Abstract
DMT1 is the major transporter for iron entrance into mammalian cells and iron exit from endosomes during the transferrin cycle. Four major mRNA isoforms correspond to 4 protein isoforms, differing at 5'/3' and N-/C- termini, respectively. Isoforms are designated 1A vs. 1B reflecting where transcription starts or +IRE vs. −IRE reflecting the presence / absence of an iron responsive element in the 3' end of the mRNA. These differences imply regulation at transcriptional and posttranscriptional levels.
Many proteins are degraded by a ubiquitination-dependent mechanism. Two different ubiquitin ligases (E3s) appear to be involved in DMT1 ubiquitination: Parkin or Nedd4 family E3s which often utilize Nedd4 family interacting protein-1 and -2 (Ndfip1 & 2) to ubiquitinate their substrate proteins. Prior data suggest that parkin ubiquitinates 1B DMT1 but not 1A DMT1 while Nedd4/Ndfips ligate ubiquitin to DMT1 in the duodenum where 1A/+IRE DMT1 predominates. Our assay for whether these systems target DMT1 depends on two HEK293 cell lines that express permanently transfected 1A/+IRE DMT1 or 1B/−IRE DMT1 after induction by doxycycline. Transient transfection with a parkin construct before induction diminishes 1B/−IRE DMT1 detected by immune-blots but not 1A/+IRE DMT1. Mutant parkin serves as a control that does not affect DMT1 levels. Thus DMT1 regulation in an isoform specific fashion can occur by ubiquitination and the events involved have implications for DMT1 function and disease processes.
Keywords: ubiquitin, protein degradation, protein isoforms, iron, Parkin, Nedd4 family interacting proteins (Ndfips)
DMT1 isoforms
The divalent metal ion transporter DMT1 is an appropriate topic when one considers iron and copper among other reasons because it was postulated to transport iron, copper and multiple other metals (Gunshin et al. 1997), properties addressed at some workshops considering iron and copper (Garrick et al. 2003; Garrick et al. 2006b). Clearly DMT1 is the major duodenal iron importer, but it also plays a similar major role in export of iron from the endosome during the transferrin cycle (Garrick and Garrick 2009; Garrick 2011). DMT1 has 4 major mRNA isoforms that encode 4 protein isoforms, differing at 5'/3' and N-/C- termini, respectively. Isoforms are designated 1A vs. 1B reflecting where transcription starts or +IRE vs. −IRE reflecting the presence / absence of an iron responsive element (IRE) in the 3' region of the mRNA. Exon 1A contains an initiator codon so has 29–31 unique amino acid residues for this N-terminal extension; while exon 1B lacks such an AUG so its translation starts in exon 2; all 4 protein isoforms share 543 amino acid residues in common that start with this Methionyl residue; the −IRE protein isoform has 25 unique C-terminal residues that distinguish it from the 18 unique C-terminal residues of the +IRE isoform. Its function as a duodenal iron importer places DMT1 in that part of the intestine where the +IRE isoform is prevalent while the need for DMT1 in the transferrin cycle is high in erythroid precursors. Yet the tissue distributions of the ±IRE DMT1 mRNA forms (Tchernitchko et al. 2002) reveal the DMT1 isoforms as widespread leaving one to wonder whether DMT1 could have additional metal ion transport functions in other tissues. The distributions of 1A / 1B DMT1 isoforms (Hubert and Hentze 2002) place the 1A form primarily in the duodenum with most other tissues expressing primarily 1B DMT1 and the kidney having substantial levels of both 1A and 1B. Clearly we need to consider multiple modes for regulation of protein levels when thinking about how DMT1 achieves these distributions while noting that the cited data are only for mRNA levels.
The modes for regulation of DMT1 particularly interest us currently relative to the rise in expression of DMT1 seen in Parkinson's Disease (PD) (Salazar et al. 2008) where evidence suggests that 1B/+IRE DMT1 increases. Table 1 systematically lists possible ways that this isoform may achieve the increase. A transcriptional mode is attractive because increased Nuclear Factor-κB (NF-κB) is well established as part of the stress responses in PD (Hunot et al. 1997; Ghosh et al. 2007) and it affects 1B DMT1 transcription (Paradkar and Roth 2006a). Although Hypoxia Inducible Factors (HIFs) are also candidates with response elements postulated in the 1B promoter (Lee et al. 1998), an abundance of data indicts hypoxia as up-regulating 1A DMT1 (Lis et al. 2005; Shah et al. 2009; Mastrogiannaki et al. 2009) via HIF-2α (Shah et al. 2009; Mastrogiannaki et al. 2009). Post-transcriptional control is also attractive given that +IRE DMT1 should be stabilized via iron regulatory proteins 1 and 2 (IRPs) and there is evidence for this occurring in the intestines (Galy et al. 2008). Increased nigral iron (Hirsch et al. 1991) would itself actually diminish +IRE DMT1 levels via this mechanism; however, the increased iron interacting with endogenous nigral dopamine to generate reactive oxygen species might activate IRPs to elevate +IRE DMT1 levels. Although microRNA (miRNA) is another regulatory possibility, the only one known to affect DMT1 acts on the −IRE form (Andolfo et al. 2010). Translational regulation cannot be eliminated as a means of affecting 1B/+IRE levels; however, no precedent is currently known. Protein turnover is clearly a possibility as the potential for specific regulation is built in to the ubiquitin pathway. Thus the rest of this paper is devoted to how DMT1 turnover occurs.
Table 1.
Regulation of DMT 1 – Possibilities for why 1B/+IRE DMT1 increases in PD
| Level | Comments |
|---|---|
| Transcription | Via NF-κB is likely, but via HIF-2α is not likely. |
| Post-transcriptional | +IRE IRP stabilized but only known miRNA affects −IRE. |
| Translational | No data but a formal possibility. |
| Post-translational | Ubiquitin E3-ligases. |
Proteasomal turnover
DMT1 turnover occurs in the proteasome and also to an extent in the lysosome (Paradkar and Roth 2006b). Hence brief coverage of proteasomal degradation is in order. The existence of a subcellular alternative to lysosomal degradation has been accepted for ~35 years (Etlinger and Goldberg 1977). We now recognize that a macromolecular assembly assures the tagging of proteins with ubiquitin for degradation based on three enzymes that assure ubiquitylation named E1, E2 and E3 (Lodish et al. 2004). E1 splits ATP to adenylate ubiquitin (activation). The ubiquitin transfers to a cysteine in E2 (conjugation) then an E3 ligates ubiquitin to the target protein. Multiple ubiquitins (≥4) are required to lead to degradation. Specificity for the target depends on the E3-ligase. There are 500–700 E3-ligases. Our interest in DMT1 and its degradation stems from thinking about its isoforms: From the viewpoint of ~600 E3-ligases and ~20,000 human genes, one might conclude that a typical E3 ligase targets ~30 gene products; however, if one recognizes that there are perhaps ~100,000 human protein isoforms, then it might be better to argue that a typical E3 ligase targets ~160 human protein isoforms. So one wonders whether the specificity of E3-ligases for targets is gene-related or isoform related. The key questions from the viewpoint of DMT1 and its isoforms is whether the E3-ligase(s) that target DMT1 are isoform specific and how many E3-ligases target DMT1.
Parkin and 1B DMT1
Parkin, product of the PARK-2 gene, is both an E3-ligase and a cause of early onset PD when mutated (Kitada et al. 1998; Chung et al. 2001). Parkin also ameliorates Manganese (Mn) toxicity in neuronal cell models (Higashi et al. 2004). This finding attracted our attention (Roth et al. 2010); it was reproducible, associated with diminished Mn uptake by SH-SY5Y neuroblastoma cells that were permanently transfected with wild type (wt) Parkin compared to control SH-SY5Y neuroblastoma cells that were similarly transfected with empty vector or a T240R mutant. The controls experienced more toxicity due to Mn exposure. Immunoblots with antibodies specific for +IRE DMT1 or −IRE DMT1 revealed that SH-SY5Y neuroblastoma cells with wt Parkin had less of either isoform than did control empty vector or T240R mutant cells. Remarkably, the small levels of 1A DMT1 present in the 3 cell types were unaffected by the Parkin status. We therefore hypothesized that the lesser levels of +IRE DMT1 or −IRE DMT1 in wt Parkin cells were due to lesser levels of 1B DMT1 and confirmed this hypothesis by immunodepleting the 1A DMT1 present by immunoprecipitation and showing that the supernatant contained nearly the same pattern for the 1B DMT1 present; i.e., both +IRE DMT1 and −IRE DMT1 were depleted when cells expressed wt Parkin.
The proteasomal inhibitor MG132 inhibited the effect of wt Parkin confirming that the E3-ligase was likely acting on 1B DMT in the proteasome (Roth et al. 2010). Remarkably ±IRE DMT1 levels in a lymphocyte line from an individual with homozygosity for a PARK-2 mutation and juvenile PD were higher than in a heterozygote sib or a +/+ control. This result confirms the potential relevance of DMT1 turnover by Parkin to PD in an affected sibship and establishes that the phenotype is not confined to neuronal tissue. These characteristics reflect a close association of specific isoforms of DMT1 with Parkin as the ±IRE DMT1 forms colocalize and co-immunoprecipitate with tagged Parkin but not with 1A as Parkin ligates ubiquitin to DMT1. Yet Parkin is not the only E3-ligase involved in DMT1 turnover.
DMT1 turnover involving another E3-ligase plus accessory proteins
Prior to the work on Parkin, two groups (Foot et al. 2008; Howitt et al. 2009) found another pathway for DMT1 turnover. It involved an E3-ligase family referred to as the neuronal precursor cell-expressed developmentally downregulated 4 (Nedd4) family. Members include NEDD4, NEDD4L, ITCH and WW domain-containing protein 2 (WWP2) where the WW domain is a protein motif consisting of 35 to 40 amino acids that contains 4 conserved aromatic residues and likely responsible for specific protein–protein interactions. It binds proline-rich PPxY (PY) motifs in particular targets. Yet many targets lack PY motifs leading to dependence on an accessory protein.that contains PY motifs and binds the target, here called Nedd4 family interacting proteins 1 & 2 (Ndfip1 and Ndfip2). The two papers showed that DMT1 proteasomal turnover involved Ndfips (Foot et al. 2008; Howitt et al. 2009).
More recently, a study (Foot et al. 2011) that relied on mice with the gene for Ndfip1 ablated demonstrated that intestinal levels of DMT1 were remarkably elevated during iron deficiency as seen by immunohistochemistry and transport activity measurement. The mice had a combination of iron deficiency and inflammatory disease when fed a low iron diet leading to a severe microcytic, hypochromic anemia.
Materials and methods
Cell lines
We relied on two HEK293F cell lines (Garrick et al. 2006a) that express DMT1 in a tetracycline (doxycycline) regulated fashion to address isoform specific regulation of DMT1. One line expresses rat 1A/+IRE DMT1; the other expresses mouse 1B/-IRE DMT1.
Transient transfection
Cells were grown to ~50% confluence, transfected with DNA constructs by means of Lipofectamine 2000 (Invitrogen) using the manufacturer's procedure. After the cells recovered in Optimem (Invitrogen), increased expression of the permanently transfected DMT1 construct was induced with doxycycline (Garrick et al. 2006a). Later (48 hr) cells were lysed, extracts prepared and analyzed by immunoblots (Garrick et al. 2006a). After analysis using an antibody to DMT1, each blot was stripped once and reprobed with either anti-actin to assure that loads were equal or anti-FLAG to detect the Parkin constructs.
Results
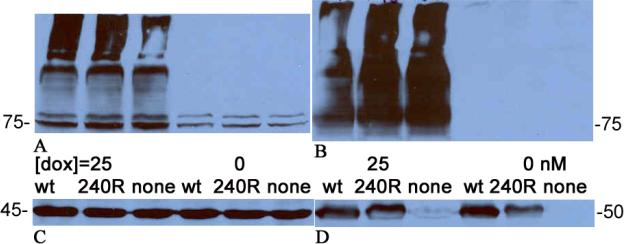
Figure 1 depicts a series of western blots that confirm that 1A/+IRE DMT1 is not degraded by Parkin. Comparison of the 3 leftmost lanes of parts A and B to those on their right verifies that induction of DMT1 by doxycycline is very effective. Each band represents DMT1; the ~75 kD band at the bottom has nearly the expected size and the ~95 kD band could represent a glycosylated form with all of the rest representing oligomers of DMT1 formed because the extracts were boiled and the effective DMT1 concentration was high. The load controls and the transient transfection controls make it clear that the Parkin constructs were expressed but have no effect on DMT1 levels.
Figure 1.
Parkin does not target 1A/+IRE DMT1. Keys for the levels of doxycycline used to induce DMT1 expression (0 or 25 nM) and the Parkin construct used for transient transfection (wt, T240R or none = empty vector) are present between parts A & C and parts B & D. A. Blot probed with anti-DMT1 directed against the 4th extracellular region. B. Blot probed with anti-DMT1 directed against exons 2–3. In parts A and B, the lowest DMT1 band is ~75kDa. C. Blot from A after it was stripped and reprobed with anti-actin (load control). The actin band is ~45 kDa. There was some ineffective transfer in the high molecular weight region of the left lane. D. Blot from B after it was stripped and reprobed with anti-FLAG (tests for successful transient transfection with FLAG-tagged-Parkin construct). The FLAG-tagged-Parkin band is ~50 kDa; it is absent from the 3rd and 6th lanes because the DNA construct was empty vector.
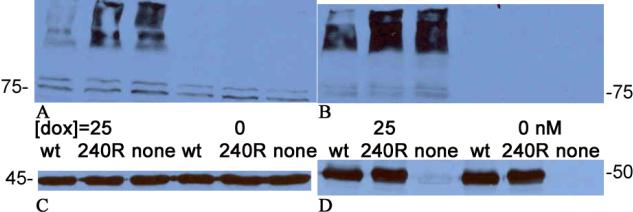
Figure 2 shows a similar series of western blots that verify that 1B/-IRE DMT1 is a Parkin ubiquitylation and degradation target. Again comparison of the 3 leftmost lanes of parts A and B to those on their right verifies that induction of DMT1 by doxycycline is very effective. Each band represents DMT1 here too; again the ~75 kD band at the bottom has nearly the expected size and the ~95 kD band could represent a glycosylated form with all of the rest representing oligomers of DMT1 formed because the extracts were boiled and the effective DMT1 concentration was high. The load controls and the transient transfection controls make it clear that the Parkin constructs were expressed so that this time wt Parkin expression led to the degradation of more than half of the DMT1.
Figure 2.
Parkin does target 1B/−IRE DMT1. Keys for the levels of doxycycline used to induce DMT1 expression (0 or 25 nM) and the Parkin construct used for transient transfection (wt, T240R or none = empty vector) are present between parts A & C and parts B & D. A. Blot probed with anti-DMT1 directed against the 4th extracellular region. B. Blot probed with anti-DMT1 directed against exons 2–3. In parts A and B, the lowest DMT1 band is ~75kDa. C. Blot from A after it was stripped and reprobed with anti-actin (load control). The actin band is ~45 kDa. D. Blot from B after it was stripped and reprobed with anti-FLAG (tests for successful transient transfection with FLAG-tagged-Parkin construct). The FLAG-tagged-Parkin band is ~50 kDa; it is absent from the 3rd and 6th lanes because the DNA construct was empty vector.
Discussion
1B DMT1 and Parkin
The data in the two immunoblot figures are entirely consistent with earlier results (Roth et al. 2010) showing that 1A DMT1 is unaffected by Parkin while 1B DMT1 is a target for this E3-ligase. The earlier results relied on an indirect method – immunoprecipitation to remove 1A DMT1 and leave 1B DMT1 in the supernatant – to reach the conclusion whereas the results presented here are a direct demonstration. Because neuronal cells express very little 1A DMT1 while the HEK293 line expresses this isoform very effectively, confirmation that it was not a Parkin target is useful. Antibody to 1A epitopes could hypothetically fail to precipitate product of 1A mRNA that has not acquired the AUG codon due to ragged mRNA ends (a possibility that does occur – results not shown) or that exhibits loss of part of the N-terminal sequence. The 1B/-IRE DMT1 producing HEK293 cells express the intended target for Parkin to show that it is indeed the target.
Our data thus verify and extend those already published (Roth et al. 2010). Considering just the data in Figures 1 and 2, it remains a formal possibility, however, that specificity is determined by the +IRE vs. −IRE difference. Hence the data here and those already published should be considered in combination. They also point out how one can do similar tests for Ndfip specificity for targeting of DMT1 isoforms and indicate that E3-ligases target specific protein isoforms rather than all isoforms produced by a particular gene.
Ndfips, Parkin and DMT1 isoforms
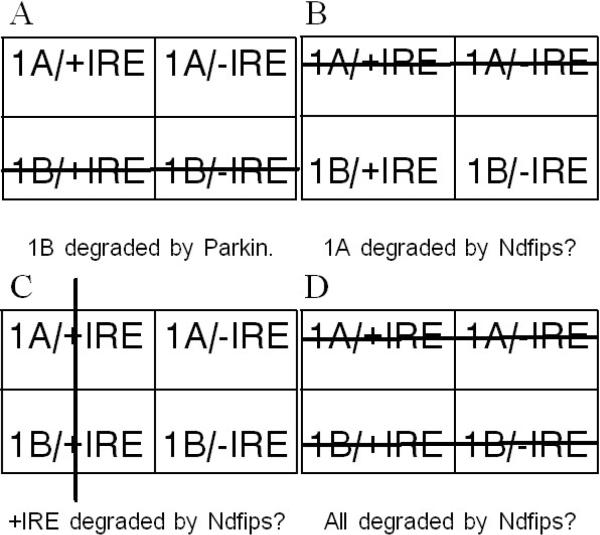
Although final answers for the targeting specificity of Ndfip 1 and 2 remain to be obtained, it is worthwhile to consider the main likelihoods (Fig 3). Parkin ubiquitinates 1B DMT1 (Fig 3A). A substantial rise in DMT1 level occurs in intestinal villi during iron deficiency in Ndfip 1 −/− mice (Foot et al. 2011). The main isoform available in this tissue is 1A/+IRE DMT1 so it should be largely responsible for the increase. If so, there are 3 probable specificities for Ndfip 1 (or 2): They could aid in ligating ubiquitin to 1A DMT1 (Fig 3B); this specificity is intellectually satisfying as all 4 forms of DMT1 would be accounted for by 2 E3-ligase systems. Or they could specify +IRE DMT1 (Fig 3C); if so, 1A/-IRE DMT1 could be the target of yet another E3-ligase. Or Ndfip 1 / 2 could target all 4 isoforms (Fig 3D).
Figure 3.
How E3-ligases could target DMT1 isoforms. All 4 parts depict the 4 DMT1 isoforms in a tabular array: 1A isoforms above 1B; +IRE isoforms to the left of −IRE. Large lines strike through the isoforms that are / might be degraded. A. Parkin targets 1B DMT1. B. Ndfips might target 1A DMT1. C. Ndfips might target +IRE DMT1. D. Ndfips might target all 4 DMT1 isoforms.
Changed regulation of DMT1 contributes to the etiological pathway for PD (Salazar et al. 2008). Parkin could mediate these inflammatory changes (Roth et al. 2010) although this conclusion does not have to account for all DMT1 mediated events much less all PD. Interestingly, inflammation has also been noted in relation to DMT1 changes via Ndfip-associated changes (Howitt et al. 2009), but this relationship appears to be more indirect (Foot et al. 2011).
Acknowledgments
We thank Mr. Fabian Pirrman for technical assistance preparing DNA constructs.
Abbreviations
- DMT1
divalent metal ion transporter
- IRE
iron responsive element
- PD
Parkinson's Disease
- NF-κB
Nuclear Factor-κB
- HIFs
hypoxia inducible factors
- IRPs
iron regulatory proteins
- miRNA
microRNA
- Mn
Manganese
- wt
wild type
- Nedd4
Neuronal precursor cell-expressed developmentally downregulated 4
- WWP2
WW domain-containing protein 2
- PY
PPxY motifs
- Ndfip1 and Ndfip2
Nedd4 family interacting proteins 1 & 2
References
- Andolfo I, Falco LD, Asci R, Russo R, Colucci S, Gorrese M, Zollo M, Iolascon A. Regulation of divalent metal transporter 1 (DMT1) non-IRE isoform by the microRNA Let-7d in erythroid cells. Haematologica. 2010;95(8):1244–1252. doi: 10.3324/haematol.2009.020685. doi:10.3324/haematol.2009.020685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung KKK, Zhang Y, Lim KL, Tanaka Y, Huang H, Gao J, Ross CA, Dawson VL, Dawson TM. Parkin ubiquitinates the [alpha]-synuclein-interacting protein, synphilin-1: implications for Lewy-body formation in Parkinson disease. Nat Med. 2001;7(10):1144–1150. doi: 10.1038/nm1001-1144. [DOI] [PubMed] [Google Scholar]
- Etlinger JD, Goldberg AL. A soluble ATP-dependent proteolytic system responsible for the degradation of abnormal proteins in reticulocytes. Proceedings of the National Academy of Sciences. 1977;74(1):54–58. doi: 10.1073/pnas.74.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foot NJ, Dalton HE, Shearwin-Whyatt LM, Dorstyn L, Tan S-S, Yang B, Kumar S. Regulation of the divalent metal ion transporter DMT1 and iron homeostasis by a ubiquitin-dependent mechanism involving Ndfips and WWP2. Blood. 2008;112(10):4268–4275. doi: 10.1182/blood-2008-04-150953. doi:10.1182/blood-2008-04-150953. [DOI] [PubMed] [Google Scholar]
- Foot NJ, Leong YA, Dorstyn LE, Dalton HE, Ho K, Zhao L, Garrick MD, Yang B, Hiwase D, Kumar S. Ndfip1-deficient mice have impaired DMT1 regulation and iron homeostasis. Blood. 2011;117(2):638–646. doi: 10.1182/blood-2010-07-295287. doi:10.1182/blood-2010-07-295287. [DOI] [PubMed] [Google Scholar]
- Galy B, Ferring-Appel D, Kaden S, Gröne H-J, Hentze MW. Iron Regulatory Proteins Are Essential for Intestinal Function and Control Key Iron Absorption Molecules in the Duodenum. Cell Metabolism. 2008;7(1):79–85. doi: 10.1016/j.cmet.2007.10.006. [DOI] [PubMed] [Google Scholar]
- Garrick M. Human iron transporters. Genes & Nutrition. 2011;6(1):45–54. doi: 10.1007/s12263-010-0184-8. doi:10.1007/s12263-010-0184-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrick MD, Dolan KG, Ghio A, Horbinski C, Higgins D, Porubcin M, Moore EG, Hainsworth LN, Umbreit JN, Conrad ME, Feng L, Lis A, Roth JE, Singleton S, Garrick LM. DMT1 (Divalent Metal Transporter 1): A mammalian transporter for multiple metals. BioMetals. 2003;16:41–54. doi: 10.1023/a:1020702213099. [DOI] [PubMed] [Google Scholar]
- Garrick MD, Garrick LM. Cellular iron transport. Biochimica et Biophysica Acta (BBA) - General Subjects. 2009;1790(5):309–325. doi: 10.1016/j.bbagen.2009.03.018. [DOI] [PubMed] [Google Scholar]
- Garrick MD, Kuo H-C, Vargas F, Singleton S, Zhao L, Smith JJ, Paradkar P, Roth JA, Garrick LM. Comparison of mammalian cell lines expressing distinct isoforms of divalent metal transporter 1 in a tetracycline-regulated fashion. Biochem J. 2006a;398(3):539–546. doi: 10.1042/BJ20051987. doi:10.1042/bj20051987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrick MD, Singleton ST, Vargas F, Kuo H-C, Zhao L, Knöpfel M, Davidson T, Costa M, Paradkar P, Roth JA, Garrick LM. DMT1: Which metals does it transport? Biol Res. 2006b;39:79–85. doi: 10.4067/s0716-97602006000100009. [DOI] [PubMed] [Google Scholar]
- Ghosh A, Roy A, Liu X, Kordower JH, Mufson EJ, Hartley DM, Ghosh S, Mosley RL, Gendelman HE, Pahan K. Selective inhibition of NF-κB activation prevents dopaminergic neuronal loss in a mouse model of Parkinson's disease. Proceedings of the National Academy of Sciences. 2007;104(47):18754–18759. doi: 10.1073/pnas.0704908104. doi:10.1073/pnas.0704908104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunshin H, Mackenzie B, Berger UV, Gunshin Y, Romero MF, Boron WF, Nussberger S, Gollan JL, Hediger MA. Cloning and characterization of a mammalian proton-coupled metal-ion transporter. Nature. 1997;388(6641):482–488. doi: 10.1038/41343. [DOI] [PubMed] [Google Scholar]
- Higashi Y, Asanuma M, Miyazaki I, Hattori N, Mizuno Y, Ogawa N. Parkin attenuates manganese-induced dopaminergic cell death. J Neurochem. 2004;89(6):1490–1497. doi: 10.1111/j.1471-4159.2004.02445.x. [DOI] [PubMed] [Google Scholar]
- Hirsch EC, Brandel JP, Galle P, Javoyagid F, Agid Y. Iron and aluminum increase in the substantia-nigra of patients with parkinsons-disease - an x-ray-microanalysis. J Neurochem. 1991;56(2):446–451. doi: 10.1111/j.1471-4159.1991.tb08170.x. [DOI] [PubMed] [Google Scholar]
- Howitt J, Putz U, Lackovic J, Doan A, Dorstyn L, Cheng H, Yang B, Chan-Ling T, Silke J, Kumar S, Tan S-S. Divalent metal transporter 1 (DMT1) regulation by Ndfip1 prevents metal toxicity in human neurons. Proceedings of the National Academy of Sciences. 2009;106(36):15489–15494. doi: 10.1073/pnas.0904880106. doi:10.1073/pnas.0904880106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubert N, Hentze MW. Previously uncharacterized isoforms of divalent metal transporter (DMT)-1: Implications for regulation and cellular function. Proc Natl Acad Sci USA. 2002;99(19):12345–12350. doi: 10.1073/pnas.192423399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunot S, Brugg B, Ricard D, Michel PP, Muriel M-P, Ruberg M, Faucheux BA, Agid Y, Hirsch EC. Nuclear translocation of NF-κB is increased in dopaminergic neurons of patients with Parkinson disease. Proceedings of the National Academy of Sciences. 1997;94(14):7531–7536. doi: 10.1073/pnas.94.14.7531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitada T, Asakawa S, Hattori N, Matsumine H, Yamamura Y, Minoshima S, Yokochi M, Mizuno Y, Shimizu N. Mutations in the parkin gene cause autosomal recessive juvenile parkinsonism. Nature. 1998;392(6676):605–608. doi: 10.1038/33416. doi:http://www.nature.com/nature/journal/v392/n6676/suppinfo/392605a0_S1.html. [DOI] [PubMed] [Google Scholar]
- Lee PL, Gelbart T, West C, Halloran C, Beutler E. The human Nramp2 gene: Characterization of the gene structure, alternative splicing, promoter region and polymorphisms. Blood Cells Mol Dis. 1998;24(9):199–215. doi: 10.1006/bcmd.1998.0186. [DOI] [PubMed] [Google Scholar]
- Lis A, Paradkar PN, Singleton S, Kuo HC, Garrick MD, Roth JA. Hypoxia induces changes in expression of isoforms of the divalent metal transporter (DMT1) in rat pheochromocytoma (PC12) cells. Biochemical Pharmacoly. 2005;69(11):1647–1655. doi: 10.1016/j.bcp.2005.03.023. [DOI] [PubMed] [Google Scholar]
- Lodish H, Berk A, Matsudaira P, Kaiser C, Krieger M, Scott M, Zipursky S, Darnell J. Molecular cell biology. 5th edn. W.H. Freeman; New York: 2004. pp. 66–72. [Google Scholar]
- Mastrogiannaki M, Matak P, Keith B, Simon C, Vaulont S, Peyssonnaux C. HIF-2α, but not HIF-1α, promotes iron absorption in mice. J Clin Invest. 2009;119(5):1159–1166. doi: 10.1172/JCI38499. doi:10.1172/JCI38499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paradkar PN, Roth JA. Nitric oxide transcriptionally down-regulates specific isoforms of divalent metal transporter (DMT1) via NF-κB. Journal of Neurochemistry. 2006a;96(6):1768–1777. doi: 10.1111/j.1471-4159.2006.03702.x. [DOI] [PubMed] [Google Scholar]
- Paradkar PN, Roth JA. Post-translational and transcriptional regulation of DMT1 during P19 embryonic carcinoma cell differentiation by retinoic acid. Biochem J. 2006b;394(1):173–183. doi: 10.1042/BJ20051296. doi:10.1042/bj20051296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth JA, Singleton S, Feng J, Garrick M, Paradkar PN. Parkin regulates metal transport via proteasomal degradation of the 1B isoforms of divalent metal transporter 1. Journal of Neurochemistry. 2010;113:454–464. doi: 10.1111/j.1471-4159.2010.06607.x. doi:10.1111/j.1471-4159.2010.06607.x. [DOI] [PubMed] [Google Scholar]
- Salazar J, Mena N, Hunot S, Prigent A, Alvarez-Fischer D, Arredondo M, Duyckaerts C, Sazdovitch V, Zhao L, Garrick LM, Nuñez MT, Garrick MD, Raisman-Vozari R, Hirsch EC. Divalent metal transporter 1 (DMT1) contributes to neurodegeneration in animal models of Parkinson's disease. Proceedings of the National Academy of Sciences. 2008;105(47):18578–18583. doi: 10.1073/pnas.0804373105. doi:10.1073/pnas.0804373105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah YM, Matsubara T, Ito S, Yim S-H, Gonzalez FJ. Intestinal Hypoxia-Inducible Transcription Factors Are Essential for Iron Absorption following Iron Deficiency. Cell Metabolism. 2009;9(2):152–164. doi: 10.1016/j.cmet.2008.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tchernitchko D, Bourgeois M, Martin ME, Beaumont C. Expression of the two mRNA isoforms of the iron transporter Nrmap2/DMTI in mice and function of the iron responsive element. Biochem J. 2002;363(3):449–455. doi: 10.1042/0264-6021:3630449. [DOI] [PMC free article] [PubMed] [Google Scholar]