentials (e.g., the auditory brainstem response, or ABR) of tigers and other felids, providing estimates of their frequency ranges of hearing (Walsh et al. 2008, 2011a). Although ABR potentials indicate that frequency ranges are broadly similar across felids, tigers have a lower upper frequency limit of hearing than domestic cats and may have specializations geared towards low-frequency sensitivity (Huang et al. 2000; Walsh et al. 2011a). Such specializations are consistent with the spectral content of tiger vocalizations, which typically contain significant low-frequency (<1 kHz) energy (Walsh et al. 2011b), a finding congruous with observations from an excised larynx preparation (Titze et al. 2010). Despite these efforts, much is still unknown about auditory physiology in tigers, including cochlear tuning.
The present study exploits the fact that the ear not only responds to sound, but emits it as well. Sounds emitted by the ear, called otoacoustic emissions (OAEs), can be measured noninvasively in the ear canal using a sensitive, low-noise microphone. Arising either spontaneously or in response to an evoking stimulus, OAEs are generally believed to be a by-product of amplification mechanisms at work in the inner ear (Kemp 1986). Consistent with this notion, healthy ears produce robust emissions, while impaired ears generate little or no sound (Probst et al. 1991). In addition to providing an assay for hearing impairment, OAEs offer a noninvasive means of probing normal cochlear function when direct physiological measurement is otherwise undesirable or impossible. For example, the delays of emissions evoked by pure tones (so-called stimulus-frequency otoacoustic emissions, or SFO-AEs) have been shown to predict tuning bandwidths obtained from auditory-nerve recordings in both mammals and non-mammals (Shera et al. 2002, 2010; Bergevin and Shera 2010; Joris et al. 2011).
Our focus here is on tiger SFOAEs. The goal is both to better characterize peripheral auditory function in the tiger and to address several unresolved questions regarding cochlear mechanics and OAE generation. Members of the cat family possess a wide range of basilar-membrane (BM) lengths and, therefore, offer a compelling opportunity to explore whether BM length influences OAEs. Tigers, whose BM extends some 36–39 mm in length (Ứlehlová et al. 1984; Walsh et al. 2004)—longer than the nominal values of 35 mm for human and 25 mm for domestic cat—present an especially interesting case to examine how BM length correlates with SFOAE delays and related estimates of cochlear tuning. Because peripheral auditory properties such as the tonotopic map (Liberman 1982) and SFOAEs (Guinan 1990); Shera and Guinan 2003) have been well characterized in domestic cats, comparing responses from domestic cat and tiger provides an opportunity to explore the relationship between cochlear morphology (e.g., BM length) and function (e.g., SFOAE delay, sharpness of tuning) (West 1985).
Methods
Measurements were performed at the Henry Doorly Zoo in Omaha, NE. All procedures were approved by the Institutional Animal Care and Use Committee. Data were collected from 9 ears of 5 tigers (Panthera tigris). Three subspecies were examined: the Bengal (P. tigris tigris), the Malayan (P. tigris jacksoni), and the Amur (P. tigris altaica). Two of the animals examined were white-coated tigers having Bengal ancestry. Both male and female individuals were studied, ranging in age from 3 to 10 years. Animals were immobilized by zoo veterinarians using a blow dart containing a combination of ketamine (2–4 mg/ kg IM), medetomidine (0.05 mg/kg IM), and midazolam (0.1 mg/kg IM). Supplemental doses of ketamine (1 mg/kg IM) were administered as necessary to maintain an immobilized state. Following induction of anesthesia, animals were transported to the Zoo hospital and recording sessions were conducted in an IAC sound-attenuating chamber. After intubation, the inhalation agent Sevoflurane mixed with oxygen was used to maintain general anesthesia throughout the measurement session. Recording sessions typically lasted 0.5–2.5 h. At the completion of each recording session, animals were returned to the holding cage and monitored until fully recovered from anesthesia.
Otoacoustic emissions were measured using Etymōtic ER-10C or ER-10A/ER2 probe systems. A mushroom shaped, flexible probe, roughly 20 mm in diameter, was gently inserted at least 16 mm beyond the entrance to the external ear canal proper (i.e., the length of the flexible probe). The probe was coated with vaseline to ensure the formation of a closed acoustic coupling. Stimuli were generated and recorded digitally using Lynx L22 sound cards controlled by custom software using a sampling rate of 44.1 kHz. The microphone response was amplified by 40 dB and high-pass filtered with a cut-off frequency of 0.41 kHz to reduce the effects of noise. The probe earphones were calibrated in situ using flat-spectrum, random-phase noise. Calibrations were repeated throughout the measurement session. In control experiments performed in tubes with dimensions and calibration curves similar to the tiger ear canal, we verified that system distortion was at or below the noise floor for the stimulus parameters used here.
Stimulus-frequency OAEs were measured using a suppression paradigm (Shera and Guinan 1999), noting that SFOAE responses are robust to changes in the paradigm used to evoke and extract them (Kalluri and Shera 2007). A probe stimulus level of Lp = 40 dB SPL was employed, along with the following suppressor parameters: fs = fp + 40 Hz and Ls = Lp + 15 dB. This choice of stimulus levels matches previous SFOAE studies in mammals (Shera and Guinan 1999, 2003) that produced acceptable signal-to-noise ratios (SNR); the levels were also low enough to avoid nonlinear effects commonly observed at higher levels (Bergevin et al. 2010). Emissions were measured over the frequency range of 0.7–13 kHz; the lower frequency limit was determined by the acoustic noise floor and the upper limit by the microphone calibration. As a consequence of recording session time limits, we were unable to measure all frequencies in all animals. We sought to optimize the frequency coverage of the total data set by dividing the frequency range into short, 1-kHz segments and measuring them in an irregular order, rather than working systematically from low to high.
At each probe frequency, 35 artifact-free waveforms, each consisting of 8,192 samples (approximately 186 ms), were averaged. Waveforms flagged by the artifact-rejection paradigm (Shera and Guinan 1999) were excluded. A settling period of ~20 ms after stimulus onset was inserted before the start of the sampling window to allow the response to reach steady state. All stimulus frequencies were quantized so that the sample window contained an integral number of stimulus cycles. Frequency step size varied from about 30 Hz at low frequencies to about 60 Hz at high, a resolution fine enough to avoid ambiguity in phase unwrapping. This frequency spacing yielded about 200 data points over the entire frequency range, a resolution equivalent to about 6–7 points per cycle of the emission phase. The noise floor, defined as the average sound-pressure level centered about (but excluding) the frequency of interest, was quantified via averaging the magnitudes of the adjacent ±3 bins in the fast Fourier transform of the response.
SFOAE data were collected from a single ear of the first tiger examined (a 3-year-old male). However, given the limited access to animals and the short duration of the recording sessions, a dual-recording method was adopted for the subsequent animals. Using this method, OAEs were collected simultaneously from both ears using two separate measurement systems. Data collection for each ear was managed independently by separate investigators; consequently, stimulus presentation across the two ears was unsynchronized. Although the data are limited, SFOAE properties (magnitude, phase, and phase-gradient delay) from the single-ear experiment are comparable to data collected using the dual-ear method in other experiments. This observation, in combination with the relatively low stimulus levels used, suggests that the dual-recording method introduced no significant systematic effects due to efferent activation. This is consistent with previous studies in humans, which show that the effects of contralateral stimulation on ipsilaterally evoked OAEs is small (Guinan et al. 2003; Backus and Guinan 2007; Francis and Guinan 2010).
At the beginning of the measurement session, all animals were screened for the presence of spontaneous OAEs by averaging the spectral magnitudes of 60 artifact-free waveforms, each consisting of 32,768 samples (about 743 ms) of data. In the two older tigers, we had time to measure distortion-product OAEs (DPOAEs) using the paradigm described by Bergevin et al. (2008). The primary tones (at frequencies f1 and f2, with f2/f1 = 1.22), and levels L1 = L2 = 65 dB SPL) were produced by separate earphones using the Etymōtic ER10C probe system. The measurement frequency range, buffer lengths, artifact-rejection and averaging procedures, and noise-floor estimation were all similar to those used for the SFOAE measurements.
Phase-gradient delays were computed via centered-differences (Shera and Guinan 2003). Delays associated with the ear-canal length (0.4-ms round-trip along the roughly 7 cm long external ear canal, the length measured in one specimen) and the data-acquisition system were subtracted from the total delay.1 Trends were computed using locally weighted regression (loess). Confidence intervals (95 %) for the trends were computed using bootstrap resampling to help determine statistical significance when comparing across species. Previous studies have shown that SFOAE delays computed using either a phase-gradient method or measured directly in the time domain match well (Meenderink and Narins 2006; Sisto et al. 2007).
Results
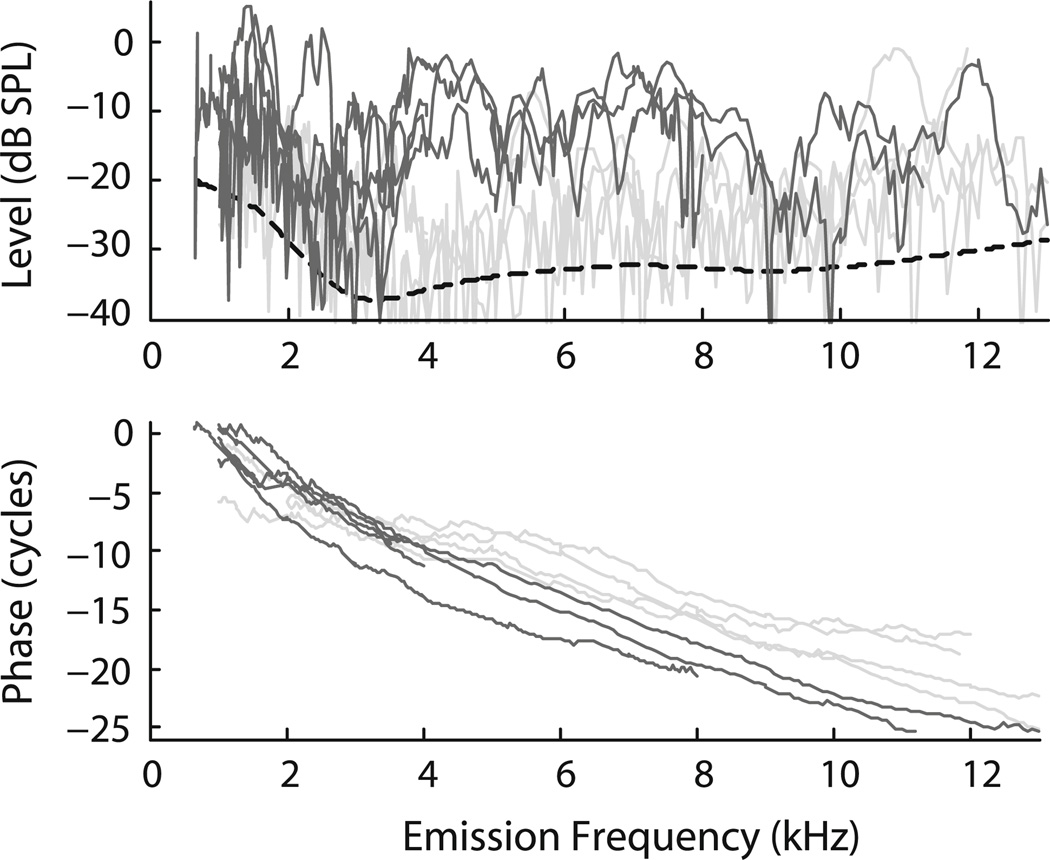
Typical examples of in situ earphone calibration curves obtained using the two probe systems are shown in Fig. 1. Figure 2 shows SFOAE magnitudes and phases from individual tigers. SFOAEs were recorded in all animals, each tiger exhibiting a unique and reproducible pattern of magnitude peaks and valleys across frequency. In the three younger animals emission levels were 5–10 dB lower than typically found in humans (Bergevin et al. 2008) and domestic cats (Guinan 1990). In the two older animals (both 10 years old), SFOAE levels were even smaller. We found no age-related effect on SFOAE phase. Although the sample pool is limited, no significant differences in SFOAE properties were apparent with regard to sex, subspecies, or background coat color (i.e., white vs. orange).
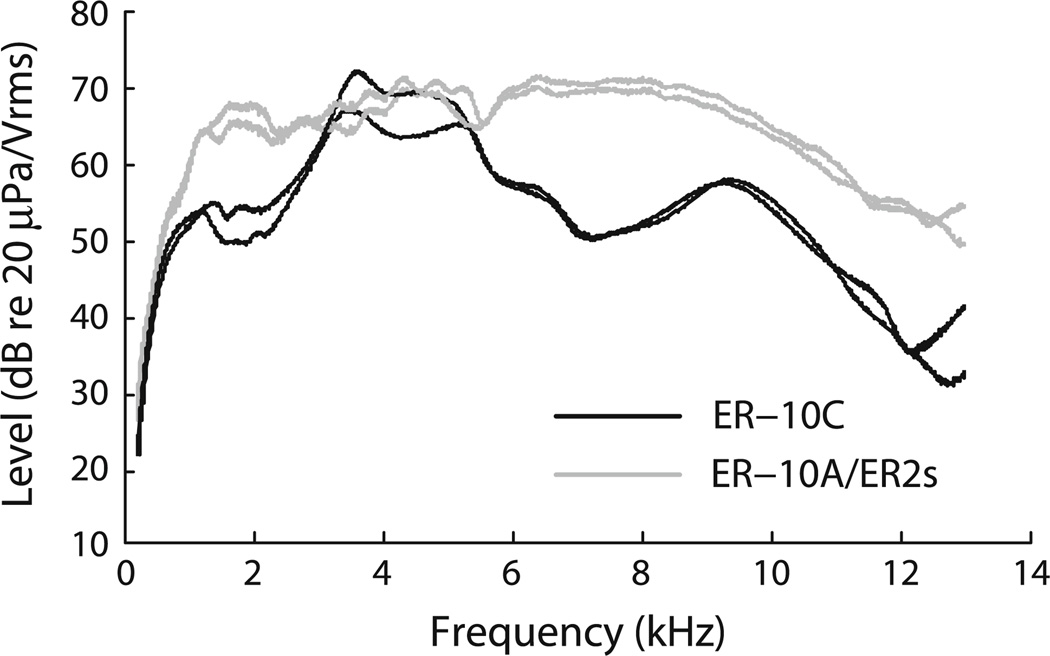
Fig. 1.
Earphone calibration curves obtained in situ from two different tigers using two different OAE probe assemblies. The ordinate indicates output for a 1 V rms drive. Dark curves are for an Etymōtic ER-10C, while the grey curves are from an assembly housing an Etymōtic ER-10A microphone and two Etymōtic ER2 earphones
Fig. 2.
Tiger SFOAEs (magnitude and phase) measured at Lp = 40 dB SPL. Data are from 9 ears from 5 tigers. The dashed curve shows an estimate of the mean noise floor obtained by loess smoothing the noise floors measured in the individual animals. Data from the three younger tigers (one 3-year-old and two 5-year-olds) are indicated by darker shading and the data from the older tigers (two 10-year-old tigers) by lighter shading. Individual phase curves were shifted vertically by an integer number of cycles to maximize their overlap
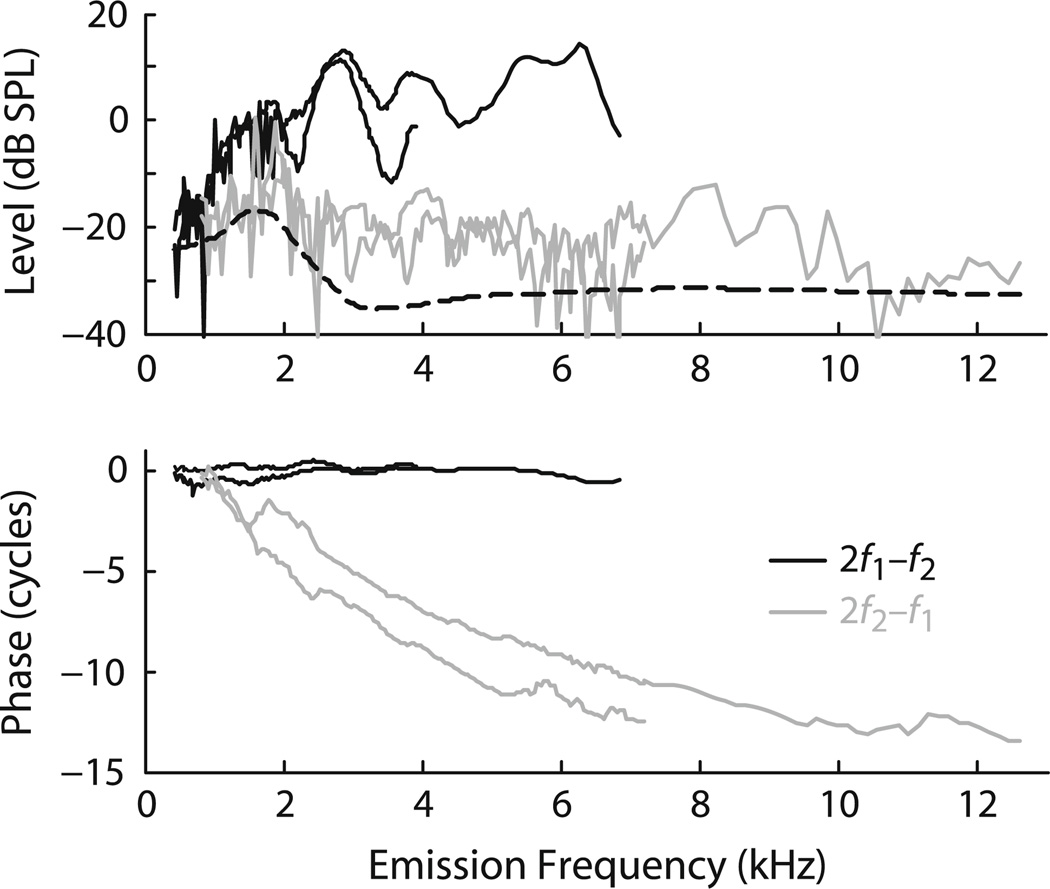
When our time with the tigers permitted, we made measurements of several other emission types. Spontaneous OAEs were looked for in all tigers but never observed. Distortion-product emissions (DPOAEs), evoked by two tones and occurring at intermodulation distortion frequencies, were examined in the two older tigers (10 years old) over a limited frequency range (Fig. 3). The magnitudes of the 2f2 - f1 DPOAE were smaller than the 2f1 - f2 but were similar to those of the SFOAEs. At the fixed f2/f1 ratio employed, the 2f1 - f2 phase remains almost constant with frequency while 2f2 - f1 exhibits significant phase accumulation. Finally, the tiger 2f1 - f2 distortion product showed no pronounced fine structure, a finding that is consistent with the relatively small magnitude of the SFOAEs observed in these same ears.
Fig. 3.
Tiger DPOAEs from the two older animals. The figure shows the magnitude and phase of both the lower- and upper-side band cubic distortion products (2f1 - f2 dark curves, 2f2 - f1 grey curves). The dashed line provides an estimate of the mean noise floor obtained by loess smoothing. The phase curves have been corrected for approximate ear-canal delays. The stimulus parameters were L1 = L2 = 65 dB SPL and f2/f1 = 1.22
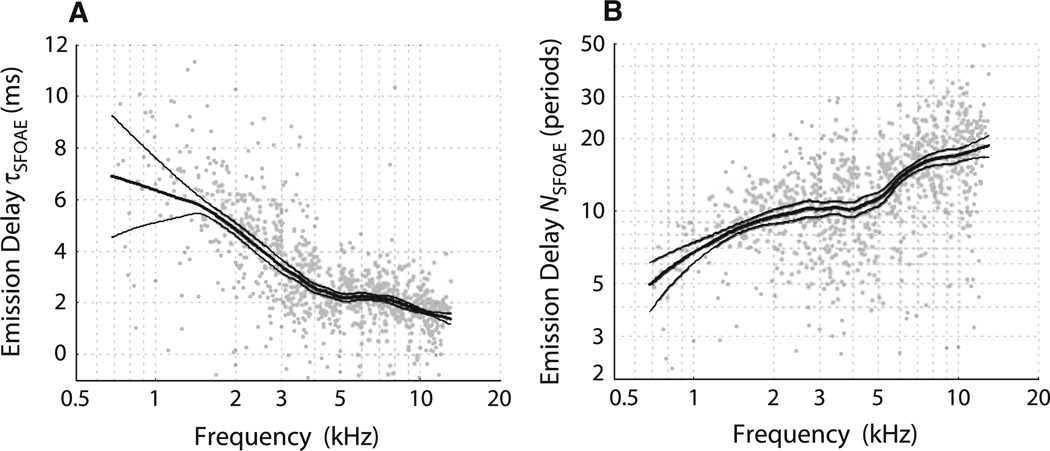
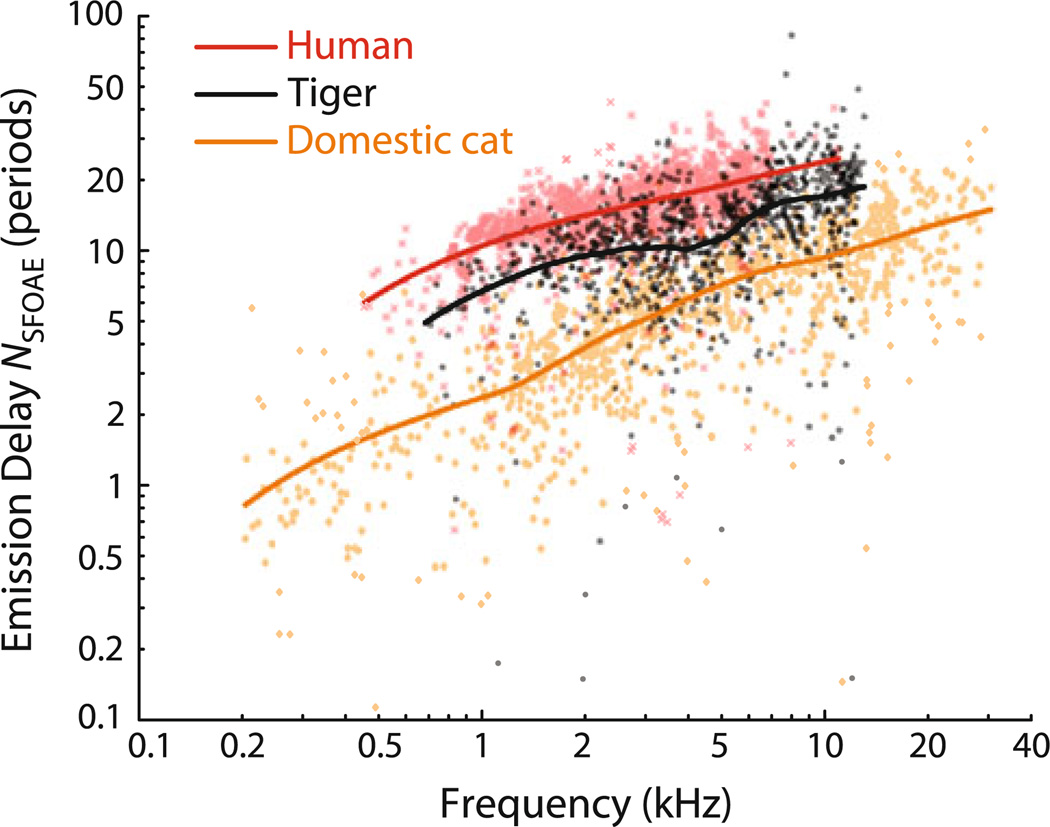
Figure 4 shows the rate of phase accumulation with frequency, also known as the phase-gradient delay. We plot these delays, computed from the slopes of the phase curves shown in Fig. 2, in two different ways: as physical latencies (τSFOAE in ms) and as the equivalent number of stimulus periods (NSFOAE). [The two are related by the equation NSFOAE = fp τSFOAE, where fp is the probe (emission) frequency.] NSFOAE is a dimensionless quantity useful when comparing delays with the quality factors (Q) commonly used to report tuning bandwidths (e.g., Shera et al. 2002). Although tiger phase-gradient delays NSFOAE(f) are shorter than those measured in humans (Fig. 5), they are significantly longer than those measured in domestic cats and many other mammals, including chinchillas and guinea pigs (Shera et al. 2010).
Fig. 4.
Tiger SFOAE phase-gradient delays computed from the phases shown in Fig. 2, expressed either in milliseconds (a) or stimulus periods (b). Only points whose corresponding magnitude was at least 10 dB above the noise floor are included. Thick lines indicate loess trends; thin lines indicate 95 % confidence intervals computed via bootstrap resampling
Fig. 5.
Tiger SFOAE phase-gradient delays (NSFOAE, in periods) compared to those in humans and domestic cats. in stimulus periods (NSFOAE). Data points and loess trends are shown for each species. The data for human and domestic cat are from Shera and Guinan (2003) and were measured using the same paradigms and stimulus levels (40 dB SPL) used here for tiger. Although the confidence intervals for the trends are excluded for clarity, there is no overlap between them for any of the three species at any of the frequencies tested
Discussion
The goal of the present study was to use OAEs to characterize peripheral auditory function in the tiger. As detailed below, our finding that tiger SFOAE delays are substantially longer than those of domestic cat suggests that tigers have significantly sharper cochlear tuning and a larger tonotopic mapping constant than domestic cats. Although tiger SFOAE delays are longer than those of many small mammals, they are shorter than those of humans. Because the tiger basilar membrane is longer than that of humans (Ứlehlová et al. 1984; Walsh et al. 2004), BM length alone cannot explain the long delays observed in humans (Shera et al. 2002). Since members of the cat family possess a wide range of BM lengths, our results can be extended and tested by measuring SFOAE properties in other felid species (e.g., those intermediate between domestic cats and tigers, such as lynx and caracal).
Ear-canal calibration curves
Earphone calibration curves are included in this report (Fig. 1) because we were surprised not to see evidence of ear-canal standing waves (e.g., deep notches in the measured pressure) due to reflections in the ear canal. As sound propagates down the canal, energy can be reflected off of the canal wall and/or the tympanic membrane and cause standing-wave interference notches at the microphone location due to destructive interference between incident and reflected components. Since the frequencies at which these notches occur depend on the distance between the probe and reflecting surface, the long ear canal of the tiger (~7 cm) was expected to produce significant standing-wave interference in the measured frequency range. Notches created in this way can confound calibrated sound delivery via insert earphones and can lead to erroneous results (Siegel 1994).
Figure 1 shows that although output levels vary with frequency, deep notches are not observed, indicating the absence of standing-wave interference patterns commonly observed in other mammals (Siegel 1994); Ravicz et al. 2007). One possible explanation for the lack of notches is sound attenuation by the canal walls: energy reflected off the tympanic membrane could be absorbed by the tissue lining the canal on its reverse path back towards the probe. Presumably such attenuation would also affect sound traveling inwards, not an efficient design consideration for an ear canal. Another possibility is that, given the complicated canal geometry and sharp turn [roughly 90°, Huang et al. (2000)], scattering occurs that smooths out peaks and valleys in the spectral response. Finally, the reflectance at the tympanic membrane may simply be small. The quarter-wave resonance in a 7-cm canal occurs near 1.2 kHz. At frequencies in this range, the single available reflectance measurement in tiger suggests a reflectance magnitude less than 0.05 (Huang et al. 2000).
General emission properties
Since otoacoustic emissions appear to be a universal property of both vertebrate (Köppl 1995; Bergevin et al. 2008) and invertebrate ears (Kössl et al. 2008), their presence in tigers comes as no surprise. Within the present subject pool, the smaller SFOAE magnitudes in the older animals suggests mild hearing loss, perhaps due to noise exposure, or age-related changes in middle-ear transmission.2 Overall, the DPOAE phase properties appear consistent with those obtained from other species (Knight and Kemp 2000; Bergevin et al. 2008).
SFOAE phase-gradient delays
Unlike the NSFOAE values so far reported in other mammalian species, tiger NSFOAE values do not increase smoothly with frequency, but manifest a shallow “dip” or plateau near 3–5 kHz. Although our sample size is small enough that the results are subject to statistical fluctuations, the trend line reliably captures the flattening seen in the individual animals. Although the origin of the flattening remains unclear, we hypothesize that it is related to the prominent “notch” seen in measurements of the middle-ear input admittance in tigers and other felids (e.g., Huang et al. 2000). This admittance notch, which occurs just below 3 kHz in the single tiger measured by Huang et al. (2000), is believed to reflect an acoustic resonance between the tympanic cavity and the bulla. In addition to influencing the magnitude of the middle-ear admittance and pressure transfer functions, the resonance produces a rapid phase change that would affect the value of the SFOAE phase-gradient delay at nearby frequencies. Although similar admittance notches occur in domestic cats, which would therefore also be expected to manifest a mid-frequency dip or plateau in their NSFOAE values, the bulla–cavity resonance—along with any effect it may have on SFOAE delay—is eliminated when the bulla is opened widely to the atmosphere, as it was during the measurements of the SFOAE delays in domestic cat shown in Fig. 5.
Estimates of tiger cochlear tuning
Our measurements of tiger SFOAE phase-gradient delays allow us to estimate the sharpness of cochlear tuning. Motivated by principles of filter theory, the procedure takes measurements of NSFOAE and applies the quantitative relationship established in other animals between NSFOAE and the frequency tuning of auditory-nerve fibers (as quantified by values of QERB) to infer the values of QERB in tiger. The quality factor QERB is defined as CF/ERB, where CF is the center or characteristic frequency of a given auditory-nerve fiber and ERB is the fiber’s equivalent rectangular bandwidth.3 More precisely (Shera et al. 2010):
| (1) |
In this equation, the function rcat is the so-called “tuning ratio” for domestic cat, defined as r = QERB/NSFOAE and computed using trend lines fit to cat auditory-nerve and otoacoustic data (Shera and Guinan 2003; Shera et al. 2010). Previous studies have found that the tuning ratio r(f/CFa|b) has nearly the same form in all mammals studied to date, including cats, guinea pigs, chinchillas, macaques, and humans (Shera et al. 2010; Joris et al. 2011). We assume here that the tiger is no exception. The constant CFa|b is a species-dependent parameter that divides the cochlea into regions of apical-like and basal-like behavior (Shera et al. 2010). In other species, the approximate value of CFa|b can be estimated from the location of a “bend” in the function NSFOAE. In tiger, however, the presence of the mid-frequency “plateau” in NSFOAE—the plateau hypothesized to originate in a middle-ear cavity resonance—makes estimation of CFa|b difficult. We therefore compute results for two different values that span the likely range.
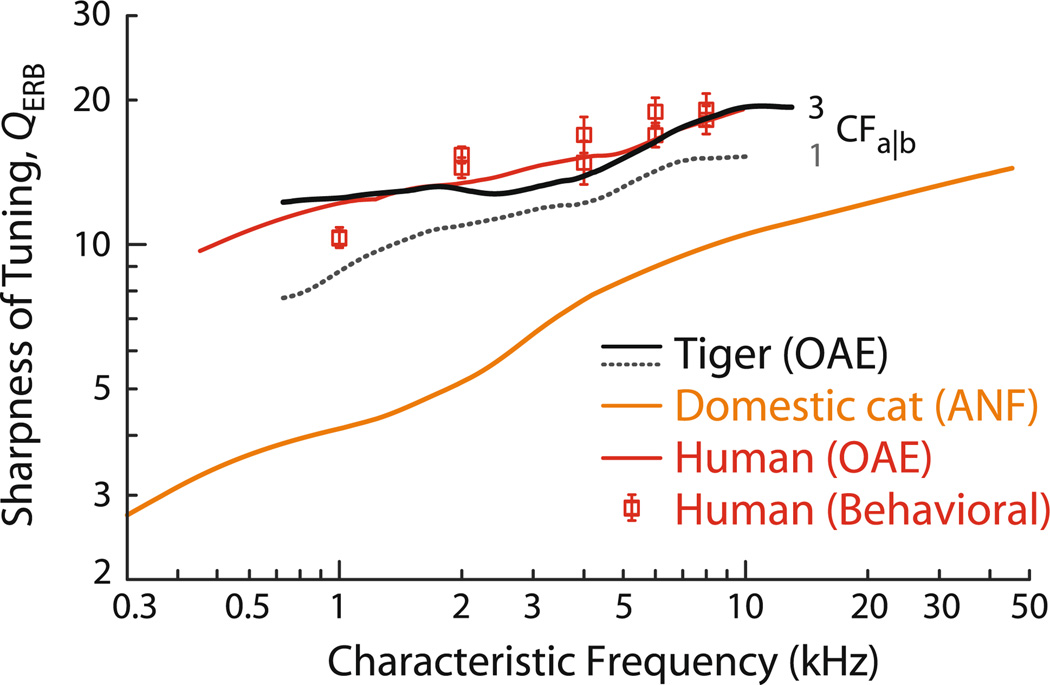
The estimates of tiger QERB obtained from Eq. (1) are given in Fig. 6 (black lines). As might be anticipated from the tiger’s longer OAE delays, our analysis predicts that cochlear tuning in tigers is significantly sharper than in domestic cats and approaches that of humans. Although these qualitative conclusions about cochlear tuning in the tiger appear robust, some quantitative details remain uncertain. For example, the figure shows that the predicted values of QERB depend on the parameter CFa|b. The solid line assumes that CFa|b in tiger is similar to that in domestic cat (i.e., about 3 kHz); the dotted line uses a smaller value similar to that in humans (i.e., near 1–1.5 kHz).
Fig. 6.
Otoacoustic estimates of QERB for tiger. Estimated values of QERB were computed from the measured values of NSFOAE using Eq. (1) and the assumption that tiger tuning ratios are the same as those of domestic cat. Results are shown for two different values of the apical—basal transition CF: the solid black line assumes CFa|b = 3 kHz, similar to domestic cat; the dotted black line assumes CFa|b = 1 kHz, similar to human. QERB trends for cat and human are shown for comparison. The cat QERB trend was obtained from auditory-nerve fiber (ANF) data (Shera and Guinan 2003; Joris et al. 2011); the human values derive from otoacoustic estimates and behavioral experiments (Shera et al. 2002, 2010)
In addition, the mid-frequency plateau or dip in the estimated values of QERB, especially evident when using CFa|b = 3 kHz, carries over from the similar plateau seen in tiger NSFOAE values. If the plateau in NSFOAE results from a middle-ear cavity resonance, or from some other cause unrelated to cochlear tuning, as we hypothesize, then the predicted plateau in QERB is entirely spurious, and no such feature would occur in measurements of tiger QERB actually obtained from the auditory nerve. Sharp tuning could have important consequences for a tiger’s ability to discern features of complex stimuli, especially in noisy or reverberant environments.
Slope of the tiger cochlear map
Returning to the theme of “size” sounded at the outset, we suggest that the relative sharpness of tuning in tiger and domestic cat is related to the parameters of their cochlear tonotopic maps. In many mammals, the sharpness of cochlear tuning correlates with the slope (in mm/octave) of the cochlear map (Shera et al. 2010).4 The reason may simply be one of real estate: a cochlea with a larger mapping constant (i.e., from a species with a longer cochlea and/or a smaller frequency range of audition) devotes more space to each octave. Since the distance between sensory hair cells (≈10 µm) varies relatively little across mammals, more millimeters per octave means more hair cells per octave—or, to put it inversely, fewer octaves per hair cell. Thus, the hair cells in a cochlea with a larger mapping constant might be expected to have sharper tuning (Bergevin 2011). Unless the tiger is an outlier, our results therefore predict that the mapping constant, or slope, of the tiger cochlear map is greater than that of domestic cat. This conclusion is consistent both with the anatomical observation that tigers have a longer BM than domestic cats (Ứehlová et al. 1984; Walsh et al. 2004) and with ABR data, which indicate that the upper frequency limit of hearing in tiger is lower than that of domestic cat (Walsh et al. 2008, 2011a).
Acknowledgments
The support, advice, and assistance of the veterinarians and staff of the Henry Doorly Zoo were invaluable to the completion of this study. All procedures were approved by the Institutional Animal Care and Use Committee. We thank Sebastiaan Meenderink and John Rosowski for helpful comments on the manuscript. The work was supported by the National Science Foundation (grants 0823417 and 0602173), the Howard Hughes Medical Institute (grant 52003749), and the National Institutes of Health (grants R01 DC003687 and P30 DC05209).
Abbreviations
- ABR
Auditory brainstem response
- BM
Basilar membrane
- DPOAE
Distortion-product otoacoustic emission
- ERB
Equivalent rectangular bandwidth
- OAE
Otoacoustic emission
- SFOAE
Stimulus-frequency otoacoustic emission
Footnotes
We were unable to measure the lengths of the external ear canals in the tigers studied here. Using a 7-cm estimate obtained previously from one specimen, and assuming an upper bound of 25 % on the tiger-to-tiger variability, yields a corresponding uncertainty of less than 0.1 ms in the round-trip emission delay. At 10 kHz, this corresponds to an uncertainty of about 1 period in NSFOAE, or about 5 %. This 5 % uncertainty carries over to our estimate of QERB.
OAEs have been suggested to be of value in clinical veterinary contexts (e.g., McBrearty and Penderis 2011).
The ERB is a parameter-free measure of tuning bandwidth commonly adopted in the psychophysical literature. For any filter, the ERB is the bandwidth of the rectangular filter with the same peak response that passes the same total power when driven by white noise (Shera et al. 2010).
Several studies examining the mammalian tonotopic map have suggested that it can deviate from a simple exponential form at frequencies below about a few hundred Hz (Greenwood 1990), potentially facilitating sensitivity and selectivity across frequency (Lepage 2003); Vater and Kössl 2011). However, deviations from an exponential map appear not to occur in several small mammals such as guinea pig (Tsuji and Liberman 1997), mouse (Müller et al. 2005), and chinchilla (Müller et al. 2010). It is not known whether significant deviations from an exponential map are present in the tiger cochlea, or if tigers can be considered ‘auditory specialists’ (Vater and Kössl 2011) for other reasons (Walsh et al. 2011a). While a transition in cochlear processing between the base and apex (e.g., loss of scaling invariance) occurs in the mammalian cochlea (Shera and Guinan 2003; Temchin et al. 2008; Shera et al. 2010), the existence of the transition has not been shown to correlate with deviations from an exponential tonotopic map.
Contributor Information
Christopher Bergevin, Department of Otolaryngology/Head and Neck Surgery, Columbia University, 630 W. 168th St., P&S 11–452, New York, NY 10032, USA, dolemitecb@gmail.com.
Edward J. Walsh, Boys Town National Research Hospital, 555 North 30th Street, Omaha, NE 68131, USA
JoAnn McGee, Boys Town National Research Hospital, 555 North 30th Street, Omaha, NE 68131, USA.
Christopher A. Shera, Eaton-Peabody Laboratories, Massachusetts Eye and Ear Infirmary, 243 Charles Street, Boston, MA 02114, USA Department of Otology and Laryngology, Harvard Medical School, Boston, MA 02115, USA.
References
- Backus BC, Guinan JJ. Measurement of the distribution of medial olivocochlear acoustic reflex strengths across normal-hearing individuals via otoacoustic emissions. J Assoc Res Otolaryngol. 2007;8:484–496. doi: 10.1007/s10162-007-0100-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergevin C. Comparison of otoacoustic emissions within gecko subfamilies: morphological implications for auditory function in lizards. J Assoc Res Otolaryngol. 2011;12:203–217. doi: 10.1007/s10162-010-0253-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergevin C, Shera CA. Coherent reflection without traveling waves: on the origin of long-latency otoacoustic emissions in lizards. J Acoust Soc Am. 2010;127:2398–2409. doi: 10.1121/1.3303977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergevin C, Freeman DM, Saunders JC, Shera CA. Otoacoustic emissions in humans, birds, lizards, and frogs: evidence for multiple generation mechanisms. J Comp Physiol A. 2008;194:665–683. doi: 10.1007/s00359-008-0338-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergevin C, Velenovsky D, Bonine KE. Tectorial membrane morphological variation: effects upon stimulus frequency otoacoustic emissions. Biophys J. 2010;99(4):1064–1072. doi: 10.1016/j.bpj.2010.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis NA, Guinan JJ. Acoustic stimulation of human medial olivocochlear efferents reduces stimulus-frequency and click-evoked otoacoustic emission delays: implications for cochlear filter bandwidths. Hear Res. 2010;267:36–45. doi: 10.1016/j.heares.2010.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood DD. A cochlear frequency-position function for several species—29 years later. J Acoust Soc Am. 1990;87:2592–2605. doi: 10.1121/1.399052. [DOI] [PubMed] [Google Scholar]
- Guinan JJ. Changes in stimulus frequency otoacoustic emissions produced by two-tone suppression and efferent stimulation in cats. In: Dallos P, Geisler CD, Matthews JW, Ruggero MA, Steele CR, editors. Mechanics and biophysics of hearing. New York: Springer; 1990. pp. 170–177. [Google Scholar]
- Guinan JJ, Backus B, Lilaonitkul W, Aharonson V. Medial olivocochlear efferent reflex in humans: otoacoustic emission (OAE) measurement issues and the advantages of stimulus frequency OAEs. J Assoc Res Otolaryngol. 2003;4:521–540. doi: 10.1007/s10162-002-3037-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang GT, Rosowski JJ, Flandermeyer DT, Lynch TJ, Peake WT. The middle ear of a lion: comparison of structure and function to domestic cat. J Acoust Soc Am. 1997;101:1532–1549. doi: 10.1121/1.418107. [DOI] [PubMed] [Google Scholar]
- Huang GT, Rosowski JJ, Peake WT. Relating middle-ear acoustic performance to body size in the cat family: measurements and models. J Comp Physiol A. 2000;186:447–465. doi: 10.1007/s003590050444. [DOI] [PubMed] [Google Scholar]
- Huang GT, Rosowski JJ, Ravicz ME, Peake WT. Mammalian ear specializations in arid habitats: structural and functional evidence from sand cat Felis margarita. J Comp Physiol A. 2002;188:663–681. doi: 10.1007/s00359-002-0332-8. [DOI] [PubMed] [Google Scholar]
- Joris PX, Bergevin C, Kalluri R, McLaughlin M, Michelet P, van der Heijden M, Shera CA. Frequency selectivity in Old-World monkeys corroborates sharp cochlear tuning in humans. Proc Nat Acad Sci USA. 2011;108:17516–17520. doi: 10.1073/pnas.1105867108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalluri R, Shera CA. Comparing stimulus-frequency otoacoustic emissions measured by compression, suppression, and spectral smoothing. J Acoust Soc Am. 2007;122:3562–3575. doi: 10.1121/1.2793604. [DOI] [PubMed] [Google Scholar]
- Kemp DT. Otoacoustic emissions, travelling waves and cochlear mechanisms. Hear Res. 1986;22:95–104. doi: 10.1016/0378-5955(86)90087-0. [DOI] [PubMed] [Google Scholar]
- Knight RD, Kemp DT. Indications of different distortion product otoacoustic emission mechanisms from a detailed f1,f2 area study. J Acoust Soc Am. 2000;107:457–473. doi: 10.1121/1.428351. [DOI] [PubMed] [Google Scholar]
- Köppl C. Otoacoustic emissions as an indicator for active cochlear mechanics: a primitive property of vertebrate auditory organs. In: Manley GA, Klump GM, Köppl K, Fastl H, Oeckinghaus H, editors. Advances in hearing research. Singapore: World Scientific; 1995. pp. 207–218. [Google Scholar]
- Kössl M, Moeckel D, Weber M, Seyfarth EA. Otoacoustic emissions from insect ears: evidence of active hearing? J Comp Physiol A. 2008;194:597–609. doi: 10.1007/s00359-008-0344-0. [DOI] [PubMed] [Google Scholar]
- Lepage EL. The mammalian cochlear map is optimally warped. J Acoust Soc Am. 2003;114:896–906. doi: 10.1121/1.1587150. [DOI] [PubMed] [Google Scholar]
- Liberman MC. The cochlear frequency map for the cat: labeling auditory-nerve fibers of known characteristic frequency. J Acoust Soc Am. 1982;72:1441–1449. doi: 10.1121/1.388677. [DOI] [PubMed] [Google Scholar]
- McBrearty AR, Penderis J. Evaluation of auditory function in a population of clinically healthy cats using evoked otoacoustic emissions. J Feline Med Surg. 2011;13:919–926. doi: 10.1016/j.jfms.2011.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meenderink SW, Narins PM. Stimulus frequency otoacoustic emissions in the Northern leopard frog, Rana pipiens pipiens: implications for inner ear mechanics. Hear Res. 2006;220:67–75. doi: 10.1016/j.heares.2006.07.009. [DOI] [PubMed] [Google Scholar]
- Müller M, von Hünerbein K, Hoidis S, Smolders JW. A physiological place-frequency map of the cochlea in the CBA/J mouse. Hear Res. 2005;202:63–73. doi: 10.1016/j.heares.2004.08.011. [DOI] [PubMed] [Google Scholar]
- Müller M, Hoidis S, Smolders JW. A physiological frequency-position map of the chinchilla cochlea. Hear Res. 2010;268:184–193. doi: 10.1016/j.heares.2010.05.021. [DOI] [PubMed] [Google Scholar]
- Probst R, Lonsbury-Martin BL, Martin GK. A review of otoacoustic emissions. J Acoust Soc Am. 1991;89:2027–2067. doi: 10.1121/1.400897. [DOI] [PubMed] [Google Scholar]
- Ravicz ME, Olson ES, Rosowski JJ. Sound pressure distribution and power flow within the gerbil ear canal from 100 Hz to 80 kHz. J Acoust Soc Am. 2007;122:2154–2173. doi: 10.1121/1.2769625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shera CA, Guinan JJ. Evoked otoacoustic emissions arise by two fundamentally different mechanisms: a taxonomy for mammalian OAEs. J Acoust Soc Am. 1999;105:782–798. doi: 10.1121/1.426948. [DOI] [PubMed] [Google Scholar]
- Shera CA, Guinan JJ. Stimulus-frequency-emission group delay: a test of coherent reflection filtering and a window on cochlear tuning. J Acoust Soc Am. 2003;113:2762–2772. doi: 10.1121/1.1557211. [DOI] [PubMed] [Google Scholar]
- Shera CA, Guinan JJ, Oxenham AJ. Revised estimates of human cochlear tuning from otoacoustic and behavioral measurements. Proc Nat Acad Sci USA. 2002;99:3318–3323. doi: 10.1073/pnas.032675099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shera CA, Guinan JJ, Oxenham AJ. Otoacoustic estimation of cochlear tuning: validation in the chinchilla. J Assoc Res Otolaryngol. 2010;11:343–365. doi: 10.1007/s10162-010-0217-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel JH. Ear-canal standing waves and high-frequency sound calibration using otoacoustic emission probes. J Acoust Soc Am. 1994;95:2589–2597. [Google Scholar]
- Sisto R, Moleti A, Shera CA. Cochlear reflectivity in transmission-line models and otoacoustic emission characteristic time delays. J Acoust Soc Am. 2007;122:3554–3561. doi: 10.1121/1.2799498. [DOI] [PubMed] [Google Scholar]
- Temchin AN, Rich NC, Ruggero MA. Threshold tuning curves of chinchilla auditory-nerve fibersIDependence on characteristic frequency and relation to the magnitudes of cochlear vibrations. J Neurophysiol. 2008;100:2889–2898. doi: 10.1152/jn.90637.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Titze IR, Fitch WT, Hunter EJ, Alipour F, Montequin D, Armstrong DL, McGee J, Walsh EJ. Vocal power and pressure-flow relationships in excised tiger larynges. J Exp Biol. 2010;213:3866–3873. doi: 10.1242/jeb.044982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuji J, Liberman MC. Intracellular labeling of auditory nerve fibers in guinea pig: central and peripheral projections. J Comp Neurol. 1997;381:188–202. [PubMed] [Google Scholar]
- Ứlehlová L, Burda H, Voldich L. Involution of the auditory neuro-epithelium in a tiger (Panthera tigris) and a jaguar (Panthera onca) J Comp Pathol. 1984;94:153–157. doi: 10.1016/0021-9975(84)90017-3. [DOI] [PubMed] [Google Scholar]
- Vater M, Kössl M. Comparative aspects of cochlear functional organization in mammals. Hear Res. 2011;273:89–99. doi: 10.1016/j.heares.2010.05.018. [DOI] [PubMed] [Google Scholar]
- Walsh EJ, Ketten DR, Arruda J, Armstrong DL, Curro T, Simmons LG, Wang LM, McGee J. Temporal bone anatomy inPanthera tigris . J Acoust Soc Am. 2004;115:2485–2486. [Google Scholar]
- Walsh EJ, Armstrong DL, Napier J, Simmons LG, Korte M, McGee J. Acoustic communication inPanthera tigris: a study of tiger vocalization and auditory receptivity revisited. J Acoust Soc Am. 2008;123:3507. [Google Scholar]
- Walsh EJ, Armstrong DL, McGee J. Comparative cat studies: are tigers auditory specialists? J Acoust Soc Am. 2011a;129:2447. [Google Scholar]
- Walsh EJ, Armstrong DL, McGee J. Tiger bioacoustics: an overview of vocalization acoustics and hearing in Panthera tigris. 3rd symposium on acoustic communication by animals; Ithaca, NY: Cornell University; 2011b. pp. 173–174. [Google Scholar]
- West CD. The relationship of the spiral turns of the cochlea and the length of the basilar membrane to the range of audible frequencies in ground dwelling mammals. J Acoust Soc Am. 1985;77:1091–1101. doi: 10.1121/1.392227. [DOI] [PubMed] [Google Scholar]