Abstract
The developmental origins of disease or fetal programming model predict that early exposures to threat or adverse conditions have lifelong consequences that result in harmful outcomes for health. The maternal endocrine ‘fight or flight’ system is a source of programming information for the human fetus to detect threats and adjust their developmental trajectory for survival. Fetal exposures to intrauterine conditions including elevated stress hormones increase the risk for a spectrum of health outcomes depending on the timing of exposure, the timetable of organogenesis and the developmental milestones assessed. Recent prospective studies, reviewed here, have documented the neurodevelopmental consequences of fetal exposures to the trajectory of stress hormones over the course of gestation. These studies have shown that fetal exposures to biological markers of adversity have significant and largely negative consequences for fetal, infant and child emotional and cognitive regulation and reduced volume in specific brain structures.
Keywords: cortisol, CRH, developmental origins of disease, fetal development, fetal programming, infant development, predictive adaptive response, pregnancy, prenatal stress, stress
The developmental origins of disease or fetal programming model predicts that early exposures to threat or adverse conditions have lifelong consequences that result in poor health outcomes [1]. The fetal period in the life cycle is unmatched by any other in growth and development, and it is the period in the human lifespan that is most vulnerable to both organizing and disorganizing influences. The human fetal brain is a primary target for programming influences because it is undergoing dramatic growth over a prolonged period of time. From 8 to 16 weeks gestational age (GA), neurons migrate to form the subplate zone and await connections from afferent neurons originating in the thalamus, basal forebrain and brainstem. At the same time, cells collect in the outer cerebral wall to form the cortical plate, which eventually will become the cerebral cortex. By gestational week 20, axons form synapses with the cortical plate, and by gestational week 24, cortical circuits are organized [2,3]. The growth of the nervous system is distinguished by the proliferation of neurons. Remarkably, by gestational week 28, the number of neurons in the human fetal brain is 40% greater than that in the adult [3–6]. The rate of synaptogenesis reaches an astonishing peak so that at gestational week 34 to 24 months post-partum, there is an increase of 40,000 synapses per second [7]. Thus, prenatal life is a time of enormous neurological change and because of that the fetal nervous system is principally susceptible to programming influences.
The basic assumption of the fetal programming model of disease is that developing organisms, including the human fetus, play a dynamic role in their own construction [8]. One example of this is the adjustment made by the tadpole in response to stress [9–11]. The desert-dwelling Western spadefoot toad lays its eggs in desert rainwater. If the developing tadpoles detect that the conditions for normal development and survival are unfavorable (e.g., rapid evaporation of the pool), stress hormones including corticotrophin-releasing hormone (CRH) and corticosterone (glucocorticoids) are released. These hormones influence metabolism and accelerate metamorphosis, so that the tadpole can escape the desiccating environment and avoid impending peril. If the biological stress response is blocked during this life-threatening stress, the rate of development is arrested, and the tadpole’s survival is compromised. There are penalties for the tadpole that survives under these conditions, however, because it is smaller at maturity and is at a disadvantage when competing with a normally developing toad foraging for food or reproducing.
Variations of this remarkable surveillance and response system are conserved so that many species, including the human fetus, can detect threats and adjust their development [10,12,13]. The human placenta is both a sensory and effector organ that incorporates and transduces information from its maternal host environment into the fetal developmental program. Early detection by the fetal/placental unit of stress signals from the maternal environment ‘informs’ the fetus that there is a threat to survival. The placental/fetal unit responds to this information in an unusual but adaptive way. In contrast to the inhibitory influence on the promoter region of the CRH gene in the hypothalamus (i.e., negative feedback), maternal stress signals (glucocorticoids) activate the CRH promoter region in the placenta, stimulate its synthesis, and advance the placental clock, resulting in myometrial activation and fetal escape (premature birth) from a malignant environment [14,15]. In parallel, the fetus adjusts its developmental trajectory and modifies its nervous system to ensure survival in a potentially hostile postpartum environment. Survival under these circumstances, as described below, is associated with compromised motor, cognitive and emotional function [16,17] and reduced brain gray matter volume [18–20].
The ‘fight or flight’ stress system
The ‘fight or flight’ stress system is profoundly altered during human pregnancy. The fight or flight response first described by Cannon characterizes the reaction of organisms to threat [21]. When the human fight or flight response is triggered, sequences of nerve cell firing are activated, and chemicals are released into the bloodstream. These responses cause the body to undergo a series of very dramatic changes (Box 1), which intensify awareness, sharpen sight, quicken impulses, provide a burst of energy, exaggerate fear, focus attention and diminish the perception of pain. All of these responses are adaptive when an organism is threatened because they prepare for fight or flight and increase the probability of survival.
Box 1. Changes in the body associated with the fight or flight response.
Heart rate and blood pressure increase. The heart pump rate increases from 1 to 5 gallons per minute, arteries constrict to maximize pressure and veins open out to ease return of blood to the heart
Veins in skin constrict to send more blood to major muscle groups. Pupils dilate to take in as much light as possible to improve vision
Hairs stand on end, making us more sensitive to the environment
Fat from fatty cells and glucose from the liver are metabolized to create instant energy
Muscles tense up, energized by adrenaline and glucose
Smooth muscles relax to allow more oxygen into the lungs
Lungs, throat and nostrils open up and breathing speeds up to get more air into the system, so blood flow can be reoxygenated. The blood carries oxygen to the muscles, allowing them to work harder
Blood vessels to the kidney and digestive system are constricted, effectively shutting down systems that are not essential. Shunted blood is directed to muscles and limbs to provide energy for fight or flight
Reduction of saliva in the mouth
The bowels and bladder may open to reduce the need for other internal actions
Blood vessels to the skin are constricted to reduce potential blood loss
Sweat glands are opened to provide an external cooling
Stress hormones are released
Selye recognized, however, that the fight or flight system could become maladaptive and lead to disease if it was chronically activated or if it responded to modern-day, non-life-threatening stressors (rather than the challenges experienced by primitive man or by organisms with natural predators) [22]. His theory of the general adaptation syndrome was characterized by an alarm reaction or shock phase in the immediate response to stress or threat, a stage of resistance to maintain safety and health and finally, if defenses failed, an exhaustion stage placing the organism at risk for ill health. An inadequate response or exhaustion of the endocrine system to stress and threat was central to Selye’s theory, and he proposed that it resulted in ‘wear and tear on the body’ [23].
Endocrine fight or flight system
Systemic stress activates the expression of the master stress hypothalamic hormone, CRH, which stimulates the cascade of events preparing the organism for ‘fight or flight.’ CRH, a 41-amino-acid neuropeptide, is synthesized primarily in the paraventricular nucleus (PVN) of the hypothalamus and has a major role in regulating pituitary and adrenal function and the physiological response to stress [24,25]. CRH stimulates the synthesis of a bioinactive 31 kDa pro-hormone, pro-opiomelanocortin in the pituitary, which is converted by enzymes into adrenocorticotropic hormone (ACTH) and other active peptides. ACTH enters the bloodstream and elicits secretion of glucocorticoids (cortisol in humans) from the adrenal gland. The negative feedback between the adrenal gland and both the hypothalamus and pituitary gland shuts down the endocrine stress response under normal conditions. In addition, cortisol crosses the blood–brain barrier and activates specific receptors in limbic brain structures and in the cortex. The limbic structures, especially the hippocampus, prefrontal cortex (PFC) and amygdala, have both excitatory and inhibitory connections with the hypothalamic–pituitary–adrenal (HPA) axis [26].
Stimulation of the amygdala promotes synthesis and release of CRH from the hypothalamus and begins the sequence of events, that ultimately results in corticosteroid biosynthesis and secretion in the adrenal gland. In contrast to the amygdala, activation of the hippocampus terminates the HPA axis responses to stress. Hippocampal lesions are associated with basal hypersecretion of glucocorticoids [27], enhanced basal CRH mRNA expression [28,29], increased ACTH secretion in PVN neurons and prolonged cortico sterone and ACTH release following exposure to a variety of stressors [30,31]. The PFC also plays an important role in negative-feedback regulation of the HPA axis [32]. Studies in rats [33–35] and humans [36,37] show that the PFC is a significant target for the negative-feedback actions of circulating corticosteroids. Direct implants of corticosterone into the medial prefrontal region decrease stress-induced ACTH and corticosterone secretion following acute or repeated restraint [38,39]. Administration of CRH enhances CRF1 RNA expression throughout the medial PFC. There is evidence that the CRH peptide interacts with CRH neurons in the PFC to inhibit the HPA axis via indirect pathways, reducing CRH release from the PVN [40].
Alterations of the endocrine fight or flight system during pregnancy
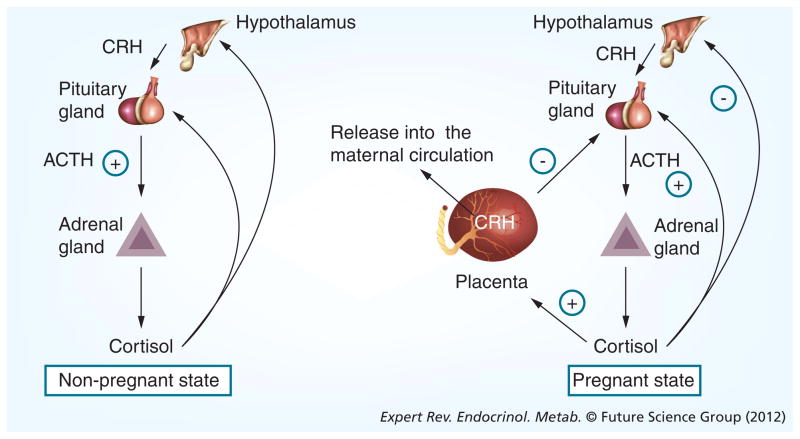
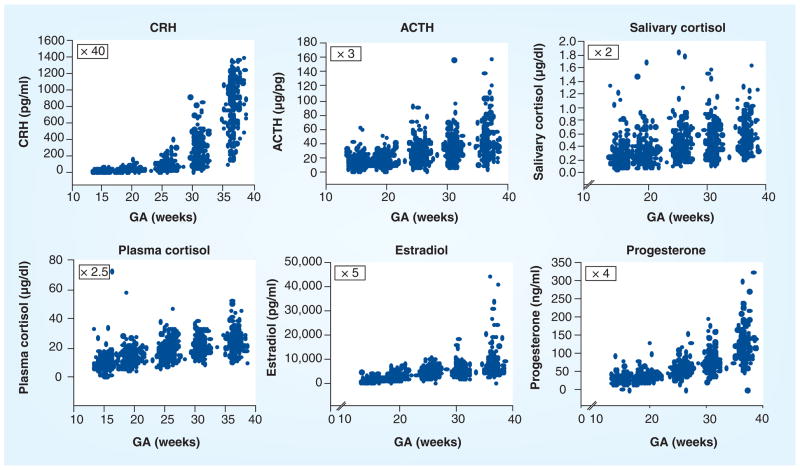
The endocrine stress or fight or flight system is profoundly altered during human pregnancy. The maternal pituitary gland doubles in size, and the output of peptides and hormones from the HPA and placental axis increases several fold as gestation progresses (Figure 1). However, it is the growth and development of a new organ, the placenta, in primates that is primarily responsible for the profound changes in the stress circuit. The human placenta and amniotic membrane express the genes for the major stress hormones CRH (hCRH mRNA) and pro-opiomelanocortin by the seventh week of gestation. As presented in Figure 2, all of the HPA and placental stress hormones increase as pregnancy advances, but the increase in placental CRH in maternal plasma is especially remarkable, reaching levels observed only in the hypothalamic portal system during physiological stress [41]. The levels of hCRH mRNA increase more than 20-fold in the 5 weeks preceding delivery [42], resulting in a significant elevation in maternal CRH plasma concentrations during the second half of pregnancy [15]. Levels rise exponentially as pregnancy advances, peaking during labor and falling to very low or undetectable levels within 24 h after delivery [43–47].
Figure 1. Changes in the hypothalamic–pituitary–adrenal axis during human pregnancy.
The growth and development of the placenta alters the feedback relationships among the hypothalamus, pituitary and adrenal gland.
ACTH: Adrenocorticotropic hormone; CRH: Corticotrophin-releasing hormone.
Figure 2. Increases in hypothalamic–pituitary–adrenal and placental hormones/peptides as a function of human gestational age.
ACTH: Adrenocorticotropic hormone; CRH: Corticotrophin-releasing hormone; GA: Gestational age.
Placental CRH is identical to hypothalamic CRH in structure, immunoreactivity and bioactivity [48,49]; however, in contrast to the inhibitory influence on the promoter region of the CRH gene in the hypothalamus, maternal stress signals (cortisol) from the adrenal glands activate the promoter region in the placenta and stimulate the expression of hCRH mRNA. This establishes a feedback loop that results in the simultaneous stimulation, synthesis and release of cortisol, ACTH and CRH over the course of gestation (Figure 2). The different, nearly opposite, activities of the CRH gene in the placenta and hypothalamus are because of the expression of different transcription factors, coactivators and corepressors in these two tissues [50]. The increase of CRH especially over the latter part of human gestation plays a fundamental role in the organization and programming of the fetal nervous system [51], influences the timing of the onset of spontaneous labor and delivery [14,15,52–54], and alters maternal adaptation during pregnancy, including dampening the effects of psychological stress [55].
Endocrine risk
The human placenta integrates numerous sources of maternal stress signals, including cortisol, and responds with a dose-dependent release of placental CRH. The surge in placental CRH is produced by syncytial cells (which can be created in vitro by fusion of purified cytotrophoblast cells) [49]. As described above, there is a positive or feed-forward loop between maternal cortisol and placental CRH, in contrast to the negative-feedback system controlling hypothalamic CRH synthesis and release. The positive relationship between cortisol and CRH release from the placenta is similar to the stimulating effect of cortisol on the amygdala release of CRH [56]. In placental tissue, glucocorticoids stimulate CRH gene expression by interacting with proteins that bind to the cAMP response site of the CRH promoter [57].
Evidence suggests that the normal trajectory of placental CRH production over the course of gestation may be accelerated by an adverse intrauterine environment characterized by physiological stress. For example, elevated placental CRH has been observed in pregnancies complicated by preeclampsia, reduced utero–placental perfusion, intrauterine infection and in cases where fetal distress has led to elective preterm delivery [58]. A series of in vitro studies have shown that CRH is released from cultured human placental cells in a dose-response manner in response to all the major biological effectors of stress, including cortisol, catecholamines and proinflammatory cytokines [49,59,60]. The authors have in vivo evidence in humans that elevated levels of maternal cortisol early in gestation are associated with a more rapid rise in placental CRH concentrations [15]. Not only is placental CRH responsive to stress-related increases in maternal cortisol, but the administration of synthetic glucocorticoids for fetal lung maturation in pregnant women at risk for preterm delivery is similarly associated with significant increases in circulating placental CRH. Placental CRH concentrations increase by 1.5-fold within 12 h in response to the administration of synthetic glucocorticoids, such as betamethasone [61,62].
The placental detection of stress or adversity results in a rapid rise in CRH and primes or advances the ‘placental clock’ and begins the cascade of events influencing myometria [54] and in extreme cases, precipitating preterm birth. Moreover, placental CRH has been shown to increase the placental production of estrogens and to inhibit the synthesis of progesterone [63,64]. Placental CRH is additionally released into the fetal compartment, where it stimulates the fetal adrenal to release dehydroe-piandrosterone sulfate, an obligate precursor for placental estriol production [65]. Alterations in the production of progesterone and estriol may be one pathway by which placental CRH regulates the timing of delivery [66].
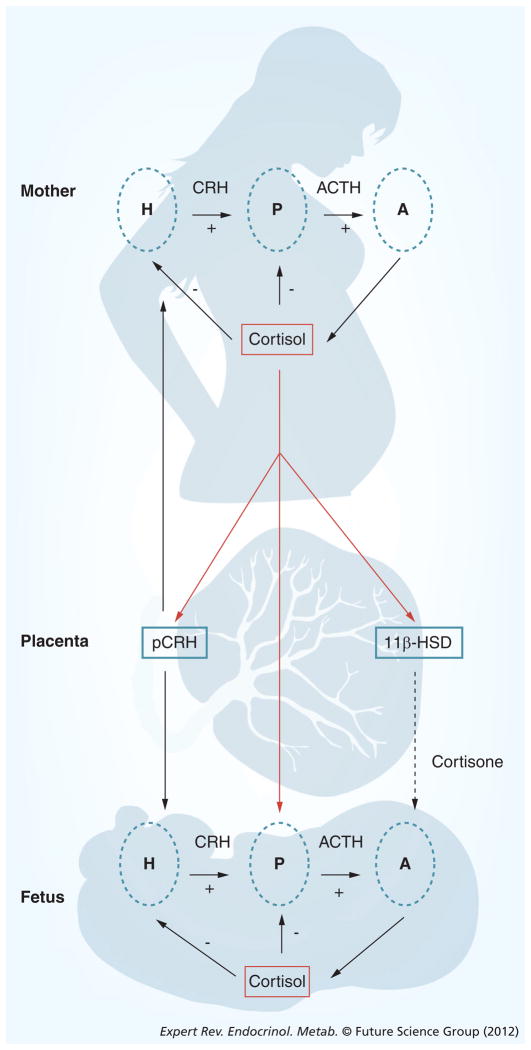
There is evidence that it is the trajectory of placental CRH and other hormone production over gestation, rather than their absolute concentrations, that best predicts preterm delivery, suggesting that target cells are highly responsive to relative changes in circulating concentrations. The effects of HPA and placental axis hormones on gestational length are modulated further by the activities of binding proteins and enzymes. For example, concurrent with increases in circulating levels of placental CRH, a CRH-binding protein (CRH-BP) is produced in the liver and also in the trophoblast and intrauterine tissues during pregnancy, and binds to circulating CRH, reducing its biological action [67–69]. In contrast to the increasing levels of circulating placental CRH over the course of gestation, CRH-BP levels are constant in the first, second and early third trimester (and are not significantly different from nonpregnant levels), but fall by approximately 30% as birth approaches [70]. The consequence of these changes in the levels of CRH and CRH-BP is a large increase of free and bioactive CRH during the last part of gestation. Maternal plasma cortisol-binding globulin (CBG) levels also increase progressively with advancing gestation until the end of gestation, when there is a significant decline in CBG leading to an increase in bioactive cortisol [71]. Moreover, levels of placental 11β-HSD2 (which oxidizes cortisol into its inactive form, cortisone) also increase as gestation progresses before falling abruptly near term (Figure 3) [72]. The decrease in CBG and the decrease in the activity of placental 11β-HSD2 toward the end of gestation increase fetal exposure to maternal cortisol, ensuring maturation of the fetal lungs, CNS and other organ systems in full-term births [73,74]. These exposures to elevated cortisol toward the end of gestation are normative and necessary for fetal maturation. Excessive exposures such as in the case of maternal stress or synthetic glucocorticoid treatment, however, are detrimental. Maternal stress may result in fetal exposure to excess cortisol both by increasing cortisol production and downregulating the activity of 11β-HSD2 [75].
Figure 3. The regulation of the hypothalamic–pituitary–adrenal axis changes dramatically over the course of gestation.
Uniquely during primate pregnancy, cortisol stimulates the synthesis and release of placental corticotrophin-releasing hormone. These two hormones are important stress signals that can influence the timing of birth and alter the neurological development of the fetus.
A: Adrenal gland; ACTH: Adrenocorticotropic hormone; CRH: Corticotrophin-releasing hormone; H: Hypothalamus; P: Pituitary gland; pCRH: Placental corticotrophin-releasing hormone.
Evidence for prenatal exposure to elevated HPA influences on health outcomes
There is compelling evidence from both animal and human studies that exposure to excess glucocorticoids during the prenatal period is a risk factor for adverse neurodevelopmental outcomes, including suppressed growth [76–80], HPA axis dysregulation [81–85], increased fear and anxiety [86–89], and impaired cognitive functioning [87,90,91]. There is evidence from animal models that prenatal treatment with synthetic glucocorticoids has persisting and programming effects on brain development [92–97].
Exposure to stress and manipulations that elevate CRH, including exogenous administration, particularly early in development, have pervasive programming consequences for both brain and behavior [98]. CRH affects the developing brain directly due to extensive expression of CRH receptors throughout the brain [99], as well as through effects on the fetal HPA axis [100]. CRH has neurotoxic effects on hippocampal neurons [101–105]; effects that are more pronounced in the immature brain [103,104,106] and be prevented by selective blockade of the CRH1 can receptor [107]. Furthermore, exogenously administered CRH increases limbic neuronal excitation leading to seizures [108–110] and participates in mechanisms of neuronal injury [103].
Consistent with the clear evidence that elevated concentrations of CRH impair the developing brain in animal models, the authors present recent evidence that prenatal exposure to elevations in maternal stress hormones influences the maturation of the human fetal nervous system with long-term consequences for child development. These data suggest that fetal exposure to maternal stress hormones shape or program the developing fetal nervous system with consequences for impaired stress regulation, fearful temperament, neuromotor functioning and brain development.
Our approach
The research team has been exploring the consequences of early and gestational exposures in animal and human models to endocrine markers of stress for over 30 years and have concluded that fetal exposure to maternal and placental hormones has a lasting influence on neurological function [8,55,111,112]. These initial studies were among the first to describe the long-lasting (perhaps permanent and programming) effects of fetal and neonatal exposure to ACTH and β-endorphin on the brain and behavior of rats [113–115]. The authors reported that fetal exposure to stress hormones increased their expression [116] and downregulated receptors in the brain [117] of these animals as adults.
Over the past 15 years or longer, the group has conducted prospective, longitudinal studies of over 800 mother/fetal dyads. The protocol for the assessment of prenatal exposure to maternal stress and stress hormones on fetal, infant and child development includes maternal psychosocial and biological stress measures collected at five gestational intervals beginning between 14 and 16 weeks. Maternal/fetal dyads are assessed at 15, 20, 25, 31 and 36 weeks of gestation. At approximately 25, 31 and 36 gestational weeks, fetal neurodevelopment is evaluated with a measure of startle and habituation. At delivery, information on length of gestation and birthweight is abstracted from medical records. Infant assessments begin 24 h postdelivery with the collection of cortisol and behavioral responses to the painful stress of the heel-stick procedure and measures of neonatal neuromuscular maturity. Infant cognitive, neuromotor development, stress and emotional regulation are evaluated at 3, 6, 12 and 24 months of age. Maternal psychosocial stress and demographic information is collected in parallel with infant assessments. Child neurodevelopment is assessed between 6 and 9 years of age with cognitive tests, measures of adjustment and brain imaging. The authors believe that it is essential to make multiple assessments during gestation and follow-up because these are critical periods both for the effects of programming on the nervous system and for the expression of subsequent behaviors. For instance, during gestation, organ systems may be most vulnerable during periods of rapid development. Furthermore, as cognitive functions come ‘on line,’ the authors may begin to detect delays that were not apparent previously; the effects of prenatal exposure to stress hormones on language areas cannot be detected before the child is able to talk. The authors have concluded from these studies that fetal exposures to elevated stress hormones of the HPA and placental axis and to maternal psychological stress are risk factors for adverse neurobehavioral outcomes [8,111]. All of these studies described here include normative samples of healthy mothers and infants/children. Furthermore, in all cases, the consequences of prenatal stress are present after considering postnatal influences, such as socioeconomic status (SES) and postnatal maternal stress, anxiety and depression.
In addition to the evaluation of natural variations in maternal stress and stress hormones, the authors have assessed the consequences of prenatal treatment with synthetic glucocorticoids. This approach takes advantage of a common clinical practice: administration of glucocorticoid treatment to women at risk of preterm delivery to promote fetal lung maturation. This model complements the understanding of the influence of natural variation in prenatal maternal stress hormones by testing the effect of administration of a high level of glucocorticoids on developmental trajectories. These studies are also important because this treatment is part of the standard of care for women in preterm labor and has resulted in millions of children being exposed to excess glucocorticoids during the prenatal period. Despite the beneficial effects of treatment for lung functioning and survival among preterm newborns, the long-term effects on human brain and behavioral development are not known. The authors believe that this approach provides important information about the impact of intrauterine exposure to excess glucocorticoids. A unique component of research is the inclusion of children who were born healthy and full term. This cohort is particularly important for two reasons: these children were exposed to a high dose of glucocorticoids without receiving commensurate medical benefits and the consequences of glucocorticoid treatment can be differentiated from the effects of preterm delivery.
The authors have demonstrated that prenatal glucocorticoid treatment suppresses growth [81] and alters postnatal HPA axis functioning in both preterm and full-term infants [82,83,118]. These alterations to biological systems during fetal development resulting from prenatal exposure to glucocorticoids may determine the neonate’s ability to respond physiologically to stressors in the postnatal environment and may establish a trajectory of development associated with increased cortisol reactivity to stress and a greater risk for the development of behavioral inhibition and anxiety. Ongoing studies to follow these children will determine the persisting effects of prenatal glucocorticoid treatment for brain and behavior. Furthermore, these prospective studies will identify maternal/fetal dyads who are vulnerable to the negative consequences of glucocorticoid treatment because of the associated changes in maternal and placental physiology.
Maternal fight or flight system influences fetal behavior
Measures of fetal responses to external stimulation have been used to directly assess the developmental consequences of exposure to endocrine markers of stress [119]. The authors have reported that elevated placental CRH [51] or pituitary stress hormones [120] during the third trimester is associated with dampened fetal responses to a novel stimulus and delayed habituation to repeated stimulation. In a more recent study of programming influences on the fetus, the authors found that low CRH at 15 gestational weeks, but not later, predicted a more mature fetal heart rate pattern at 25 gestational weeks [121]. This is evidence that exposure to endocrine stress exerts programming influences on the developing fetal nervous system with possible consequences for the trajectory of development and for the risk of poor neurobiological health outcomes [111,112].
Neurological risk & GA: birth phenotype or intrauterine exposures?
The important and fertile initial studies introducing the programming hypothesis were retrospective and relied on birth phenotype, either birthweight or gestational length, as markers of intrauterine exposures. In these early studies, adult health outcomes were known, but birth outcomes were obtained from a review of old medical records. The well-known findings were that poor health was associated with adverse birth outcomes. It is well established and briefly described herein that adverse birth outcomes can result from numerous intrauterine complications and sources. It is also well known that adverse birth outcomes influence the course of infant and child development. Contemporary studies, including the authors’ prospective projects, are focused on specific intrauterine conditions that influence (program) the fetus, and these conditions may or may not result in adverse birth outcomes. In every case, the authors’ projects on the effects of intrauterine fetal exposures on neurodevelopmental outcome always adjust for gestational length and size at birth.
As described earlier, the human fetus detects maternal stress and can ‘escape’ or ‘flee’ from the inhospitable maternal host by accelerating the birth process, enlisting the same stress hormones as the tadpole does to escape threats to survival. The association between preterm labor/delivery and maternal plasma concentrations of CRH (and other hormones) and levels of stress in humans and has been examined in many published studies [122]. The general findings are that plasma CRH concentrations of women in preterm labor are significantly higher than those of gestational age-matched controls, and the rate of change of CRH over gestation is accelerated in women destined to deliver early [15]. Similarly, higher levels of stress and anxiety are associated with shorter gestational length [123]. Studies measuring CRH or stress at a single point during gestation produce equivocal findings because there are wide individual differences that can only be assessed with longitudinal designs, and because it is the trajectory of these biopsychological markers over gestation that best predicts length of gestation generally and not just preterm birth [14,66].
There are well-documented increases in infant and toddler mortality and morbidity associated with preterm birth, but there are other longer-term risks associated with abbreviated gestation. Retrospective studies have concluded that fetuses born early or small for GA are at greater subsequent risk for later cardiovascular disease, hypertension, hyperlipidemia, insulin resistance, non- insulin-dependent diabetes mellitus, obesity, higher serum cholesterol concentrations, shortened lifespan and other poor health outcomes [1,124–127]. The consequences of shortened gestation are not restricted to preterm birth, but rather are apparent across the full GA range [128]. New findings from the program indicate that there are neurological advantages in 6–10-year-old children associated with longer gestation even in full-term births (greater than 37 weeks) [19]. As reviewed earlier, elevated levels of placental CRH and other hormones are a significant risk factor for shortened gestation, and the authors believe that fetal exposures to these endocrine changes during pregnancy make an independent contribution to subsequent health outcomes typically associated with preterm birth.
Maternal fight or flight system influences stress, emotional regulation & the brain
Neonates
Evidence from the project indicates that fetal exposure to maternal stress hormones exerts influences on the development of the fetal HPA axis that have consequences for neonatal functioning [129]. The authors reported that fetal exposure to maternal cortisol and psychosocial stress influences stress regulation in 24-hold neonates. Elevated maternal cortisol early in gestation and maternal psychosocial stress throughout gestation were associated with slower neonatal behavioral recovery from the painful stress of a heel-stick procedure. Elevated maternal cortisol during the second half of gestation was associated with a larger and more prolonged neonatal cortisol response to stress. These findings were consistent with evidence that prenatal exposure to synthetic glucocorticoids during the late second and early third trimester in healthy full-term infants were associated with an amplified cortisol response to stress [118]. These data are important because they indicate that prenatal exposure to glucocorticoids alters developmental trajectories of the fetal HPA axis, and these effects on the HPA axis are apparently independent of gestational length and before there is in an opportunity for postpartum influences. Moreover, these findings provide support that the timing of exposures during gestation determine the neonate’s ability to respond behaviorally and physiologically to stressors in the postnatal environment. It is possible that neonates who are more reactive because of their prenatal exposure to stress hormones carry a greater risk for developmental and other health risks independent from birth outcome.
Infants
The authors have found that the association between prenatal psychobiological stress signals and greater behavioral reactivity persists throughout infancy. Elevated maternal cortisol and placental CRH during the third trimester independently predicted infants’ reactivity to novel stimuli, indicating greater fearful temperament [130,131]. Infants who are easily aroused by varied stimulation are more likely to become behaviorally inhibited as young children [132,133] and result in higher risk for social anxiety in adolescence [134]. Thus, it is likely that prenatal exposure to elevated cortisol and placental CRH has direct implications for subsequent behavioral problems including increased risk for affective disorders. These longitudinal studies in which the authors have followed these infants into childhood support this hypothesis.
Children
Exposure to maternal psychological distress during gestation is associated with negative temperament at 2 years of age [135]. These recent data provide compelling evidence that the consequences of these intrauterine exposures persist and that they contribute to risk for anxiety disorders [136]. In this study of 178 mother/child dyads, the authors reported that prenatal exposure to elevated maternal cortisol, depression, perceived stress and pregnancy-specific anxiety was associated with increased anxiety symptoms in 6–9-year-old children. The results of this study indicate that elevated maternal cortisol levels and maternal psychological distress during gestation are associated independently with childhood anxiety as long as 9 years later, and that this association could not be explained by critical prenatal or obstetric risk factors or by postnatal influences such as maternal psychological wellbeing and SES. Specifically, children who were exposed to elevated levels of maternal cortisol during gestation are significantly more likely to fall in the borderline or clinically significant range for anxiety (odds ratio: 2.1). The association between prenatal exposure to elevated maternal cortisol and child anxiety is consistent with experimental animal studies indicating lifelong consequences of prenatal exposure to elevated glucocorticoids [137,138], and with human studies that link maternal cortisol with increased fearful or reactive behavior during infancy [131,139]. This project identifies prenatal risk factors associated with lasting consequences for child mental health. Importantly, the association between the prenatal risk factors and child outcomes described here cannot be explained by postnatal exposures such as SES or postnatal maternal psychological distress. These data raise the possibility that reducing maternal distress during the prenatal period will have long-term benefits for child wellbeing.
Exposure to maternal cortisol during gestation may influence the development of anxiety by modifying fetal development in regions such as the amygdalae [140,141] that are particularly sensitive to excessive levels of glucocorticoids [142] and play a role in the regulation of anxious behavior [143]. Findings from animal models illustrate that prenatal stress exposures, including excess glucocorticoids, alter the density of cortisol receptors [144] and increase the production of CRH in the amygdalae [145,146]. Furthermore, exposure to stress during the prenatal period is associated with an increase in amygdala volume [147], suggesting a plausible mechanism by which prenatal cortisol may influence vulnerability to the development of anxiety.
In a recent study [148], the authors evaluated the consequences of fetal exposure to maternal cortisol early in pregnancy on the volumes of the amygdala and hippocampus in 65 children. The authors found that higher maternal cortisol concentrations in early gestation were associated with a larger right amygdala volume and affective problems in girls at the age of 7 years. The magnitude of the effect was substantial; a one standard deviation increase in maternal cortisol was associated with a 6.4% increase in the size of the right amygdala. The current findings represent the first report linking maternal stress hormone levels in human pregnancy with subsequent volume of the amygdala and affective problems in childhood. The effect was significant after controlling for the effects of other pregnancy, birth and concurrent child and maternal characteristics, including maternal depression. Statistical modeling suggested that the association between maternal cortisol in early gestation and affective problems in girls is mediated by their cortisol-associated larger right amygdala.
Maternal fight or flight system influences cognition & brain development
Neonates
In a previous study of 158 newborns within 24 h after birth, the authors found that fetal exposure to increased levels of maternal cortisol at 15 and 19 weeks gestation and increased levels of placental CRH at 31-weeks gestation were associated with significant decreases in newborn physical and neuromuscular maturation [148]. These effects were observed after adjusting for length of gestation, indicating that fetal exposure to stress hormones programs neonatal neuromuscular maturation independent of GA. These observations in the neonate provide strong support for fetal programming because the consequences of gestational exposures cannot be explained by postnatal influences such as quality of maternal care.
Infants
In a large study (125 subjects) of the cognitive consequences of fetal exposures to stress hormones, the authors reported that high levels of maternal cortisol early in pregnancy resulted in significantly lower scores on measures of mental development at 12 months of age [150]. Elevated maternal cortisol late in gestation, however, was associated with significantly better scores on measures of mental development. It is important to remember that the human fetus is partially protected from elevations of maternal cortisol early in pregnancy because it is oxidized and inactivated by the enzyme 11β-HSD2. However, 11β-HSD2 is only a partial barrier, which explains why maternal and fetal cortisol levels are correlated. Thus, synthesis and release of elevated levels of maternal cortisol exposes the fetus to concentrations that may have detrimental neurological consequences, especially when these exposures occur early in gestation. As described earlier, fetal exposure to elevated cortisol is necessary for maturation of the fetal nervous system and lungs as pregnancy advances towards term. Fetal exposure to cortisol during the third trimester is ensured by the sharp drop in 11β-HSD2, which allows a greater proportion of maternal cortisol to cross the placental barrier. These results indicate that gestational exposure to maternal stress hormones has a significant influence on the fetal nervous system with potentially long-term consequences for mental processes such as memory and attention and that these consequences are determined by the timing of exposure. Maternal psychological distress signals early in gestation were also associated with delays in mental development. Consistent with the observations for stress and emotional regulation, the consequences of psychological distress were independent from the effects of maternal cortisol [150]. These data indicate that exposure to maternal psychobiological distress early in gestation is associated with an increased risk for cognitive impairments.
Children
The authors report new findings that fetal exposure to maternal anxiety exerts persisting consequences on child cognition. High levels of maternal pregnancy-specific anxiety over the course of gestation were associated with lower inhibitory control in 6–9-year-old girls and poorer visuospatial working memory performance in both girls and boys. The findings are highly consistent with the observations during infancy and contribute to the literature supporting an association between prenatal anxiety and cognitive development. This study provides evidence about the persistence of this effect until middle childhood [151].
The authors have shown that fetal exposure to maternal anxiety was related to specific changes in brain morphology of children independent of birth phenotype [152]. Specifically, maternal reports of anxiety about pregnancy early in gestation were associated with gray matter volume reductions in many areas of the brain that are associated with a variety of cognitive abilities. Specifically, prefrontal cortical areas that are responsible for executive functions such as reasoning, planning, attention, working memory and some aspects of language were reduced [153]. Moreover, structures in the medial temporal lobe were reduced, including areas connected to the hippocampus that constitute a medial temporal lobe memory system [154]. Reduced volume was observed in children whose mothers reported high levels of anxiety in the temporal polar cortex that is associated with social and emotional processing, including recognition and semantic memory [155,156]. Reduction of volume in an auditory network [157] and in brain systems involved in language learning [158] was observed in children whose mothers reported high levels of anxiety early in gestation. The prospective longitudinal studies provide evidence that exposures to both maternal cortisol and maternal psychological distress early in gestation alter trajectories of brain development and increase risk for cognitive impairments, and that these effects persist at least into pre-adolescence.
Expert commentary
Exposure to biological markers of prenatal stress, especially related to the HPA and placental axis, is an emerging risk factor affecting human development and risk for disease. Exposure to stress at any time during human development can have serious and long-term consequences for physical and emotional health; however, the effects may be most harmful when they occur early during development. It has become apparent that during pregnancy, maternal stress threatens the fetal nervous system with serious consequences that result in a spiral of increasing risk of impairment and morbidity.
It is important to acknowledge that gestation does not proceed at a uniform rate but has critical periods when there are peak activities of specific events. Maternal hormones are in flux during pregnancy and so are enzymes and binding proteins that influence their activity. Despite these known effects, there still are very few longitudinal, prospective studies of the consequences for the human fetus of changes in the endocrine stress system during human pregnancy. Only with the implementation of prospective longitudinal studies of pregnancy will it be possible to determine the developmental consequences of gestational exposure to maternal stress and stress hormones.
To further complicate the ability to predict the effects of exposures to biological markers of stress on health outcomes is the fact that organogenesis follows a developmental pattern. Different organs develop at different times, and organs are most susceptible to organizing and disorganizing influences during periods of rapid development. The authors have focused on the development of the brain and have described the effects of gestational stress on neurological outcomes, but there are influences on other organs with different profiles of risk. It is equally important to recognize that skills and abilities (motor, language and so on) are exhibited at different times during infant and child development, and assessments must to be tailored to expected outcomes. All of these facts make it essential to conduct longitudinal studies with serial measures in order to determine accurately the risk of exposures on health outcomes. Studies of a single exposure at a single time or of a single outcome are the norm and are likely to provide an incomplete picture at best.
There is growing recognition that there are sexually dimorphic responses to stress and adversity [159–161], including and perhaps especially associated with stress during the prenatal period [146,162,163]. The authors reported [164] that female fetuses displayed more mature startle responses than males at 31 and 36 gestational weeks, and were more sensitive to the effects of cortisol on the development of the startle response during this gestational period [165], and that delayed neuromuscular development associated with fetal exposure to cortisol early in gestation and placental CRH late in gestation was confined to male neonates [149]. A reduction in brain volumes in children exposed to elevated maternal anxiety early in gestation, primarily, were observed in girls [111]. Results from the studies are consistent with those that report specific trajectories of development are determined by fetal sex [166,167] and that sexually dimorphic risk of neurological impairment is associated with neonatal complications [168].
Sexually dimorphic patterns in response to stress may be developed very early in gestation. The female placenta appears to be more responsive to changes in glucocorticoid concentration than the male placenta, resulting in different patterns of growth [169]. Male fetuses invest in growth and not flexibility in response to adversity or stress. As the male fetus has not adjusted to early adversity and not conserved its resources, it is less able to adjust to later stress and is therefore at greater risk for morbidity and mortality. By contrast, the female placenta responds or adjusts to prenatal adversity in multiple ways (gene and protein changes), resulting in reduced growth. However, if exposed to stress that reduces nutrients and resources later in gestation, the female fetus has conserved its energy needs, which increases the probability of survival. Clearly, consideration of sex differences as early as fetal life is an important factor for determining risk for health outcomes. These differential responses of male and female fetuses to intrauterine signals probably contribute to sexually specific vulnerabilities to later health risk.
Five-year view
In recognition of the importance of early-life events for mental health, the National Institute of Mental Health (NIMH) advocates advancing the field of translational developmental neuroscience by identifying and understanding periods of development that exhibit the most dramatic transitions in both humans and model organisms. It was argued that particular attention should be paid to altered trajectories during periods of rapid developmental transition for the identification of individuals at risk for mental illness. As mental illnesses were defined as disorders of life trajectories that begin before birth it is critical to examine the complex processes that shape early-life neurodevelopmental trajectories and determine how these processes lead to vulnerabilities for cognitive and emotional disorders. The fetal period in the life cycle is unmatched by any other in growth and development, and it is the period in the human lifespan that is most vulnerable to both organizing and disorganizing influences. This priority of the NIMH will focus research in this rapidly expanding field in the next 5 years.
Among the questions that need to be addressed include understanding how various endocrine signals of stress interact to reach and influence the developing fetus. This review primarily focused on endocrine markers. However, vascular and immune exposures exert both independent and additive influences on fetal health and developmental outcomes. The precise mechanisms of communication between mother and fetus are undoubtedly complex and largely unknown, and in some cases, the most plausible candidates have been ruled out. This is a fertile area of research, and in the next 5 years, an understanding should emerge of how and by which mechanisms the fetal experience exerts lifelong influences on health and wellbeing.
The focus of most developmental research evaluating the consequences of early experience has been on the influence of post-natal experiences, such as nurturance and maternal sensitivity. Although there is growing evidence supporting the importance of the prenatal environment for neurobehavioral development, few studies have evaluated the joint role of the prenatal and early postnatal periods. Research in the next 5 years will advance the understanding of the modulating influences of the postnatal environment on prenatal experience. The predictive adaptive response (PAR) model [170] is an alternative to the programming hypothesis. Instead of assuming that risk for disease is the only outcome of fetal exposure to adversity, the PAR proposes that the developing organism makes adjustments based on the predicted postnatal environment. When the PAR does not match the environment (i.e., the prediction is inaccurate), the mismatch results in disease states. For example, a prescient fetus exposed to maternal depression will prepare for, and perhaps thrive, in a post-natal environment in which a mother is depressed and less able to provide emotional support [171]. However, that same fetus may have increased risk of impairment if the postnatal environment does not match the predicted one, even if it is supportive.
Key issues.
The developmental origins of disease or fetal programming model predict that early exposures to threat or adverse conditions have lifelong consequences that result in poor health outcomes.
The basic premise of the fetal programming model of disease is that developing organisms play an active role in their own construction.
A remarkable surveillance and response system has evolved so that the human fetus can detect threats to survival and adjust its developmental trajectory.
The adaptive ‘fight or flight’ system is dramatically altered during pregnancy, including the massive changes in the endocrine system characterized by the doubling in size of the pituitary gland and the growth and development of the placenta, which becomes an active endocrine organ.
Stress hormones increase adaptively over the course of human pregnancy; however, abnormal elevations can increase the risk for adverse birth outcomes and other health risks.
The consequences of exposure to stress hormones for the developing fetus are determined by the timetable for fetal organogenesis, vast changes in maternal stress physiology across gestation and changes in placental physiology.
A prospective, longitudinal research program has provided evidence that exposures to stress hormones have neurological consequences for the fetus, neonate, infant and child. The effects are observed in emotional and cognitive regulation and in distinctive patterns of brain structure.
Male and female fetuses respond differently to gestational stress exposures. Female fetuses are more likely to alter their developmental trajectory in response to moderate stressors and thus are better able to survive subsequent stress.
The fetal period in the life cycle is unmatched by any other in growth and development, and it is the period in the human lifespan that is most vulnerable to both organizing and disorganizing influences. It is anticipated that studies of the human fetus and the health consequences of exposures to markers of stress will become an area of intense research.
The predictive adaptive response is an alternative to the programming model. The premise is that health risk is associated with the match between fetal and infant/child exposures rather than just exposures to adversity. It is assumed that developing organisms accurately preparing for future environments will thrive even if the future is hostile.
Acknowledgments
The authors are grateful for the expert assistance of Kendra Leak, Cheryl Crippen, Megan Faulkner, Christina Canino and Natalie Hernandez, and to the families who participated in our studies.
Footnotes
Financial & competing interests disclosure
The authors were supported by NIH grants NS-41298, HD-51852 and HD-28413 to CA Sandman and by HD-50662 and HD-65823 to EP Davis. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
• OF INTEREST
•• OF CONSIDERABLE INTEREST
- 1••.Barker DJP. Mothers, Babies and Health in Later Life. Churchill Livingstone; Edinburgh, UK: 1998. An important document in the programming literature. It provides a compilation of studies linking birth outcome to later disease risk. [Google Scholar]
- 2.Kostovic I, Judas M, Rados M, Hrabac P. Laminar organization of the human fetal cerebrum revealed by histochemical markers and magnetic resonance imaging. Cereb Cortex. 2002;12(5):536–544. doi: 10.1093/cercor/12.5.536. [DOI] [PubMed] [Google Scholar]
- 3.Bourgeois JP, Goldman-Rakic PS, Rakic P. Synaptogenesis in the prefrontal cortex of rhesus monkeys. Cereb Cortex. 1994;4(1):78–96. doi: 10.1093/cercor/4.1.78. [DOI] [PubMed] [Google Scholar]
- 4.Huttenlocher PR, Dabholkar AS. Regional differences in synaptogenesis in human cerebral cortex. J Comp Neurol. 1997;387(2):167–178. doi: 10.1002/(sici)1096-9861(19971020)387:2<167::aid-cne1>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 5.Huttenlocher PR, de Courten C, Garey LJ, Van der Loos H. Synaptogenesis in human visual cortex–evidence for synapse elimination during normal development. Neurosci Lett. 1982;33(3):247–252. doi: 10.1016/0304-3940(82)90379-2. [DOI] [PubMed] [Google Scholar]
- 6.Becker LE, Armstrong DL, Chan F, Wood MM. Dendritic development in human occipital cortical neurons. Brain Res. 1984;315(1):117–124. doi: 10.1016/0165-3806(84)90083-x. [DOI] [PubMed] [Google Scholar]
- 7.Levitt P. Structural and functional maturation of the developing primate brain. J Pediatr. 2003;143(Suppl 4):S35–S45. doi: 10.1067/s0022-3476(03)00400-1. [DOI] [PubMed] [Google Scholar]
- 8.Sandman CA, Davis EP. Gestational stress influences cognition and behavior. Future Neurology. 2010;5(5):675–690. [Google Scholar]
- 9••.Denver RJ. Environmental stress as a developmental cue: corticotropin-releasing hormone is a proximate mediator of adaptive phenotypic plasticity in amphibian metamorphosis. Horm Behav. 1997;31(2):169–179. doi: 10.1006/hbeh.1997.1383. First article to describe the role of corticotropin-releasing hormone (CRH) in the developmental trajectory of the tadpole, with strong implications for human development. [DOI] [PubMed] [Google Scholar]
- 10.Crespi EJ, Denver RJ. Ancient origins of human developmental plasticity. Am J Hum Biol. 2005;17(1):44–54. doi: 10.1002/ajhb.20098. [DOI] [PubMed] [Google Scholar]
- 11.Boorse GC, Denver RJ. Acceleration of Ambystoma tigrinum metamorphosis by corticotropin-releasing hormone. J Exp Zool. 2002;293(1):94–98. doi: 10.1002/jez.10115. [DOI] [PubMed] [Google Scholar]
- 12.Pike IL. Maternal stress and fetal responses: evolutionary perspectives on preterm delivery. Am J Hum Biol. 2005;17(1):55–65. doi: 10.1002/ajhb.20093. [DOI] [PubMed] [Google Scholar]
- 13.Kuzawa CW. Fetal origins of developmental plasticity: are fetal cues reliable predictors of future nutritional environments? Am J Hum Biol. 2005;17(1):5–21. doi: 10.1002/ajhb.20091. [DOI] [PubMed] [Google Scholar]
- 14••.McLean M, Bisits A, Davies J, Woods R, Lowry P, Smith R. A placental clock controlling the length of human pregnancy. Nat Med. 1995;1(5):460–463. doi: 10.1038/nm0595-460. Important article first documenting the influence of circulating CRH on birth outcomes. Elevated CRH was associated with shorter gestation and increased risk for preterm birth. [DOI] [PubMed] [Google Scholar]
- 15••.Sandman CA, Glynn L, Schetter CD, et al. Elevated maternal cortisol early in pregnancy predicts third trimester levels of placental corticotropin releasing hormone (CRH): priming the placental clock. Peptides. 2006;27(6):1457–1463. doi: 10.1016/j.peptides.2005.10.002. First article in humans for in vivo evidence of the linkage between levels of cortisol and later levels of CRH influencing birth outcome. [DOI] [PubMed] [Google Scholar]
- 16.Anderson P, Doyle LW Victorian Infant Collaborative Study Group. Neurobehavioral outcomes of school-age children born extremely low birth weight or very preterm in the 1990s. JAMA. 2003;289(24):3264–3272. doi: 10.1001/jama.289.24.3264. [DOI] [PubMed] [Google Scholar]
- 17.Peterson BS, Vohr B, Staib LH, et al. Regional brain volume abnormalities and long-term cognitive outcome in preterm infants. JAMA. 2000;284(15):1939–1947. doi: 10.1001/jama.284.15.1939. [DOI] [PubMed] [Google Scholar]
- 18.Peterson BS, Anderson AW, Ehrenkranz R, et al. Regional brain volumes and their later neurodevelopmental correlates in term and preterm infants. Pediatrics. 2003;111(5 Pt 1):939–948. doi: 10.1542/peds.111.5.939. [DOI] [PubMed] [Google Scholar]
- 19.Davis EP, Buss C, Muftuler LT, et al. Children’s brain development benefits from longer gestation. Front Psychol. 2011;2:1. doi: 10.3389/fpsyg.2011.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nosarti C, Al-Asadi MH, Frangou S, Stewart AL, Rifkin L, Murray RM. Adolescents who were born very preterm have decreased brain volumes. Brain. 2000;125(7):1616–1623. doi: 10.1093/brain/awf157. [DOI] [PubMed] [Google Scholar]
- 21.Cannon WB. Bodily Changes in Pain, Hunger, Fear, and Rage. D. Appleton and Co; NY, USA: 1929. [Google Scholar]
- 22.Selye H. A syndrome produced by diverse nocuous agents. Nature. 1936;138:32. doi: 10.1176/jnp.10.2.230a. [DOI] [PubMed] [Google Scholar]
- 23••.Selye H. The Stress of Life. McGraw-Hill; NY, USA: 1956. Seminal work in the history of stress and disease. [Google Scholar]
- 24.Vale W, Spiess J, Rivier C, Rivier J. Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and β-endorphin. Science. 1981;213(4514):1394–1397. doi: 10.1126/science.6267699. [DOI] [PubMed] [Google Scholar]
- 25.Chrousos GP. Regulation and dysregulation of the hypothalamic–pituitary–adrenal axis. The corticotropin-releasing hormone perspective. Endocrinol Metab Clin North Am. 1992;21(4):833–858. [PubMed] [Google Scholar]
- 26.Avishai-Eliner S, Brunson KL, Sandman CA, Baram TZ. Stressed-out, or in (utero)? Trends Neurosci. 2002;25(10):518–524. doi: 10.1016/s0166-2236(02)02241-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Knigge KM, Hays M. Evidence of inhibitive role of hippocampus in neural regulation of ACTH release. Proc Soc Exp Biol Med. 1963;114:67–69. doi: 10.3181/00379727-114-28587. [DOI] [PubMed] [Google Scholar]
- 28.Herman JP, Schäfer MK, Young EA, et al. Evidence for hippocampal regulation of neuroendocrine neurons of the hypothalamo–pituitary–adrenocortical axis. J Neurosci. 1989;9(9):3072–3082. doi: 10.1523/JNEUROSCI.09-09-03072.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Herman JP, Cullinan WE, Morano MI, Akil H, Watson SJ. Contribution of the ventral subiculum to inhibitory regulation of the hypothalamo–pituitary–adrenocortical axis. J Neuroendocrinol. 1995;7(6):475–482. doi: 10.1111/j.1365-2826.1995.tb00784.x. [DOI] [PubMed] [Google Scholar]
- 30.Nettles KW, Pesold C, Goldman MB. Influence of the ventral hippocampal formation on plasma vasopressin, hypothalamic–pituitary–adrenal axis, and behavioral responses to novel acoustic stress. Brain Res. 2000;858(1):181–190. doi: 10.1016/s0006-8993(99)02281-7. [DOI] [PubMed] [Google Scholar]
- 31.Herman JP, Dolgas CM, Carlson SL. Ventral subiculum regulates hypothalamo–pituitary–adrenocortical and behavioural responses to cognitive stressors. Neuroscience. 1998;86(2):449–459. doi: 10.1016/s0306-4522(98)00055-4. [DOI] [PubMed] [Google Scholar]
- 32.Meaney MJ, Diorio J, Francis D, et al. Early environmental regulation of forebrain glucocorticoid receptor gene expression: implications for adrenocortical responses to stress. Dev Neurosci. 1996;18(1–2):49–72. doi: 10.1159/000111395. [DOI] [PubMed] [Google Scholar]
- 33.Feldman S, Conforti N. Modifications of adrenocortical responses following frontal cortex simulation in rats with hypothalamic deafferentations and medial forebrain bundle lesions. Neuroscience. 1985;15(4):1045–1047. doi: 10.1016/0306-4522(85)90253-2. [DOI] [PubMed] [Google Scholar]
- 34.Bagley J, Moghaddam B. Temporal dynamics of glutamate efflux in the prefrontal cortex and in the hippocampus following repeated stress: effects of pretreatment with saline or diazepam. Neuroscience. 1997;77(1):65–73. doi: 10.1016/s0306-4522(96)00435-6. [DOI] [PubMed] [Google Scholar]
- 35.Moghaddam B. Stress preferentially increases extraneuronal levels of excitatory amino acids in the prefrontal cortex: comparison to hippocampus and basal ganglia. J Neurochem. 1993;60(5):1650–1657. doi: 10.1111/j.1471-4159.1993.tb13387.x. [DOI] [PubMed] [Google Scholar]
- 36.Murros K, Fogelholm R, Kettunen S, Vuorela AL. Serum cortisol and outcome of ischemic brain infarction. J Neurol Sci. 1993;116(1):12–17. doi: 10.1016/0022-510x(93)90083-b. [DOI] [PubMed] [Google Scholar]
- 37.Shimizu E, Kodama K, Sakamoto T, et al. Recovery from neuroendocrinological abnormalities and frontal hypoperfusion after remission in a case with rapid cycling bipolar disorder. Psychiatry Clin Neurosci. 1997;51(4):207–212. doi: 10.1111/j.1440-1819.1997.tb02584.x. [DOI] [PubMed] [Google Scholar]
- 38.Diorio D, Viau V, Meaney MJ. The role of the medial prefrontal cortex (cingulate gyrus) in the regulation of hypothalamic–pituitary–adrenal responses to stress. J Neurosci. 1993;13(9):3839–3847. doi: 10.1523/JNEUROSCI.13-09-03839.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Akana SF, Chu A, Soriano L, Dallman MF. Corticosterone exerts site-specific and state-dependent effects in prefrontal cortex and amygdala on regulation of adrenocorticotropic hormone, insulin and fat depots. J Neuroendocrinol. 2001;13(7):625–637. doi: 10.1046/j.1365-2826.2001.00676.x. [DOI] [PubMed] [Google Scholar]
- 40.Brunson KL, Grigoriadis DE, Lorang MT, Baram TZ. Corticotropin-releasing hormone (CRH) downregulates the function of its receptor (CRF1) and induces CRF1 expression in hippocampal and cortical regions of the immature rat brain. Exp Neurol. 2002;176(1):75–86. doi: 10.1006/exnr.2002.7937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lowry PJ. Corticotropin-releasing factor and its binding protein in human plasma. Ciba Found Symp. 1993;172:108–15. discussion 115. [PubMed] [Google Scholar]
- 42.Frim DM, Emanuel RL, Robinson BG, Smas CM, Adler GK, Majzoub JA. Characterization and gestational regulation of corticotropin-releasing hormone messenger RNA in human placenta. J Clin Invest. 1988;82(1):287–292. doi: 10.1172/JCI113585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Campbell EA, Linton EA, Wolfe CD, Scraggs PR, Jones MT, Lowry PJ. Plasma corticotropin-releasing hormone concentrations during pregnancy and parturition. J Clin Endocrinol Metab. 1987;64(5):1054–1059. doi: 10.1210/jcem-64-5-1054. [DOI] [PubMed] [Google Scholar]
- 44.Chan EC, Smith R, Lewin T, et al. Plasma corticotropin-releasing hormone, β-endorphin and cortisol inter-relationships during human pregnancy. Acta Endocrinol. 1993;128(4):339–344. doi: 10.1530/acta.0.1280339. [DOI] [PubMed] [Google Scholar]
- 45.Goland RS, Conwell IM, Warren WB, Wardlaw SL. Placental corticotropin-releasing hormone and pituitary-adrenal function during pregnancy. Neuroendocrinol. 1992;56(5):742–749. doi: 10.1159/000126302. [DOI] [PubMed] [Google Scholar]
- 46.Wolfe CD, Patel SP, Linton EA, et al. Plasma corticotrophin-releasing factor (CRF) in abnormal pregnancy. Br J Obstet Gynaecol. 1988;95(10):1003–1006. doi: 10.1111/j.1471-0528.1988.tb06504.x. [DOI] [PubMed] [Google Scholar]
- 47.Sasaki A, Shinkawa O, Margioris AN, et al. Immunoreactive corticotropin-releasing hormone in human plasma during pregnancy, labor, and delivery. J Clin Endocrinol Metab. 1987;64(2):224–229. doi: 10.1210/jcem-64-2-224. [DOI] [PubMed] [Google Scholar]
- 48.Sasaki A, Tempst P, Liotta AS, et al. Isolation and characterization of a corticotropin-releasing hormone-like peptide from human placenta. J Clin Endocrinol Metab. 1988;67(4):768–773. doi: 10.1210/jcem-67-4-768. [DOI] [PubMed] [Google Scholar]
- 49.Petraglia F, Sutton S, Vale W. Neurotransmitters and peptides modulate the release of immunoreactive corticotropin-releasing factor from cultured human placental cells. Am J Obstet Gynecol. 1989;160(1):247–251. doi: 10.1016/0002-9378(89)90130-0. [DOI] [PubMed] [Google Scholar]
- 50.King BR, Smith R, Nicholson RC. Novel glucocorticoid and cAMP interactions on the CRH gene promoter. Mol Cell Endocrinol. 2002;194(1–2):19–28. doi: 10.1016/s0303-7207(02)00218-6. [DOI] [PubMed] [Google Scholar]
- 51•.Sandman CA, Wadhwa PD, Chicz-DeMet A, Porto M, Garite TJ. Maternal corticotropin-releasing hormone and habituation in the human fetus. Dev Psychobiol. 1999;34(3):163–173. doi: 10.1002/(sici)1098-2302(199904)34:3<163::aid-dev1>3.0.co;2-9. First evidence that circulating placental CRH had effects on the fetal nervous system. [DOI] [PubMed] [Google Scholar]
- 52.Smith R, Mesiano S, McGrath S. Hormone trajectories leading to human birth. Regul Pept. 2002;108(2–3):159–164. doi: 10.1016/s0167-0115(02)00105-2. [DOI] [PubMed] [Google Scholar]
- 53.Smith R, Nicholson RC. Corticotrophin releasing hormone and the timing of birth. Front Biosci. 2007;12:912–918. doi: 10.2741/2113. [DOI] [PubMed] [Google Scholar]
- 54.Tyson EK, Smith R, Read M. Evidence that corticotropin-releasing hormone modulates myometrial contractility during human pregnancy. Endocrinology. 2009;150(12):5617–5625. doi: 10.1210/en.2009-0348. [DOI] [PubMed] [Google Scholar]
- 55.Glynn LM, Sandman CA. Prenatal origins of neurological development: a critical period for fetus and mother. Curr Dir Psycholog Science. 2011;20:384–389. [Google Scholar]
- 56.Schulkin J. Corticotropin-releasing hormone signals adversity in both the placenta and the brain: regulation by glucocorticoids and allostatic overload. J Endocrinol. 1999;161(3):349–356. doi: 10.1677/joe.0.1610349. [DOI] [PubMed] [Google Scholar]
- 57.Cheng YH, Nicholson RC, King B, Chan EC, Fitter JT, Smith R. Glucocorticoid stimulation of corticotropin-releasing hormone gene expression requires a cyclic adenosine 3′,5′-monophosphate regulatory element in human primary placental cytotrophoblast cells. J Clin Endocrinol Metab. 2000;85(5):1937–1945. doi: 10.1210/jcem.85.5.6552. [DOI] [PubMed] [Google Scholar]
- 58.Giles WB, McLean M, Davies JJ, Smith R. Abnormal umbilical artery Doppler waveforms and cord blood corticotropin-releasing hormone. Obstet Gynecol. 1996;87(1):107–111. doi: 10.1016/0029-7844(95)00338-x. [DOI] [PubMed] [Google Scholar]
- 59.Petraglia F, Sawchenko PE, Rivier J, Vale W. Evidence for local stimulation of ACTH secretion by corticotropin-releasing factor in human placenta. Nature. 1987;328(6132):717–719. doi: 10.1038/328717a0. [DOI] [PubMed] [Google Scholar]
- 60.Petraglia F, Volpe A, Genazzani AR, Rivier J, Sawchenko PE, Vale W. Neuroendocrinology of the human placenta. Front Neuroendocrinol. 1990;11:6–37. [Google Scholar]
- 61.Marinoni E, Korebrits C, Di Iorio R, Cosmi EV, Challis JR. Effect of betamethasone in vivo on placental corticotropin-releasing hormone in human pregnancy. Am J Obstet Gynecol. 1998;178(4):770–778. doi: 10.1016/s0002-9378(98)70490-9. [DOI] [PubMed] [Google Scholar]
- 62.Korebrits C, Yu DH, Ramirez MM, Marinoni E, Bocking AD, Challis JR. Antenatal glucocorticoid administration increases corticotrophin-releasing hormone in maternal plasma. Br J Obstet Gynaecol. 1998;105(5):556–561. doi: 10.1111/j.1471-0528.1998.tb10158.x. [DOI] [PubMed] [Google Scholar]
- 63.You X, Yang R, Tang X, Gao L, Ni X. Corticotropin-releasing hormone stimulates estrogen biosynthesis in cultured human placental trophoblasts. Biol Reprod. 2006;74(6):1067–1072. doi: 10.1095/biolreprod.105.049361. [DOI] [PubMed] [Google Scholar]
- 64.Yang R, You X, Tang X, Gao L, Ni X. Corticotropin-releasing hormone inhibits progesterone production in cultured human placental trophoblasts. J Mol Endocrinol. 2006;37(3):533–540. doi: 10.1677/jme.1.02119. [DOI] [PubMed] [Google Scholar]
- 65.Smith R, Mesiano S, Chan EC, Brown S, Jaffe RB. Corticotropin-releasing hormone directly and preferentially stimulates dehydroepiandrosterone sulfate secretion by human fetal adrenal cortical cells. J Clin Endocrinol Metab. 1998;83(8):2916–2920. doi: 10.1210/jcem.83.8.5020. [DOI] [PubMed] [Google Scholar]
- 66.Smith R, Smith JI, Shen X, et al. Patterns of plasma corticotropin-releasing hormone, progesterone, estradiol, and estriol change and the onset of human labor. J Clin Endocrinol Metab. 2009;94(6):2066–2074. doi: 10.1210/jc.2008-2257. [DOI] [PubMed] [Google Scholar]
- 67.Orth DN, Mount CD. Specific high-affinity binding protein for human corticotropin-releasing hormone in normal human plasma. Biochem Biophys Res Commun. 1987;143(2):411–417. doi: 10.1016/0006-291x(87)91369-6. [DOI] [PubMed] [Google Scholar]
- 68.Petraglia F, Potter E, Cameron VA, et al. Corticotropin-releasing factor-binding protein is produced by human placenta and intrauterine tissues. J Clin Endocrinol Metab. 1993;77(4):919–924. doi: 10.1210/jcem.77.4.8408466. [DOI] [PubMed] [Google Scholar]
- 69.Petraglia F, Florio P, Nappi C, Genazzani AR. Peptide signaling in human placenta and membranes: autocrine, paracrine, and endocrine mechanisms. Endocr Rev. 1996;17(2):156–186. doi: 10.1210/edrv-17-2-156. [DOI] [PubMed] [Google Scholar]
- 70.Linton EA, Perkins AV, Woods RJ, et al. Corticotropin releasing hormone-binding protein (CRH-BP): plasma levels decrease during the third trimester of normal human pregnancy. J Clin Endocrinol Metab. 1993;76(1):260–262. doi: 10.1210/jcem.76.1.8421097. [DOI] [PubMed] [Google Scholar]
- 71.Ho JT, Lewis JG, O’Loughlin P, et al. Reduced maternal corticosteroid-binding globulin and cortisol levels in pre-eclampsia and gamete recipient pregnancies. Clin Endocrinol. 2007;66(6):869–877. doi: 10.1111/j.1365-2265.2007.02826.x. [DOI] [PubMed] [Google Scholar]
- 72.Sun K, Adamson SL, Yang K, Challis JR. Interconversion of cortisol and cortisone by 11β-hydroxysteroid dehydrogenases type 1 and 2 in the perfused human placenta. Placenta. 1999;20(1):13–19. doi: 10.1053/plac.1998.0352. [DOI] [PubMed] [Google Scholar]
- 73.Ma XH, Wu WX, Nathanielsz PW. Gestation-related and betamethasone-induced changes in 11β-hydroxysteroid dehydrogenase types 1 and 2 in the baboon placenta. Am J Obstet Gynecol. 2003;188(1):13–21. doi: 10.1067/mob.2003.62. [DOI] [PubMed] [Google Scholar]
- 74.Murphy VE, Clifton VL. Alterations in human placental 11β-hydroxysteroid dehydrogenase type 1 and 2 with gestational age and labour. Placenta. 2003;24(7):739–744. doi: 10.1016/s0143-4004(03)00103-6. [DOI] [PubMed] [Google Scholar]
- 75.O’Donnell KJ, Bugge Jensen A, Freeman L, Khalife N, O’Connor TG, Glover V. Maternal prenatal anxiety and downregulation of placental 11β-HSD2. Psychoneuroendocrinol. 2012;37(6):818–826. doi: 10.1016/j.psyneuen.2011.09.014. [DOI] [PubMed] [Google Scholar]
- 76.Bloom SL, Sheffield JS, McIntire DD, Leveno KJ. Antenatal dexamethasone and decreased birth weight. Obstet Gynecol. 2001;97(4):485–490. doi: 10.1016/s0029-7844(00)01206-0. [DOI] [PubMed] [Google Scholar]
- 77.Thorp JA, Jones PG, Knox E, Clark RH. Does antenatal corticosteroid therapy affect birth weight and head circumference? Obstet Gynecol. 2002;99(1):101–108. doi: 10.1016/s0029-7844(01)01656-8. [DOI] [PubMed] [Google Scholar]
- 78.French NP, Hagan R, Evans SF, Godfrey M, Newnham JP. Repeated antenatal corticosteroids: size at birth and subsequent development. Am J Obstet Gynecol. 1999;180(1 Pt 1):114–121. doi: 10.1016/s0002-9378(99)70160-2. [DOI] [PubMed] [Google Scholar]
- 79.Crowther CA, Haslam RR, Hiller JE, Doyle LW, Robinson JS Australasian Collaborative Trial of Repeat Doses of Steroids (ACTORDS) Study Group. Neonatal respiratory distress syndrome after repeat exposure to antenatal corticosteroids: a randomised controlled trial. Lancet. 2006;367(9526):1913–1919. doi: 10.1016/S0140-6736(06)68846-6. [DOI] [PubMed] [Google Scholar]
- 80.Piazze J, Ruozi-Berretta A, Di Cioccio A, Anceschi M. Neonatal length and cranial circumference are reduced in human pregnancies at term after antepartum administration of betamethasone. J Perinat Med. 2005;33(5):463–464. doi: 10.1515/JPM.2005.083. [DOI] [PubMed] [Google Scholar]
- 81.Davis EP, Waffarn F, Uy C, Hobel CJ, Glynn LM, Sandman CA. Effect of prenatal glucocorticoid treatment on size at birth among infants born at term gestation. J Perinatol. 2009;29(11):731–737. doi: 10.1038/jp.2009.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82•.Davis EP, Townsend EL, Gunnar MR, et al. Effects of prenatal betamethasone exposure on regulation of stress physiology in healthy premature infants. Psychoneuroendocrinol. 2004;29(8):1028–1036. doi: 10.1016/j.psyneuen.2003.10.005. Showed that prenatal exposure to synthetic glucocorticoids altered the stress reactivity of infants. [DOI] [PubMed] [Google Scholar]
- 83.Chen XK, Lougheed J, Lawson ML, et al. Effects of repeated courses of antenatal corticosteroids on somatic development in children 6 to 10 years of age. Am J Perinatol. 2008;25(1):21–28. doi: 10.1055/s-2007-995222. [DOI] [PubMed] [Google Scholar]
- 84.de Vries A, Holmes MC, Heijnis A, et al. Prenatal dexamethasone exposure induces changes in nonhuman primate offspring cardiometabolic and hypothalamic–pituitary–adrenal axis function. J Clin Invest. 2007;117(4):1058–1067. doi: 10.1172/JCI30982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chen XK, Lougheed J, Lawson ML, et al. Effects of repeated courses of antenatal corticosteroids on somatic development in children 6 to 10 years of age. Am J Perinatol. 2008;25(1):21–28. doi: 10.1055/s-2007-995222. [DOI] [PubMed] [Google Scholar]
- 86.Trautman PD, Meyer-Bahlburg HF, Postelnek J, New MI. Effects of early prenatal dexamethasone on the cognitive and behavioral development of young children: results of a pilot study. Psychoneuroendocrinol. 1995;20(4):439–449. doi: 10.1016/0306-4530(94)00070-0. [DOI] [PubMed] [Google Scholar]
- 87.Hirvikoski T, Nordenström A, Lindholm T, et al. Cognitive functions in children at risk for congenital adrenal hyperplasia treated prenatally with dexamethasone. J Clin Endocrinol Metab. 2007;92(2):542–548. doi: 10.1210/jc.2006-1340. [DOI] [PubMed] [Google Scholar]
- 88.Hauser J, Dettling-Artho A, Pilloud S, et al. Effects of prenatal dexamethasone treatment on postnatal physical, endocrine, and social development in the common marmoset monkey. Endocrinology. 2007;148(4):1813–1822. doi: 10.1210/en.2006-1306. [DOI] [PubMed] [Google Scholar]
- 89.Nagano M, Ozawa H, Suzuki H. Prenatal dexamethasone exposure affects anxiety-like behaviour and neuroendocrine systems in an age-dependent manner. Neurosci Res. 2008;60(4):364–371. doi: 10.1016/j.neures.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 90.MacArthur BA, Howie RN, Dezoete JA, Elkins J. School progress and cognitive development of 6-year-old children whose mothers were treated antenatally with betamethasone. Pediatrics. 1982;70(1):99–105. [PubMed] [Google Scholar]
- 91.Spinillo A, Viazzo F, Colleoni R, Chiara A, Maria Cerbo R, Fazzi E. Two-year infant neurodevelopmental outcome after single or multiple antenatal courses of corticosteroids to prevent complications of prematurity. Am J Obstet Gynecol. 2004;191(1):217–224. doi: 10.1016/j.ajog.2003.12.023. [DOI] [PubMed] [Google Scholar]
- 92.Setiawan E, Jackson MF, MacDonald JF, Matthews SG. Effects of repeated prenatal glucocorticoid exposure on long-term potentiation in the juvenile guinea-pig hippocampus. J Physiol. 2007;581(Pt 3):1033–1042. doi: 10.1113/jphysiol.2006.127381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Antonow-Schlorke I, Kühn B, Müller T, et al. Antenatal betamethasone treatment reduces synaptophysin immunoreactivity in presynaptic terminals in the fetal sheep brain. Neurosci Lett. 2001;297(3):147–150. doi: 10.1016/s0304-3940(00)01605-0. [DOI] [PubMed] [Google Scholar]
- 94.Raschke C, Schmidt S, Schwab M, Jirikowski G. Effects of betamethasone treatment on central myelination in fetal sheep: an electron microscopical study. Anat Histol Embryol. 2008;37(2):95–100. doi: 10.1111/j.1439-0264.2007.00807.x. [DOI] [PubMed] [Google Scholar]
- 95.Uno H, Lohmiller L, Thieme C, et al. Brain damage induced by prenatal exposure to dexamethasone in fetal macaques. I Hippocampus. Brain Res Dev Brain Res. 1990;53:157–167. doi: 10.1016/0165-3806(90)90002-g. [DOI] [PubMed] [Google Scholar]
- 96.Moss TJ, Doherty DA, Nitsos I, Sloboda DM, Harding R, Newnham JP. Effects into adulthood of single or repeated antenatal corticosteroids in sheep. Am J Obstet Gynecol. 2005;192(1):146–152. doi: 10.1016/j.ajog.2004.06.065. [DOI] [PubMed] [Google Scholar]
- 97.Coe CL, Lubach GR. Developmental consequences of antenatal dexamethasone treatment in nonhuman primates. Neurosci Biobehav Rev. 2005;29(2):227–235. doi: 10.1016/j.neubiorev.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 98.Williams MT, Hennessy MB, Davis HN. CRF administered to pregnant rats alters offspring behavior and morphology. Pharmacol Biochem Behav. 1995;52(1):161–167. doi: 10.1016/0091-3057(95)00082-8. [DOI] [PubMed] [Google Scholar]
- 99.Avishai-Eliner S, Yi SJ, Baram TZ. Developmental profile of messenger RNA for the corticotropin-releasing hormone receptor in the rat limbic system. Brain Res Dev Brain Res. 1996;91(2):159–163. doi: 10.1016/0165-3806(95)00158-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sirianni R, Rehman KS, Carr BR, Parker CR, Jr, Rainey WE. Corticotropin-releasing hormone directly stimulates cortisol and the cortisol biosynthetic pathway in human fetal adrenal cells. J Clin Endocrinol Metab. 2005;90(1):279–285. doi: 10.1210/jc.2004-0865. [DOI] [PubMed] [Google Scholar]
- 101.Maecker H, Desai A, Dash R, Rivier J, Vale W, Sapolsky R. Astressin, a novel and potent CRF antagonist, is neuroprotective in the hippocampus when administered after a seizure. Brain Res. 1997;744(1):166–170. doi: 10.1016/s0006-8993(96)01207-3. [DOI] [PubMed] [Google Scholar]
- 102.Strijbos PJ, Relton JK, Rothwell NJ. Corticotrophin-releasing factor antagonist inhibits neuronal damage induced by focal cerebral ischaemia or activation of NMDA receptors in the rat brain. Brain Res. 1994;656(2):405–408. doi: 10.1016/0006-8993(94)91485-0. [DOI] [PubMed] [Google Scholar]
- 103.Baram TZ, Hatalski CG. Neuropeptide-mediated excitability: a key triggering mechanism for seizure generation in the developing brain. Trends Neurosci. 1998;21(11):471–476. doi: 10.1016/s0166-2236(98)01275-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Baram TZ, Ribak CE. Peptide-induced infant status epilepticus causes neuronal death and synaptic reorganization. Neuroreport. 1995;6(2):277–280. doi: 10.1097/00001756-199501000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Brunson KL, Eghbal-Ahmadi M, Bender R, Chen Y, Baram TZ. Long-term, progressive hippocampal cell loss and dysfunction induced by early-life administration of corticotropin-releasing hormone reproduce the effects of early-life stress. Proc Natl Acad Sci USA. 2001;98(15):8856–8861. doi: 10.1073/pnas.151224898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hollrigel GS, Chen K, Baram TZ, Soltesz I. The pro-convulsant actions of corticotropin-releasing hormone in the hippocampus of infant rats. Neuroscience. 1998;84(1):71–79. doi: 10.1016/s0306-4522(97)00499-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chen Y, Dubé CM, Rice CJ, Baram TZ. Rapid loss of dendritic spines after stress involves derangement of spine dynamics by corticotropin-releasing hormone. J Neurosci. 2008;28(11):2903–2911. doi: 10.1523/JNEUROSCI.0225-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ehlers CL, Henriksen SJ, Wang M, Rivier J, Vale W, Bloom FE. Corticotropin releasing factor produces increases in brain excitability and convulsive seizures in rats. Brain Res. 1983;278(1–2):332–336. doi: 10.1016/0006-8993(83)90266-4. [DOI] [PubMed] [Google Scholar]
- 109.Baram TZ, Chalmers DT, Chen C, Koutsoukos Y, De Souza EB. The CRF1 receptor mediates the excitatory actions of corticotropin releasing factor (CRF) in the developing rat brain: in vivo evidence using a novel, selective, non-peptide CRF receptor antagonist. Brain Res. 1997;770(1–2):89–95. doi: 10.1016/s0006-8993(97)00759-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Baram TZ, Hirsch E, Snead OC, 3rd, Schultz L. Corticotropin-releasing hormone-induced seizures in infant rats originate in the amygdala. Ann Neurol. 1992;31(5):488–494. doi: 10.1002/ana.410310505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Sandman CA, Davis EP, Buss C, Glynn LM. Exposure to prenatal psychobiological stress exerts programming influences on the mother and her fetus. Neuroendocrinol. 2011;95:1–3. doi: 10.1159/000327017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sandman CA, Davis EP, Buss C, Glynn LM. Prenatal programming of human neurological function. Int J Pept. 2011;2011:837596. doi: 10.1155/2011/837596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sandman CA, Kastin AJ. Intraventricular administration of MSH induces hyperalgesia in rats. Peptides. 1981;2(2):231–233. doi: 10.1016/s0196-9781(81)80040-x. [DOI] [PubMed] [Google Scholar]
- 114.Beckwith BE, Sandman CA, Hothersall D, Kastin AJ. Influence of neonatal injections of alpha-MSH on learning, memory and attention in rats. Physiol Behav. 1977;18(1):63–71. doi: 10.1016/0031-9384(77)90095-6. [DOI] [PubMed] [Google Scholar]
- 115.Sandman CA, O’Halloran JP. Pro-opiomelanocortin, learning, memory and attention. In: Dewied D, Gispen WH, Van Wimersma Greidanus TB, editors. Encyclopedia on Pharmacology and Therapeutics. Pergamon Press; Oxford, UK: 1986. pp. 397–420. [Google Scholar]
- 116.Moldow RL, Kastin AJ, Hollander CS, Coy DH, Sandman CA. Brain β-endorphin-like immunoreactivity in adult rats given β-endorphin neonatally. Brain Res Bull. 1981;7(6):683–686. doi: 10.1016/0361-9230(81)90118-0. [DOI] [PubMed] [Google Scholar]
- 117.Sandman CA, Yessaian N. Persisting subsensitivity of the striatal dopamine system after fetal exposure to β-endorphin. Life Sci. 1986;39(19):1755–1763. doi: 10.1016/0024-3205(86)90095-0. [DOI] [PubMed] [Google Scholar]
- 118.Davis EP, Waffarn F, Sandman CA. Prenatal treatment with glucocorticoids sensitizes the HPA axis response to stress among full-term infants. Dev Psychobiol. 2011;53(2):175–183. doi: 10.1002/dev.20510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Sandman CA, Wadhwa PD, Chicz-DeMet A, Dunkel-Schetter C, Porto M. Maternal stress, HPA activity, and fetal/infant outcome. Ann NY Acad Sci. 1997;814:266–275. doi: 10.1111/j.1749-6632.1997.tb46162.x. [DOI] [PubMed] [Google Scholar]
- 120.Sandman CA, Glynn L, Wadhwa PD, Chicz-DeMet A, Porto M, Garite T. Maternal hypothalamic–pituitary–adrenal disregulation during the third trimester influences human fetal responses. Dev Neurosci. 2003;25(1):41–49. doi: 10.1159/000071467. [DOI] [PubMed] [Google Scholar]
- 121••.Class QA, Buss C, Davis EP, et al. Low levels of corticotropin-releasing hormone during early pregnancy are associated with precocious maturation of the human fetus. Dev Neurosci. 2008;30(6):419–426. doi: 10.1159/000191213. First illustration in human subjects that the human fetal response to ex utero stimulation was influenced by very early exposure to stress hormones. First article to demonstrate a programming effect that was independent of postnatal influences. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Markovic D, Vatish M, Gu M, et al. The onset of labor alters corticotropin-releasing hormone type 1 receptor variant expression in human myometrium: putative role of interleukin-1β. Endocrinology. 2007;148(7):3205–3213. doi: 10.1210/en.2007-0095. [DOI] [PubMed] [Google Scholar]
- 123.Sandman CA, Davis EP, Glynn LM. Psychobiological stress and preterm birth. In: Morrison JC, editor. Preterm Birth: Mother and Child. Intech Publishing; Rijeka, Croatia: 2012. [Google Scholar]
- 124.Barker DJ, Osmond C, Simmonds SJ, Wield GA. The relation of small head circumference and thinness at birth to death from cardiovascular disease in adult life. BMJ. 1993;306(6875):422–426. doi: 10.1136/bmj.306.6875.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.McCormack VA, dos Santos Silva I, De Stavola BL, Mohsen R, Leon DA, Lithell HO. Fetal growth and subsequent risk of breast cancer: results from long term follow up of Swedish cohort. BMJ. 2003;326(7383):248. doi: 10.1136/bmj.326.7383.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Roseboom TJ, van der Meulen JH, Osmond C, et al. Coronary heart disease after prenatal exposure to the Dutch famine, 1944–45. Heart. 2000;84(6):595–598. doi: 10.1136/heart.84.6.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Richards M, Hardy R, Kuh D, Wadsworth ME. Birth weight and cognitive function in the British 1946 birth cohort: longitudinal population based study. BMJ. 2001;322(7280):199–203. doi: 10.1136/bmj.322.7280.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Tita AT, Landon MB, Spong CY, et al. Eunice Kennedy Shriver NICHD Maternal-Fetal Medicine Units Network. Timing of elective repeat cesarean delivery at term and neonatal outcomes. N Engl J Med. 2009;360(2):111–120. doi: 10.1056/NEJMoa0803267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Davis EP, Glynn LM, Waffarn F, Sandman CA. Prenatal maternal stress programs infant stress regulation. J Child Psychol Psychiatry. 2011;52(2):119–129. doi: 10.1111/j.1469-7610.2010.02314.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Davis EP, Glynn LM, Dunkel-Schetter C, Hobel C, Chicz-Demet A, Sandman CA. Corticotropin-releasing hormone during pregnancy is associated with infant temperament. Dev Neurosci. 2005;27(5):299–305. doi: 10.1159/000086709. [DOI] [PubMed] [Google Scholar]
- 131•.Davis EP, Glynn LM, Schetter CD, Hobel C, Chicz-Demet A, Sandman CA. Prenatal exposure to maternal depression and cortisol influences infant temperament. J Am Acad Child Adolesc Psychiatry. 2007;46(6):737–746. doi: 10.1097/chi.0b013e318047b775. Describes the joint and independent effects of prenatal exposure to maternal cortisol and depression on infant temperament. [DOI] [PubMed] [Google Scholar]
- 132.Kagan J, Snidman N, Arcus D. Childhood derivatives of high and low reactivity in infancy. Child Dev. 1998;69(6):1483–1493. [PubMed] [Google Scholar]
- 133.Pfeifer M, Goldsmith HH, Davidson RJ, Rickman M. Continuity and change in inhibited and uninhibited children. Child Dev. 2002;73(5):1474–1485. doi: 10.1111/1467-8624.00484. [DOI] [PubMed] [Google Scholar]
- 134.Schwartz CE, Snidman N, Kagan J. Adolescent social anxiety as an outcome of inhibited temperament in childhood. J Am Acad Child Adolesc Psychiatry. 1999;38(8):1008–1015. doi: 10.1097/00004583-199908000-00017. [DOI] [PubMed] [Google Scholar]
- 135.Blair MM, Glynn LM, Sandman CA, Davis EP. Prenatal maternal anxiety and early childhood temperament. Stress. 2011;14(6):644–651. doi: 10.3109/10253890.2011.594121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Davis EP, Sandman CA. Prenatal psychobiological predictors of anxiety risk in preadolescent children. Psychoneuroendocrinol. 2012;37:1224–1233. doi: 10.1016/j.psyneuen.2011.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kapoor M, Liu S, Huh K, Parapuram S, Kennedy L, Leask A. Connective tissue growth factor promoter activity in normal and wounded skin. Fibrogenesis Tissue Repair. 2008;1(1):3. doi: 10.1186/1755-1536-1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Seckl JR. Glucocorticoids, developmental ‘programming’ and the risk of affective dysfunction. Prog Brain Res. 2008;167:17–34. doi: 10.1016/S0079-6123(07)67002-2. [DOI] [PubMed] [Google Scholar]
- 139.de Weerth C, van Hees Y, Buitelaar JK. Prenatal maternal cortisol levels and infant behavior during the first 5 months. Early Hum Dev. 2003;74(2):139–151. doi: 10.1016/s0378-3782(03)00088-4. [DOI] [PubMed] [Google Scholar]
- 140.Herman JP, Cullinan WE. Neurocircuitry of stress: central control of the hypothalamo-pituitary-adrenocortical axis. Trends Neurosci. 1997;20(2):78–84. doi: 10.1016/s0166-2236(96)10069-2. [DOI] [PubMed] [Google Scholar]
- 141.Joëls M, Baram TZ. The neuro-symphony of stress. Nat Rev Neurosci. 2009;10(6):459–466. doi: 10.1038/nrn2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Rodrigues SM, LeDoux JE, Sapolsky RM. The influence of stress hormones on fear circuitry. Annu Rev Neurosci. 2009;32:289–313. doi: 10.1146/annurev.neuro.051508.135620. [DOI] [PubMed] [Google Scholar]
- 143.Schulkin J. Angst and the amygdala. Dialogues Clin Neurosci. 2006;8(4):407–416. doi: 10.31887/DCNS.2006.8.4/jschulkin. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Kapoor A, Dunn E, Kostaki A, Andrews MH, Matthews SG. Fetal programming of hypothalamo-pituitary-adrenal function: prenatal stress and glucocorticoids. J Physiol. 2006;572(Pt 1):31–44. doi: 10.1113/jphysiol.2006.105254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Cratty MS, Ward HE, Johnson EA, Azzaro AJ, Birkle DL. Prenatal stress increases corticotropin-releasing factor (CRF) content and release in rat amygdala minces. Brain Res. 1995;675(1–2):297–302. doi: 10.1016/0006-8993(95)00087-7. [DOI] [PubMed] [Google Scholar]
- 146.Mueller BR, Bale TL. Sex-specific programming of offspring emotionality after stress early in pregnancy. J Neurosci. 2008;28(36):9055–9065. doi: 10.1523/JNEUROSCI.1424-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Salm AK, Pavelko M, Krouse EM, Webster W, Kraszpulski M, Birkle DL. Lateral amygdaloid nucleus expansion in adult rats is associated with exposure to prenatal stress. Brain Res Dev Brain Res. 2004;148(2):159–167. doi: 10.1016/j.devbrainres.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 148••.Buss C, Davis EP, Shahbaba B, Pruessner JC, Head K, Sandman CA. Maternal cortisol over the course of pregnancy and subsequent child amygdala and hippocampus volumes and affective problems. Proc Natl Acad Sci. 2012;109:E1312–E1319. doi: 10.1073/pnas.1201295109. First and only study to link exposure to stress hormones early in human pregnancy with discrete changes in brain structures and affective impairment in children. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Ellman LM, Schetter CD, Hobel CJ, Chicz-Demet A, Glynn LM, Sandman CA. Timing of fetal exposure to stress hormones: effects on newborn physical and neuromuscular maturation. Dev Psychobiol. 2008;50(3):232–241. doi: 10.1002/dev.20293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150••.Davis EP, Sandman CA. The timing of prenatal exposure to maternal cortisol and psychosocial stress is associated with human infant cognitive development. Child Dev. 2010;81(1):131–148. doi: 10.1111/j.1467-8624.2009.01385.x. Demonstrated that the trajectory of maternal psychosocial and biological stress signals are associated with human cognitive development and that the same stress signal can have different consequences depending on the gestational period of exposure. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Buss C, Davis EP, Hobel CJ, Sandman CA. Maternal pregnancy-specific anxiety is associated with child executive function at 6–9 years age. Stress. 2011;14(6):665–676. doi: 10.3109/10253890.2011.623250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152••.Buss C, Davis EP, Muftuler LT, Head K, Sandman CA. High pregnancy anxiety during mid-gestation is associated with decreased gray matter density in 6–9-year-old children. Psychoneuroendocrinol. 2010;35(1):141–153. doi: 10.1016/j.psyneuen.2009.07.010. Presented the first evidence that prenatal maternal anxiety, measured prospectively, has consequences for child brain development. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Connolly JD, Goodale MA, Menon RS, Munoz DP. Human fMRI evidence for the neural correlates of preparatory set. Nat Neurosci. 2002;5(12):1345–1352. doi: 10.1038/nn969. [DOI] [PubMed] [Google Scholar]
- 154.Squire LR, Stark CE, Clark RE. The medial temporal lobe. Annu Rev Neurosci. 2004;27:279–306. doi: 10.1146/annurev.neuro.27.070203.144130. [DOI] [PubMed] [Google Scholar]
- 155.Höistad M, Barbas H. Sequence of information processing for emotions through pathways linking temporal and insular cortices with the amygdala. Neuroimage. 2008;40(3):1016–1033. doi: 10.1016/j.neuroimage.2007.12.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Nakamura K, Kubota K. The primate temporal pole: its putative role in object recognition and memory. Behav Brain Res. 1996;77(1–2):53–77. doi: 10.1016/0166-4328(95)00227-8. [DOI] [PubMed] [Google Scholar]
- 157.Ahmad Z, Balsamo LM, Sachs BC, Xu B, Gaillard WD. Auditory comprehension of language in young children: neural networks identified with fMRI. Neurology. 2003;60(10):1598–1605. doi: 10.1212/01.wnl.0000059865.32155.86. [DOI] [PubMed] [Google Scholar]
- 158.Mestres-Missé A, Càmara E, Rodriguez-Fornells A, Rotte M, Münte TF. Functional neuroanatomy of meaning acquisition from context. J Cogn Neurosci. 2008;20(12):2153–2166. doi: 10.1162/jocn.2008.20150. [DOI] [PubMed] [Google Scholar]
- 159••.Bale TL. Neuroendocrine and immune influences on the CNS: it’s a matter of sex. Neuron. 2009;64(1):13–16. doi: 10.1016/j.neuron.2009.09.036. Important review of the sex-specific effects of metabolic influences on the developing brain. [DOI] [PubMed] [Google Scholar]
- 160.Lin Y, Ter Horst GJ, Wichmann R, et al. Sex differences in the effects of acute and chronic stress and recovery after long-term stress on stress-related brain regions of rats. Cereb Cortex. 2009;19(9):1978–1989. doi: 10.1093/cercor/bhn225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Boukouvalas G, Gerozissis K, Markaki E, Kitraki E. High-fat feeding influences the endocrine responses of pubertal rats to an acute stress. Neuroendocrinol. 2010;92(4):235–245. doi: 10.1159/000321393. [DOI] [PubMed] [Google Scholar]
- 162.Goel N, Bale TL. Identifying early behavioral and molecular markers of future stress sensitivity. Endocrinology. 2007;148(10):4585–4591. doi: 10.1210/en.2007-0479. [DOI] [PubMed] [Google Scholar]
- 163.Gabory A, Attig L, Junien C. Sexual dimorphism in environmental epigenetic programming. Mol Cell Endocrinol. 2009;304(1–2):8–18. doi: 10.1016/j.mce.2009.02.015. [DOI] [PubMed] [Google Scholar]
- 164.Buss C, Davis EP, Class QA, et al. Maturation of the human fetal startle response: evidence for sex-specific maturation of the human fetus. Early Hum Dev. 2009;85(10):633–638. doi: 10.1016/j.earlhumdev.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Glynn LM, Sandman CA. Sex moderates associations between prenatal glucocorticoid exposure and human fetal neurological development. Dev Science. 2012 doi: 10.1111/j.1467-7687.2012.01159.x. (In Press) [DOI] [PubMed] [Google Scholar]
- 166.Bernardes J, Gonçalves H, Ayres-de-Campos D, Rocha AP. Linear and complex heart rate dynamics vary with sex in relation to fetal behavioural states. Early Hum Dev. 2008;84(7):433–439. doi: 10.1016/j.earlhumdev.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 167.DiPietro JA, Costigan KA, Shupe AK, Pressman EK, Johnson TR. Fetal neurobehavioral development: associations with socioeconomic class and fetal sex. Dev Psychobiol. 1998;33(1):79–91. [PubMed] [Google Scholar]
- 168.Kesler SR, Reiss AL, Vohr B, et al. Brain volume reductions within multiple cognitive systems in male preterm children at age twelve. J Pediatr. 2008;152(4):513–520. doi: 10.1016/j.jpeds.2007.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Clifton VL. Review: sex and the human placenta: mediating differential strategies of fetal growth and survival. Placenta. 2010;31:S33–S39. doi: 10.1016/j.placenta.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 170••.Gluckman PD, Hanson MA. Developmental origins of disease paradigm: a mechanistic and evolutionary perspective. Pediatr Res. 2004;56(3):311–317. doi: 10.1203/01.PDR.0000135998.08025.FB. Significant review of the developmental origins of disease model with special emphasis on the predictive adaptive response. [DOI] [PubMed] [Google Scholar]
- 171••.Sandman CA, Davis EP, Glynn LM. Prescient human fetuses thrive. Psychol Sci. 2012;23(1):93–100. doi: 10.1177/0956797611422073. Only study that describes the predictive adaptive response in human subjects exposed to maternal depression. [DOI] [PMC free article] [PubMed] [Google Scholar]