Abstract
Fetal detection of adversity is a conserved trait that allows many species to adapt their early developmental trajectories to ensure survival. According to the fetal-programming model, exposure to stressful or hostile conditions in utero is associated with compromised development and a lifelong risk of adverse health outcomes. In a longitudinal study, we examined the consequences of prenatal and postnatal exposure to adversity for infant development. We found increased motor and mental development during the 1st year of life among infants whose mothers experienced congruent levels of depressive symptoms during and after pregnancy, even when the levels of symptoms were relatively high and the prenatal and postnatal environments were unfavorable. Congruence between prenatal and postnatal environments prepares the fetus for postnatal life and confers an adaptive advantage for critical survival functions during early development.
Keywords: predictive adaptive response, fetal programming, depression, stress, HPA axis, infant development, stress reactions
Remarkable surveillance and response systems have evolved and are conserved in many species, ranging from desert-dwelling Western Spadefoot toads to humans, so that they can detect threats to survival during early development and adjust their developmental trajectories (Boorse & Denver, 2002). When tadpoles detect the evaporation of life-sustaining pools of desert water, their metamorphosis accelerates to ensure their survival (Denver, 1997, 1999). The human fetal-placental complex has evolved similar mechanisms to sample information from maternal circulation: If the prenatal environment is perceived to be stressful or hostile, the fetal-placental complex may promote accelerated developmental trajectories, such as preterm birth, that ensure short-term survival (Dunkel Schetter, 2009). Competing models provide different frameworks for understanding the longer-term consequences associated with fetal adaption to maternal signals in utero. In this article, we provide support for one model on the basis of our findings that congruous prenatal and postnatal environments confer an adaptive advantage in motor and mental development during an infant’s 1st year of life, even when the environments are unfavorable.
The influential developmental-origins-of-disease model (Barker, 1998), also known as the fetal-programming model (Lucas, Fewtrell, & Cole, 1999), predicts that early exposures to threat or adversity have lifelong negative consequences for health (Gluckman & Hanson, 2004; Gluckman, Hanson, & Spencer, 2005). Fetal exposure to adversity increases subsequent risk for numerous poor health outcomes, including cardiovascular disease, non-insulin-dependent diabetes mellitus, obesity, and vulnerability to stressful life events (Barker, 1998; Kjaer, Wegener, Rosenberg, Lund, & Hougaard, 2010; McCormack et al., 2003; Roseboom et al., 2000). Although an impressive body of literature supports this model, most studies examining fetal programming in human subjects have been retrospective and have used measures of birth phenotype (e.g., birth weight, length of gestation) as surrogate indicators of prenatal adversity and as predictors of subsequent health outcomes (Bohner & Breslau, 2008; Lucas et al., 1999).
Rather than assuming that disease and pathology constitute the only outcomes for fetal or early developmental exposure to trauma, a second model proposes a provocative and perhaps radical alternative. The predictive-adaptive-response (PAR) model (Gluckman et al., 2005; Gluckman & Hanson, 2004), also known as the weather-forecasting model (Bateson et al., 2004), predicts that, under certain conditions, organisms that are stressed in utero may have an adaptive advantage if they are confronted with stress later in development but an increased risk for disease if the conditions of their postnatal environment are favorable (Bogin, Silva, & Rios, 2007). Studies that have examined the consequences of discordance between prenatal and postnatal nutrient environments have provided persuasive support for the PAR model. For instance, increasing the discrepancy between prenatal and postnatal nutrition has been shown to increase the risk for altered cardiovascular function and cardiac hypertrophy in sheep (Cleal et al., 2007), and humans provided with sufficient nutrition after near-starvation in utero have been shown to have an increased risk of developing metabolic diseases (Gluckman et al., 2005). Prior prospective investigations of the PAR model as it relates to human development have been limited to studies of nutritional adversity.
Adequate maternal care is as critical to infant development as nutrition (Chisholm, 1998; Chugani et al., 2001). When the quality of maternal care is compromised by postpartum depression, infants suffer pervasive negative consequences for health and development (Fihrer, McMahon, & Taylor, 2009; Kurstjens & Wolke, 2001), even if their mothers’ depressive symptoms are subclinical (Moehler et al., 2007). Our extension of the PAR model predicts that a fetus exposed to maternal depressive symptoms in utero will have an adaptive advantage if it is exposed to the adverse conditions associated with maternal depressive symptoms in infancy. We assessed the consequences of prenatal and early postnatal exposure to maternal depressive symptoms for infants’ mental and psychomotor development.
Method
To obtain medical and psychosocial information and to assess symptoms of maternal depression, we administered comprehensive interviews and questionnaires at regular intervals throughout the pregnancies of a sample of 221 healthy pregnant women (Sandman & Davis, 2010). After delivery, mothers and infants were evaluated at regular intervals for 12 months. For analysis, infants were separated into four groups. Two groups included infants whose mothers had concordant prenatal and early postnatal depressive symptoms: either high prenatal and postnatal symptoms (concordant adversity) or low prenatal and postnatal symptoms (concordant favorability) The other two groups included infants whose mothers had discrepant prenatal and postnatal depressive symptoms: either high levels of prenatal symptoms and low levels of postnatal symptoms (prenatal-only adversity) or low levels of prenatal symptoms and high levels of postnatal symptoms (postnatal-only adversity; Table 1).
Table 1.
Assignment of Infants to Groups on the Basis of Maternal Levels of Depression During the Prenatal and Postnatal Assessments
| Postnatal assessment | Prenatal assessment
|
|
|---|---|---|
| Not depressed | Depressed | |
| Not depressed | n = 82 | n = 32 |
| Depressed | n = 38 | n = 69 |
Note: Groups with concordant prenatal and early postnatal maternal depressive symptoms are shown in boldface.
All subjects in our sample of pregnant women were recruited from a large university medical center. To participate in the study, subjects had to be pregnant with only one fetus, English speaking, over the age of 18 years, nonsmoking, free of conditions that could complicate pregnancy outcomes (e.g., endocrine, hepatic, or renal disorders; use of corticosteroid medications), and free of uterine or cervical abnormalities. To obtain medical, biological, and psychosocial information, we examined subjects during laboratory visits performed over the course of pregnancy at systematic intervals: at 14 to 16 weeks’ gestation (M = 15.31 weeks, SD = 0.92), 24 to 26 weeks’ gestation (M = 25.55 weeks, SD = 0.93), 30 to 32 weeks’ gestation (M = 30.96 weeks, SD = 0.77), and 36 or more weeks’ gestation (M = 36.7 weeks, SD = 0.83). A research nurse reviewed maternal medical records to assess prenatal medical history and birth outcomes. No subjects reported using alcohol or other drugs during pregnancy. Fourteen subjects reported taking antidepressant (SSRI) medications; 11 of these subjects were in the concordant-adversity group. Postnatal assessments of mothers and their infants were conducted at 3 months, 6 months, and 12 months postpartum.
Psychosocial measures
Maternal depressive symptoms were assessed with a nine-item version (Santor & Coyne, 1997) of the Center for Epidemiological Studies Depression Scale (CES-D; Radloff, 1977). At each prenatal time point, subjects indicated how often they had experienced each listed symptom of depression (e.g., “I feel depressed”) during the past week, using a 4-point scale (from 0, rarely or none of the time, to 3, most or all of the time). This instrument has good internal consistency (Kuder-Richardson 20 = .87), and raw total scores from the nine-item version correlate highly with scores from the original scale (r = .97; Santor & Coyne, 1997). We also administered the Perceived Stress Scale at each prenatal and postnatal laboratory visit (Cohen, Kamarck, & Mermelstein, 1983). This brief, valid, and reliable scale measures general perceptions of stress (i.e., the degree to which respondents perceive their lives to be unpredictable, uncontrollable, or overwhelming) without reference to the sources of stress (Cohen, 1986; Cohen et al., 1983). Reported estimates have demonstrated the significant reliability and predictive and concurrent validity of the scale (Cohen et al., 1983).
Infant development
Infant development was assessed with the Bayley Scales of Infant Development, Second Edition (BSID; Bayley, 1993), which yields two primary scores: the Mental Developmental Index (MDI) and Psychomotor Developmental Index (PDI). We obtained infants’ MDI and PDI scores at 3, 6, and 12 months of age. Following conventional methods of scoring of the BSID, we created composite MDI and PDI scaled scores by summing the total number of items achieved on each index at each time point, converting raw scores to scaled scores by reference to a chronological table, and correcting for age-related effects. Examiners were trained by a clinician who had more than 15 years of experience with the BSID and were directly supervised by a clinical psychologist. An independent observer reviewed 20% of the digitally recorded assessments from each postnatal time point. Interrater reliability was significant for all three time points (95% at 3 and 6 months and 93% at 12 months).
Cortisol assessment
In the afternoon during each laboratory visit, blood samples (20 ml) were drawn from the women by antecubital venipuncture into purple-top EDTA Vacutainers for plasma and red-top Vacutainers for serum collection. EDTA Vacutainers were chilled on ice immediately, and aprotinin (Sigma Chemical Co., St. Louis, MO) was added at 500 KIU/ml. Blood samples in red-top Vacutainers sat at room temperature until clotted. Samples were then centrifuged at 2000 × g for 15 min. Plasma and serum were decanted into polypropylene tubes and stored at −70 °C until assayed.
Plasma cortisol levels were determined with a competitive-binding, solid-phase, enzyme-linked immunosorbent assay (IBL America, Minneapolis, MN). Plasma samples (20 μl) and enzyme conjugate (200 μl) were added to the antibody-coated microtiter wells, thoroughly mixed, and incubated for 60 min at room temperature. Each well was washed three times with wash solution (400 μl per well) and struck to remove residual droplets. Substrate solution (100 μl) was added to each well and incubated for 15 min at room temperature. The absorbance units were measured at 450 nm within 10 min after the stop solution (100 μl) had been added. The assay has less than 9% cross-reactivity with progesterone and less than 2% cross-reactivity with five other naturally occurring steroids. The interassay and intra-assay coefficients of variance are reported as less than 8%, and the minimum detectable level of the assay was 0.25 μg/dL.
Data analysis
We employed a nonlinear, categorical analysis of maternal depressive symptoms for several reasons. First, the prenatal and postpartum measures of depression were significantly skewed, and the postpartum measures exhibited a bimodal distribution. The median z score for the postpartum measures was −0.38, indicating nonnormal distribution. Several transformations of the postpartum data, including a logarithmic transformation, did not result in normalization and did not eliminate the bimodal distribution. Second, levels of depressive symptoms are typically higher during pregnancy than they are postpartum. We therefore examined how subjects ranked in the shifting, bimodal distributions by assessing subjects’ CES-D scores in relation to the median scores. Third, previous research demonstrating the adaptive response we expected to observe has suggested that such effects are not linear. For instance, variables used to compare prenatal and postpartum conditions typically have been categorical (Bogin et al., 2007; Jasienska, Thune, & Ellison, 2006; Roth, Lubin, Funk, & Sweatt, 2009; Silverira, Portella, Goldani, & Barbieri, 2007).
For these reasons, and because of the semicontinuity of the distributions (Delucchi & Bostrom, 2004), we assigned infants to groups on the basis of whether their average prenatal CES-D score across pregnancy and their CES-D score at 3 months postpartum were above or below the median (Stowe, Hostetter, & Newport, 2005; Table 1). In subsequent analyses, we assessed depressive symptoms at each of the prenatal time points to determine the influence of the timing of exposures to adversity. The effects of prenatal and postnatal exposures to depression on motor and mental development at each postnatal assessment were tested with two-way analyses of covariance (ANCOVAs). Main and interaction effects for prenatal and postnatal exposures were computed. Potential covariates in these models included ethnicity, maternal age, maternal level of income, maternal level of education, obstetric risk associated with the pregnancy, length of gestation, infant’s birth order, and infant’s sex. At each postnatal assessment, we included variables that were statistically significant predictors of MDI or PDI scores in the corresponding ANCOVA model to adjust for their contribution to main and interaction effects. In addition, at 6 and 12 months after birth, the concurrent maternal depression score was included as a covariate.
Results
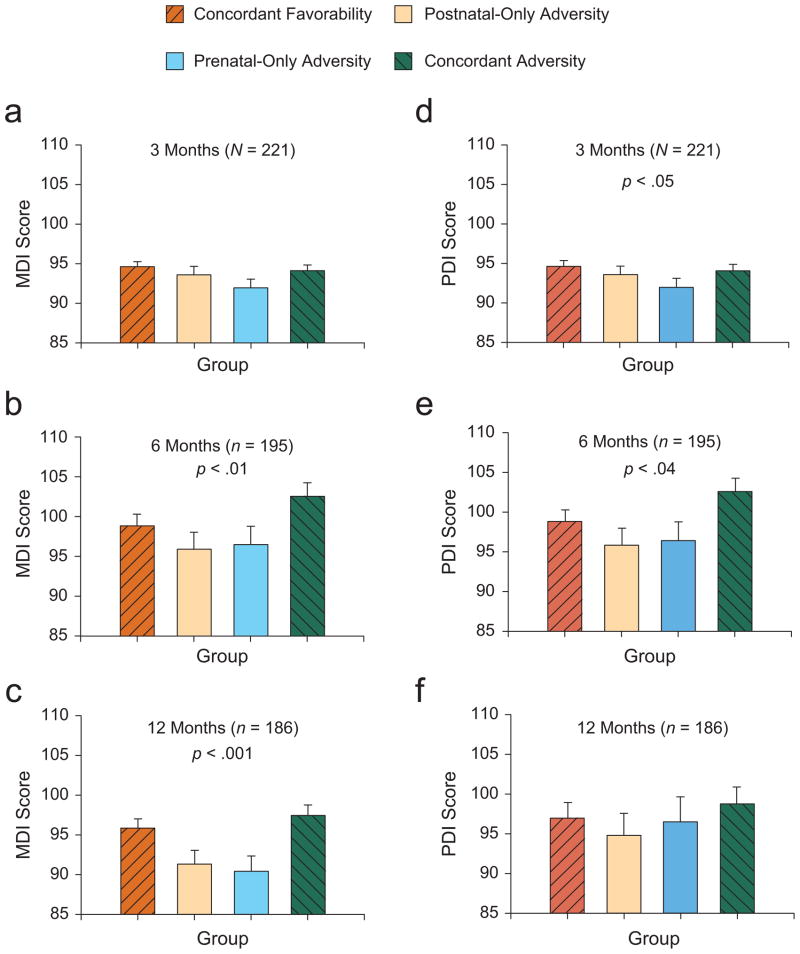
At 3 months of age, infants in the two concordant groups performed better than infants in the two discrepant groups did on measures of psychomotor development, F(1, 215) = 3.89, p < .05 (Fig. 1d), but we found no differences between the groups on measures of mental development, F(1, 215) = 2.46, p = .11 (Fig. 1a). By 6 months of age, infants in the concordant groups achieved superior performance on measures of both psychomotor development, F(1, 186) = 4.24, p < .04 (Fig. 1e) and mental development, F(1, 186) = 6.87, p < .01 (Fig. 1b), compared with infants in the two discrepant groups. By 12 months of age, infants in the two concordant groups continued to have higher mental development scores, F(1, 178) = 11.95, p < .001 (Fig. 1c), but not higher psychomotor scores (Fig. 1f), compared with infants in the two discrepant groups. We found no main effects to indicate that exposure to maternal depression alone influenced mental or psychomotor development at any age.
Fig. 1.
Mean scores on the Mental Developmental Index (MDI) and Psychomotor Developmental Index (PDI) of the Bayley Scales of Infant Development (Bayley, 1993) for infants in the concordant-favorability, postnatal-only-adversity, prenatal-only-adversity, and concordant-adversity groups. The three panels on the left present the mean MDI scores for (a) 3-month-old, (b) 6-month-old, and (c) 12-month-old infants, and the three panels on the right present the mean PDI scores for (d) 3-month-old, (e) 6-month-old, and (f) 12-month-old infants. The p values indicate significant differences between the concordant and the discrepant groups. Error bars indicate standard errors of the mean.
Because previous research has demonstrated that fetal vulnerability to adverse prenatal environments varies according to the stage of fetal maturation (Davis & Sandman, 2010), we examined the effects of concordance between prenatal depressive symptoms at particular prenatal time points (14 to 16 weeks’ gestation, 24 to 26 weeks’ gestation, 30 to 32 weeks’ gestation, and 36 or more weeks’ gestation) and post-partum maternal depressive symptoms on infants’ developmental trajectories. Maternal prenatal depressive symptoms at approximately 25 weeks’ gestation had effects consistent with the PAR model. Among infants who were exposed to congruent symptoms of maternal depression at approximately 25 weeks’ gestation and postpartum, we observed superior mental development at 3 months, F(1, 212) = 5.69, p < .02; 6 months, F(1, 184) = 4.35, p < .04; and 12 months, F(1, 176) = 4.93, p < .03. We also observed superior psychomotor development in 6-month-old infants whose mothers reported congruent levels of depressive symptoms at approximately 25 weeks’ gestation and postpartum, F(1, 184) = 5.35, p < .02. No other single prenatal time points were consistently associated with the effects of prenatal and postnatal congruence.
To investigate whether congruence between prenatal and postnatal maternal depressive symptoms exerts unique influences on child development, we assessed whether the degree and continuity of maternal perceptions of recent stress also affected the infants’ development. Despite our findings for maternal depression, we found no evidence that congruence between maternal perceptions of stress during and after pregnancy influenced infants’ mental or motor development. In addition, we investigated the possibility that stress hormones from the hypothalamic-pituitary-adrenal (HPA) axis were associated with prenatal maternal depression. The effects of maternal cortisol were examined in a series of 2 (period: prenatal, postnatal) × 2 (symptoms: depression, no depression) ANCOVAs, one for each prenatal time point. Congruence between prenatal and postnatal maternal depression was not associated with maternal level of cortisol at any gestational age.
Discussion
This prospective study is the first to evaluate the effects of congruence between prenatal and postnatal levels of maternal depression on human infant development. We obtained support for the PAR model with our counterintuitive finding that infants thrive, at least on dimensions of critical psychomotor and mental development, when their prenatal and postnatal environments are congruent, even if the conditions of those environments are adverse (Kurstjens & Wolke, 2001; Moehler et al., 2007). Moreover, postpartum euthymia in mothers did not benefit the psychomotor and mental development of infants who had been exposed to maternal depressive symptoms in utero; this finding is consistent with the predictions of the PAR model. Specifically, infants’ mental and motor functions were relatively impaired when their mothers exhibited symptoms of depression during pregnancy but became euthymic during the first 3 months after birth. These results are consistent both with reports that exposure to incongruent prenatal and postnatal nutritional conditions increases infants’ risk of developing metabolic disease and with findings of adaptive advantages among infants exposed to matching prenatal and postnatal nutritional environments (Cleal et al., 2007; Gluckman & Hanson, 2004).
Our findings indicate that stability between the prenatal and the postnatal environments prepares the fetus for postnatal life and that this preparation confers an advantage in critical survival functions during early development. The progression from superior psychomotor ability to superior mental ability during the 1st year of life among infants in the two concordant groups might indicate both a short-term adaptive response and the selection of a special characteristic that may be critical for survival (Bateson et al., 2004). Advanced psychomotor skills are advantageous early in development, when infants’ capacities for language and reasoning are undeveloped. As infants approach 1 year of age, they begin to use rudimentary language and reasoning, and it is reasonable to assume that advanced mental abilities increase infants’ opportunity for survival. It is possible that a progression of special skill advantages continues throughout the life span of infants such as those in our concordant groups and that accelerated psychomotor and mental development is replaced by advantages in other proficiencies.
The observed effects that were consistent with the PAR model were largely attributable to congruence between maternal depressive symptoms at midgestation (approximately 25 weeks) and postpartum. The human fetus may be especially sensitive or vulnerable to adversity during this period, as has been reported previously (Davis et al., 2005; DiPietro, Novak, Costigan, Atella, & Reusing, 2006; Glynn, Wadhwa, Dunkel Schetter, & Sandman, 2001; Sandman, Davis, Buss, & Glynn, 2011). Moreover, in a study of another large cohort of women, we found that biological and psychological symptoms of maternal depression at approximately 25 weeks’ gestation were the strongest predictors of postpartum depression (Yim et al., 2010; Yim, Glynn, Dunkel Schetter, Chicz-DeMet, & Sandman, 2009). These findings indicate that the fetus is most sensitive to maternal signals of adversity when those signals are the most predictive of future outcomes.
Without question, congruently favorable conditions before and after birth are beneficial for developing infants and children (Ainsworth, Blehar, Waters, & Wall, 1978; Calkins, Graziano, Berdan, Keane, & Degnan, 2008; Kochanska, Philibert, & Barry, 2009). Our finding that congruently unfavorable circumstances can also convey adaptive advantages may be at odds with the predominant view of development, which assumes that early exposures to adversity are associated with significant lifelong costs to survival, growth, and reproduction. Other models of development, however, propose that early exposures to stress have beneficial consequences later in life. For instance, the stress-inoculation model predicts that exposure to mild stress during early development promotes resilience in the face of stressful (i.e., congruent) circumstances later in life (Lyons & Parker, 2007). The theory of biological sensitivity to context (Ellis, Boyce, Belsky, Bakermans-Kranenburg, & van Ijzendoorn, 2011) posits that early exposure to stress increases the lability of responses to subsequent adversity. This increased lability results in increased impairment after exposure to stress later in life but also an enhanced ability to benefit from supportive and protective features of the environment.
The debate about the adaptive consequences of early exposure to adversity, specifically in relation to the rate of maturation, is yet unresolved. According to one view, the delayed growth and reproduction following early exposure to adversity may be adaptive, given that delayed growth and reproduction will reduce nutritional requirements if resources are scarce during early development (Bogin et al., 2007). There is some evidence, however, that early exposure to psychosocial adversity accelerates maturation, especially in the area of reproductive development. For example, exposures to psychosocial adversity during early development and again later in life are associated with accelerated sexual maturation in girls (Coall & Chisholm, 2003, 2010; Nettle, Coall, & Dickins, 2010). Researchers have argued that such early maturation maximizes the probability of reproduction and allows females to increase their number of offspring; in conditions of increased health risk, this greater number of offspring will ensure that more offspring survive.
Considering our findings in the context of this debate suggests competing explanations for the similarity between the concordant groups in our study. First, it is possible that the concordant-adversity group exhibited the accelerated maturation associated with early adversity but that the mental and motor advantages observed among infants in the concordant-favorability group reflected the benefits of relatively favorable prenatal and postnatal environments. Second, the similarities between the two concordant groups may have resulted from the same factors. If so, either both groups or neither group exhibited accelerated maturation. Subsequent follow-up studies of the subjects in these groups could determine whether either group exhibited the life-history costs typically associated with accelerated maturation. However, the improved motor and cognitive skills in the two concordant groups may have resulted not from accelerated maturation but rather from fetal prediction of and preparation for postnatal life. We believe that our findings support the conclusion that it was the congruence between prenatal and postnatal exposures to maternal depression, and not the exposure to maternal depression itself, that determined infants’ outcomes.
The precise mechanism by which a pregnant woman communicates her psychological state to her fetus is unknown. One possibility is that maternal stress and depression expose the fetus to elevated levels of stress hormones. The relation between psychosocial measures of adversity, including depression, and HPA activity during pregnancy was nonsignificant in our study and has been found to be low or nonsignificant in a number of other studies (Davis & Sandman, 2010; Harville, Savitz, Dole, Herring, & Thorp, 2009; Kramer et al., 2009); these findings suggest that stress hormones alone are not the mechanism by which maternal signals of psychosocial adversity are communicated to the fetus.
Fetal exposure to maternal depression has been shown to be associated with increased methylation of the glucocorticoid receptor gene in neonates and with increased HPA responses to stress or adversity; this finding suggests that an epigenetic mechanism may underlie both the maternal communication of adversity to the fetus and the persistent influence of the exposure (Oberlander et al., 2008). In addition, animal studies have indicated that specific patterns of maternal care during early development can alter the methylation of the nerve growth factor-inducible protein A (NGFI-A) binding site in a region of the Nr3c1 promoter responsible for the control of hippocampal glucocorticoid receptor expression; such findings support the possibility of this epigenetic effect and point to its direct implications for areas of the nervous system involved with mental development (Weaver et al., 2004). These findings, and findings from other studies reporting a link between persisting altered expression of the brain-derived neurotrophic factor in the prefrontal cortex and exposure to early adversity (Roth et al., 2009), suggest possible routes of maternal influence on fetal and early infant neurological development that have profound implications for infants’ adjustments to life challenges.
Acknowledgments
This project was conceived and designed by C. A. S., E. P. D., and L. M. G. Biological and medical reviews were conducted by C. A. S. and L. M. G. Maternal postnatal assessments were made by L. M. G. Longitudinal analysis of infant behavior was carried out by E. P. D. Statistical analyses was performed by L. M. G. and C. A. S. The authors are grateful for the outstanding assistance of Cheryl Crippen, Carol Holliday, Christina Canino, Christine Cordova, and Natalie Hernandez. The authors wish to thank the families who participated in this longitudinal project.
Funding
This research was supported by awards from the National Institutes of Health (NS-41298, HD-51852, and HD-28413 to C. A. S.; HD-40967 to L. M. G.).
Footnotes
Reprints and permission: sagepub.com/journalsPermissions.nav
Declaration of Conflicting Interests
The authors declared that they had no conflicts of interest with respect to their authorship or the publication of this article.
References
- Ainsworth MS, Blehar MC, Waters E, Wall S. Patterns of attachment: A psychological study of the strange situation. Oxford, England: Erlbaum; 1978. [Google Scholar]
- Barker DJP. Mothers, babies and health in later life. Edinburgh, Scotland: Churchill Livingston; 1998. [Google Scholar]
- Bateson P, Barker D, Clutton-Brock T, Deb D, D’Udine B, Foley RA, Sultan SE. Developmental plasticity and human health. Nature. 2004;430:419–421. doi: 10.1038/nature02725. [DOI] [PubMed] [Google Scholar]
- Bayley N. Bayley Scales of Infant Development. 2. San Antonio, TX: Psychological Corp; 1993. [Google Scholar]
- Bogin B, Silva MI, Rios L. Life history trade-offs in human growth: Adaptation or pathology? American Journal of Human Biology. 2007;19:631–642. doi: 10.1002/ajhb.20666. [DOI] [PubMed] [Google Scholar]
- Bohner KM, Breslau N. Stability of psychiatric outcomes of low birth weight: A longitudinal investigation. Archives of General Psychiatry. 2008;65:1080–1086. doi: 10.1001/archpsyc.65.9.1080. [DOI] [PubMed] [Google Scholar]
- Boorse GC, Denver RJ. Acceleration of Ambystoma tigrinum metamorphosis by corticotropin-releasing hormone. Journal of Experimental Zoology. 2002;293:94–98. doi: 10.1002/jez.10115. [DOI] [PubMed] [Google Scholar]
- Calkins SD, Graziano PA, Berdan LE, Keane SP, Degnan KA. Predicting cardiac vagal regulation in early childhood from maternal-child relationship quality during toddler-hood. Developmental Psychobiology. 2008;50:751–766. doi: 10.1002/ dev.20344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chisholm KA. A three year follow-up of attachment and indiscriminate friendliness in children adopted from Romanian orphanages. Child Development. 1998;69:1092–1106. [PubMed] [Google Scholar]
- Chugani HT, Behen ME, Muzik O, Juhász C, Nagy F, Chugani DC. Local brain functional activity following early deprivation: A study of postinstitutionalized Romanian orphans. NeuroImage. 2001;14:1290–1301. doi: 10.1006/nimg.2001.0917. [DOI] [PubMed] [Google Scholar]
- Cleal JK, Poore KR, Boullin JP, Khan O, Chau R, Hambidge O, Green LR. Mismatched pre- and postnatal nutrition leads to cardiovascular dysfunction and altered renal function in adulthood. Proceedings of the National Academy of Sciences, USA. 2007;104:9529–9533. doi: 10.1073/pnas.0610373104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coall DA, Chisholm JS. Evolutionary perspectives on pregnancy: Maternal age at menarche and infant birth weight. Social Science & Medicine. 2003;57:1771–1781. doi: 10.1016/s0277-9536(03)00022-4. [DOI] [PubMed] [Google Scholar]
- Coall DA, Chisholm JS. Reproductive development and parental investment during pregnancy: Moderating influence of mother’s early environment. American Journal of Human Biology. 2010;22:143–153. doi: 10.1002/ajhb.20965. [DOI] [PubMed] [Google Scholar]
- Cohen S. Contrasting the Hassles Scale and the Perceived Stress Scale: Who’s really measuring appraised stress? American Psychologist. 1986;41:717–718. [Google Scholar]
- Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. Journal of Health and Social Behavior. 1983;24:385–396. [PubMed] [Google Scholar]
- Davis EP, Glynn LM, Dunkel Schetter C, Hobel C, Chicz-Demet A, Sandman CA. Corticotropin-releasing hormone during pregnancy is associated with infant temperament. Developmental Neuroscience. 2005;27:299–305. doi: 10.1159/000086709. [DOI] [PubMed] [Google Scholar]
- Davis EP, Sandman CA. The timing of prenatal exposure to maternal cortisol and psychosocial stress is associated with human infant cognitive development. Child Development. 2010;81:131–148. doi: 10.1111/j.1467-8624.2009.01385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delucchi K, Bostrom AL. Methods for analysis of skewed data distributions in psychiatric clinical studies: Working with many zero values. American Journal of Psychiatry. 2004;161:1159–1168. doi: 10.1176/appi.ajp.161.7.1159. [DOI] [PubMed] [Google Scholar]
- Denver RJ. Environmental stress as a developmental cue: Corticotropin-releasing hormone is a proximate mediator of adaptive phenotypic plasticity in amphibian metamorphosis. Hormones and Behavior. 1997;31:169–179. doi: 10.1006/hbeh.1997.1383. [DOI] [PubMed] [Google Scholar]
- Denver RJ. Evolution of the corticotropin-releasing hormone signaling system and its role in stress-induced phenotypic plasticity. Annals of the New York Academy of Sciences. 1999;897:46–53. doi: 10.1111/j.1749-6632.1999.tb07877.x. [DOI] [PubMed] [Google Scholar]
- DiPietro JA, Novak MF, Costigan KA, Atella LD, Reusing SP. Maternal psychological distress during pregnancy in relation to child development at age two. Child Development. 2006;77:573–587. doi: 10.1111/j.1467-8624.2006.00891.x. [DOI] [PubMed] [Google Scholar]
- Dunkel Schetter C. Stress processes in pregnancy and pre-term birth. Current Directions in Psychological Science. 2009;18:204–209. [Google Scholar]
- Ellis BJ, Boyce WT, Belsky J, Bakermans-Kranenburg MJ, van Ijzendoorn MH. Differential susceptibility to the environment: An evolutionary-neurodevelopmental theory. Development and Psychopathology. 2011;23:7–28. doi: 10.1017/S0954579410000611. [DOI] [PubMed] [Google Scholar]
- Fihrer I, McMahon CA, Taylor AJ. The impact of postnatal and concurrent maternal depression on child behaviour during the early school years. Journal of Affective Disorders. 2009;119:116–123. doi: 10.1016/j.jad.2009.03.001. [DOI] [PubMed] [Google Scholar]
- Gluckman PD, Hanson MA. Living with the past: Evolution, development, and patterns of disease. Science. 2004;305:1733–1736. doi: 10.1126/science.1095292. [DOI] [PubMed] [Google Scholar]
- Gluckman PD, Hanson MA, Spencer HG. Predictive adaptive responses and human evolution. Trends in Ecology & Evolution. 2005;20:527–533. doi: 10.1016/j.tree.2005.08.001. [DOI] [PubMed] [Google Scholar]
- Glynn L, Wadhwa PD, Dunkel Schetter C, Sandman CA. When stress happens matters: The effects of earthquake timing on stress responsivity in pregnancy. American Journal of Obstetrics & Gynecology. 2001;184:637–642. doi: 10.1067/mob.2001.111066. [DOI] [PubMed] [Google Scholar]
- Harville EW, Savitz DA, Dole N, Herring AH, Thorp JM. Stress questionnaires and stress biomarkers during pregnancy. Journal of Women’s Health. 2009;18:1425–1433. doi: 10.1089/jwh.2008.1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasienska G, Thune I, Ellison PT. Fatness at birth predicts adult susceptibility to ovarian suppression: An empirical test of the predictive adaptive response hypothesis. Proceedings of the National Academy of Sciences, USA. 2006;103:12759–12762. doi: 10.1073/pnas.0605488103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjaer SL, Wegener G, Rosenberg R, Lund SP, Hougaard KS. Prenatal and adult stress interplay: Behavioral implications. Brain Research. 2010;1320:106–113. doi: 10.1016/j.brainres.2010.01.008. [DOI] [PubMed] [Google Scholar]
- Kochanska G, Philibert RA, Barry RA. Interplay of genes and early mother–child relationship in the development of self-regulation from toddler to preschool age. Journal of Child Psychology and Psychiatry. 2009;50:1331–1338. doi: 10.1111/j.1469-7610.2008.02050.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer MS, Lydon J, Seguin L, Goulet L, Kahn SR, McNamara H, Platt RW. Stress pathways to spontaneous preterm birth: The role of stressors, psychological distress, and stress hormones. American Journal of Epidemiology. 2009;169:1319–1326. doi: 10.1093/aje/kwp061. [DOI] [PubMed] [Google Scholar]
- Kurstjens S, Wolke D. Effects of maternal depression on cognitive development of children over the first 7 years of life. Journal of Child Psychology and Psychiatry. 2001;42:623–636. [PubMed] [Google Scholar]
- Lucas A, Fewtrell MS, Cole TJ. Fetal origins of adult disease—the hypothesis revisited. British Medical Journal. 1999;319:245–249. doi: 10.1136/bmj.319.7204.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons DM, Parker KJ. Stress inoculation-induced indications of resilience in monkeys. Journal of Traumatic Stress. 2007;20:423–433. doi: 10.1002/jts.20265. [DOI] [PubMed] [Google Scholar]
- McCormack VA, Dos Santos Silva I, De Stavola BL, Mohsen R, Leon DA, Lithell HO. Fetal growth and subsequent risk of breast cancer: Results from long term follow up of Swedish cohort. British Medical Journal. 2003;326:248. doi: 10.1136/bmj.326.7383.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moehler E, Kagan J, Parzer P, Brunner R, Reck C, Wiebel A, Resch F. Childhood behavioral inhibition and maternal symptoms of depression. Psychopathology. 2007;40:446–452. doi: 10.1159/000107429. [DOI] [PubMed] [Google Scholar]
- Nettle D, Coall DA, Dickins TE. Birthweight and paternal involvement predict early reproduction in British women: Evidence from the National Child Development Study. American Journal of Human Biology. 2010;22:172–179. doi: 10.1002/ajhb.20970. [DOI] [PubMed] [Google Scholar]
- Oberlander TF, Weinberg J, Papsdorf M, Grunau R, Misri S, Devlin AM. Prenatal exposure to maternal depression, neonatal methylation of human glucocorticoid receptor gene (NR3C1) and infant cortisol stress responses. Epigenetics. 2008;3:97–106. doi: 10.4161/epi.3.2.6034. [DOI] [PubMed] [Google Scholar]
- Radloff LS. The CESD Scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- Roseboom TJ, van der Meulen JH, Osmond C, Barker DJ, Ravelli AC, Schroeder-Tanka JM, Bleker OP. Coronary heart disease after prenatal exposure to the Dutch famine, 1944–45. Heart. 2000;84:595–598. doi: 10.1136/heart.84.6.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth TL, Lubin FD, Funk AJ, Sweatt JD. Lasting epigenetic influence of early-life adversity on the BDNF gene. Biological Psychiatry. 2009;65:760–769. doi: 10.1016/j.biopsych.2008.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandman CA, Davis EP. Gestational stress influences cognition and behavior. Future Neurology. 2010;5:675–690. [Google Scholar]
- Sandman CA, Davis EP, Buss C, Glynn LM. Exposure to prenatal psychobiological stress exerts programming influences on the mother and her fetus. Neuroendocrinology. 2011 doi: 10.1159/000327017. Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santor DA, Coyne JC. Shortening the CES-D to improve its ability to detect cases of depression. Psychological Assessment. 1997;9:233–243. [Google Scholar]
- Silverira PP, Portella AK, Goldani MZ, Barbieri MA. Developmental origins of health and disease (DOHaD) Journal de Pediatria. 2007;83:494–504. doi: 10.2223/JPED.1728. [DOI] [PubMed] [Google Scholar]
- Stowe ZNA, Hostetter AL, Newport DJ. The onset of postpartum depression: Implications for clinical screening in obstetrical and primary care. American Journal of Obstetrics & Gynecology. 2005;192:522–526. doi: 10.1016/j.ajog.2004.07.054. [DOI] [PubMed] [Google Scholar]
- Weaver IC, Cervoni N, Champagne FA, D’Alessio AC, Sharma S, Seckl JR, Meaney MJ. Epigenetic programming by maternal behavior. Nature Neuroscience. 2004;7:847–854. doi: 10.1038/nn1276. [DOI] [PubMed] [Google Scholar]
- Yim IS, Glynn LM, Dunkel Schetter C, Chicz-DeMet A, Sandman CA. Elevated corticotropin-releasing hormone in human pregnancy increases the risk of postpartum depressive symptoms. Archives of General Psychiatry. 2009;66:162–169. doi: 10.1001/archgenpsychiatry.2008.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yim IS, Glynn LM, Dunkel Schetter C, Hobel CJ, Chicz-DeMet A, Sandman CA. Prenatal B-endorphin as an early predictor of postpartum depressive symptoms in euthymic women. Journal of Affective Disorders. 2010;125:128–133. doi: 10.1016/j.jad.2009.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]