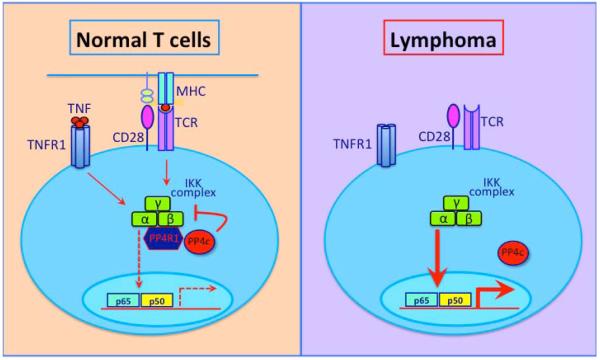
The NF-κB signaling pathway is important in the regulation of physiological and malignant hematopoiesis. In this issue, Brechmann et al. (2012) identify a phosphatase, PP4R1, that inhibit NF-kB activation in T cells and T cell lymphoma.
The regulated expression and activation of the transcription factor NF-κB plays a critical role in cellular responses to external stimuli by modulating a broad spectrum of target genes that control cell proliferation, differentiation, and survival (Hayden and Ghosh, 2012). To no surprise, deregulation of NF-κB signaling results in devastating consequences. Aberrant NF-κB activity has been shown to induce a wide spectrum of diseases that include cancer, neurodegeneration, arthritis, and atherosclerosis (Gamble et al., 2012). In the context of immune system, NF-κB functions as a key molecular mediator in development and differentiation of T lymphocytes. Stimulation through the T cell receptor (TCR) or TNF receptor 1 (TNFR1) leads to IkB kinase (IKK) complex mediated phosphorylation and subsequent proteasome degradation of inhibitory kB proteins (IkBs), allowing for translocation of NF-κB proteins to the nucleus, where they bind to DNA and initiate transcriptional machinery (Hacker and Karin, 2006). It was previously shown that several immune malignancies depend on NF-κB activation, including multiple myeloma, distinct lymphoma types and acute T cell leukemia (Lim et al., 2012). Targeting of NF-κB presents to be an attractive therapeutic avenue aimed to either inhibit tumor growth or “weaken” tumor cells, making them more susceptible to chemotherapy. Several NF-κB small molecule inhibitors that directly target the IKK complex are currently under intense investigation and have been introduced in clinical trials (Gamble et al., 2012).
NF-κB signaling is modulated by an intricate network of regulatory elements. For example, a number of kinases such as PI3K, PDk1, PKCθ, TAK1 and MEKK3, were shown to regulate NF-κB (Hacker and Karin, 2006). It is generally believed that de-phosphorylation, which antagonizes protein phosphorylation, plays an important role in fine-tuning of NF-κB signaling; yet, after a couple decades of extensive research, much less is known about phosphatases involved in regulating machinery of NF-κB activation (Li et al., 2008). In this issue of Immunity, Brechmann et al (2012) demonstrates that the PP4R1-PP4c complex is an important negative regulator of canonical NF-κB signaling in T cells.
To identify phosphatases that modulate NF-κB signaling, the authors performed an RNAi screen and identified siRNAs that enhanced NF-κB reporter activity in Jurkat T cell leukemia line upon TCR-CD28 or TNFR1 stimulation. One of the strongest “hits” of this siRNA screen was Protein phosphatase 4, regulatory subunit 1 (PP4R1), a non-catalytic molecule that acts as an interaction partner of the catalytic protein phosphatase 4 (PP4c). PP4c is a serine - threonine phosphatase that belongs to PP2A phosphatase family. PP4R1 is ubiquitously expressed and is highly conserved between species. In 1999, Kloeker and Wadzinski, while looking for physiological substrates of PP4c, purified the PP4R1-PP4 heterodimer (Kloeker and Wadzinski, 1999). Since then, a list of PP4c-interacting partners has grown to include several PP4R proteins (PP4R4, PP4R2, and PP4R3), elucidating an assortment of phophatase complexes. The biological function of PP4R molecules began to surface only recently and has been mainly linked to repair of DNA double strand breaks (Lee et al., 2010).
Upon examination of PP4R1 expression in T cells, the authors found that the PP4R1 protein was undetectable in resting cells and its expression increased significantly upon T cell stimulation, while PP4c expression remained constant regardless of cell activation state. They then showed that in expanded human T cells, silencing of PP4R1 expression led to activation of NF-κB pathway, whereas overexpression of PP4R1 resulted in NF-κB inhibition. Subsequent to performing a series of methodical experiments, Brechmann et al concluded that PP4R1 acted as a negative regulator of canonical NF-κB signaling in TCR-CD28 or TNFR1-triggered T cells. The group then hypothesized that PP4R1 acted on a common convergent of NF-κB signaling. Indeed, they discovered that in activated T cells PP4R1 directly interacted with IKKα□□□□□□β, the enzymatic subunits of IKK complex. The authors meticulously dissected the mechanism of that interaction and showed that PP4c catalytic subunit associated with IKK only in the presence of PP4R1. Those results indicated that PP4R1 linked PP4c with IKK complex, allowing its dephosphorylation and subsequent NF-κB inhibition.
Brechmann et al further assessed a role of PP4R1 in T cell tumors. Cutaneous T cell Lymphoma (CTCL) encompasses a heterogenous group of cutaneous malignancies manifested by clonal proliferation of CD4+ T cells that home to patient’s skin. The two most common forms of CTCL are mycosis fungoides, which presents in the skin with erythematous plaques and Sezary syndrome characterized by substantial leukemic T cell burden in the blood as well as erythroderma. Currently a combination of different non-targeted therapies is used to target both of these CTCL forms. Despite the ongoing efforts, however, those diseases are rarely curable (Wong et al., 2011). Atypical NF-κB activity has been previously implicated to play a key role in CTCL progression. Specifically, constitutively activated NF-κB signaling was shown to promote resistance to apoptosis in CTCL cell lines as well as peripheral blood lymphocytes from patients with Sezary syndrome. Importantly, targeted inhibition of NF-κB pathway in CTCL cell lines using proteosome 26S inhibitors, that restrained NF-κB translocation to the nucleus, resulted decreased proliferation and cell viability though induction of apoptosis (Sors et al., 2006). To date, the mechanisms mediating NF-κB deregulation in CTCL remain elusive. Given role of PP4R1 in negative regulation NF-κB in T cells, Brechmann et al examined a relationship between NF-κB activity in CTCL cells and PP4R1 expression. Interestingly, the authors found that primary tumor cells from peripheral blood of Sezary syndrome patients as well as several CTCL cell lines had markedly reduced expression as well as protein amounts of PP4R1. Specifically, a CTCL cell line, HH, derived from primary Sezary cells completely lacked PP4R1 expression and displayed constitutive IKKβ kinase activity. To address weather forced re-expression of PP4R1 altered the proliferation of malignant cells, the authors retrovirally transduced PP4R1 into CTCL cell lines. Tumor cells that displayed expression of PP4R1 prior to retroviral transduction were unaffected by overexpression of PP4R1. In contrast, CTCL cell lines, that had decreased levels of PP4R1 or lacked it entirely to begin with, displayed significantly reduced cell abundance as a result of PP4R1 overexpression. Taken together, those studies suggest a role for PP4R1as a suppressor of aberrant NF-κB signaling in CTCL malignancy (see also Figure 1).
Figure 1. The PP4R1-PP4c complex is a negative regulator of NF-κB signaling in T cells.
Signaling though TCR-CD28 or TNFR1 leads to phosphorylation of IKK complex and subsequent NF-κB activation as well as PP4R1 expression. PP4R1 molecule links IKK with phophatase PP4c resulting in partial dephosphorylation of IKK and dampening of NF-κB activity. In T cell lymphoma, absence of PP4R1 leads to inability of PP4c to associate and dephosphorylate the IKK complex, leading to untamed IKK phosphorylation and aberrant NF-κB activity.
The present work illuminates the mechanism of NF-κB suppression by the PP4R1/PP4c complex and raises a number of questions regarding regulation of PP4R1. Specifically, given the variety of feedback mechanisms enveloping NF-κB activity it would be interesting to know if PP4R1 expression is modulated by NF-κB itself. Moreover, physiological functions of the PP4R1-PP4c complex should be further elucidated. Also, It is not clear weather decreased expression of PP4R1 in CTCL cells is caused by genetic lesions or a result of posttranscriptional and/or posttranslational modification. Thus, targeted exon sequencing of PP4R1, putative PP4R1 expression regulators and other members of the PP4c complex would be important to determine whether these genes are targeted in CTLC. Finally, these studies propose further focus on PP4c complex function in additional types of lymphomas where previously NF-κB-specific activity was discovered (Lim et al., 2012) especially as such enzymatic complexes could be targeted using molecularly designed small molecules.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Brechmann, et al. This issue. 2012 [Google Scholar]
- Gamble C, McIntosh K, Scott R, Ho KH, Plevin R, Paul A. Inhibitory kappa B Kinases as targets for pharmacological regulation. Br J Pharmacol. 2012;165:802–819. doi: 10.1111/j.1476-5381.2011.01608.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hacker H, Karin M. Regulation and function of IKK and IKK-related kinases. Sci STKE. 2006;2006:re13. doi: 10.1126/stke.3572006re13. [DOI] [PubMed] [Google Scholar]
- Hayden MS, Ghosh S. NF-kappaB, the first quarter-century: remarkable progress and outstanding questions. Genes Dev. 2012;26:203–234. doi: 10.1101/gad.183434.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloeker S, Wadzinski BE. Purification and identification of a novel subunit of protein serine/threonine phosphatase 4. J Biol Chem. 1999;274:5339–5347. doi: 10.1074/jbc.274.9.5339. [DOI] [PubMed] [Google Scholar]
- Lee DH, Pan Y, Kanner S, Sung P, Borowiec JA, Chowdhury D. A PP4 phosphatase complex dephosphorylates RPA2 to facilitate DNA repair via homologous recombination. Nat Struct Mol Biol. 2010;17:365–372. doi: 10.1038/nsmb.1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li HY, Liu H, Wang CH, Zhang JY, Man JH, Gao YF, Zhang PJ, Li WH, Zhao J, Pan X, et al. Deactivation of the kinase IKK by CUEDC2 through recruitment of the phosphatase PP1. Nat Immunol. 2008;9:533–541. doi: 10.1038/ni.1600. [DOI] [PubMed] [Google Scholar]
- Lim KH, Yang Y, Staudt LM. Pathogenetic importance and therapeutic implications of NF-kappaB in lymphoid malignancies. Immunol Rev. 2012;246:359–378. doi: 10.1111/j.1600-065X.2012.01105.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sors A, Jean-Louis F, Pellet C, Laroche L, Dubertret L, Courtois G, Bachelez H, Michel L. Down-regulating constitutive activation of the NF-kappaB canonical pathway overcomes the resistance of cutaneous T-cell lymphoma to apoptosis. Blood. 2006;107:2354–2363. doi: 10.1182/blood-2005-06-2536. [DOI] [PubMed] [Google Scholar]
- Wong HK, Mishra A, Hake T, Porcu P. Evolving insights in the pathogenesis and therapy of cutaneous T-cell lymphoma (mycosis fungoides and Sezary syndrome) Br J Haematol. 2011;155:150–166. doi: 10.1111/j.1365-2141.2011.08852.x. [DOI] [PMC free article] [PubMed] [Google Scholar]