Abstract

L-Alanine is an important amino acid that plays a key role in the molecular structure of many proteins. Crystallized forms of this molecule are currently in high demand in chemical, pharmaceutics, and food industries. However, the traditional evaporative crystallization method takes up to several hours to complete and does not always consistently yield usable crystals. Using the metal-assisted and microwave-accelerated evaporative crystallization (MA-MAEC) technique, larger and better-organized L-alanine crystals were formed in a fraction of the time using room temperature crystallization. This technique may be applicable to organic molecules other than amino acids and thus will be able to produce the large amount of molecular crystals desired by industries today.
Crystallography has become a very useful tool for scientists in recent years due to its success in contributing to the understanding of molecular structures. While crystals of all molecular types are helping to recognize biological significances, proteins and amino acids are the primary molecules that are being focused on today. Amino acids are of particular importance because of their solubility and stabilizing properties that allow them to create multitudes of distinctive proteins.1 Along with this, they also can serve as either intermediate or end products of biological functions and have a wide range of applications in the chemical, food, cosmetic, and pharmaceutical industries.2
L-Alanine is one of the most abundant amino acids used in the synthesis of proteins.3 Because of its structural simplicity and importance in protein construction, it is also a key molecule in crystallization research. Furthermore, because hydrogen bonding plays a large role in alanine’s molecular structure, research concerning this particular amino acid can lead to a better understanding of the structural dimensions of macromolecules such as peptides and proteins.4 A number of studies have been conducted on the properties of crystallized L-alanine, including studies about its vibrational spectra,5 morphology,6 and thermal properties.4 However, a majority of these studies utilized the traditional room temperature evaporative crystallization method, which can take up to several days to complete.
In this study, the application of a previously described method, called the metal-assisted and microwave-accelerated evaporative crystallization (MA-MAEC),7 to rapid crystallization of L-ala-nine, is presented. The MA-MAEC technique is based on the combined use of microwave heating (for speeding up the crystallization process) and plasmonic nanostructures (silver island films, SIFs as selective nucleation sites) for L-alanine crystal growth. The MA-MAEC technique is a promising new method for rapid molecular crystallization that significantly decreases the amount of time required for complete evaporation and crystallization to occur.
The effect of using SIFs and evaporative crystallization conditions (room temperature and microwave-accelerated) on the time of crystallization and type of crystals of L-alanine was studied. Table 1 summarizes the results for the crystallization of L-alanine at room temperature and using the MA-MAEC technique. For a fixed volume (20 μL) and concentration (2.70 M, pH = 5.3) of L-alanine (minimum of five samples were used), the crystallization process on blank glass slides and SIFs took 50 ± 3 min and 41 ± 13 min on average at room temperature, respectively. Complete L-alanine crystallization required 38 s to 6.5 min when using the microwave-accelerated evaporative crystallization (MAEC) technique on blank glass slides. Observable crystals formed on 25 of 31 blank glass slides, which is consistent with previously published results for L-glycine. Average crystallization time decreased as the microwave power level was increased when using the MA-MAEC technique. For example, crystallization of L-alanine was completed in only 22 s on SIFs when using the MA-MAEC technique at microwave power level 10 and in 7 min on SIFs at microwave power level 1. It is also important to note that all SIFs surfaces yielded observable L-alanine crystals. The α-form of L-alanine crystals was observed by optical microscopy in all samples in this study, which is similar to observations made by other groups.6,8 Powder X-ray diffraction studies (Supporting Information, Figure S8) shows that L-alanine crystals grown in this study had the identical pattern, which implies that microwave heating does not affect the structure of L-alanine crystals.
Table 1.
Summary of Results for the Crystallization of 20 μL of L-Alanine from 2.70 M Solution on Glass Slides and Silver Island Films (SIFs) at Room Temperature and Using MA-MAEC Techniquea
| glass | SIFs | type of crystal | |
|---|---|---|---|
| room temperature | 50 ± 3 min | 41 ± 13 min | a |
| microwave power level 1 | 6.5 ± 1 min | 7 ± 1 min | a |
| microwave power level 5 | 41 ± 3 s | 45 ± 6 s | a |
| microwave power level 10 | 38 ± 2 s | 22 ± 3 s | a |
N = 5 samples.
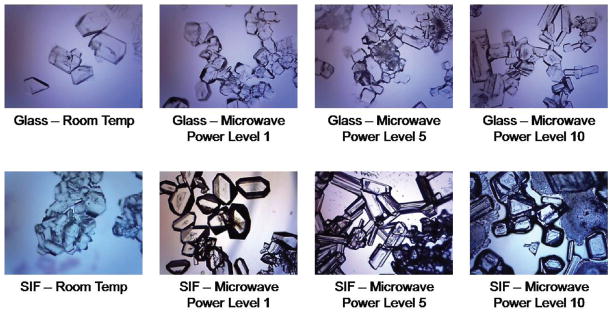
Figure 1 shows the visual comparison of L-alanine crystals formed using room temperature and MA-MAEC techniques on both blank glass slides and on SIFs. For a majority of the samples, crystals grown using the MA-MAEC technique were consistently larger than those grown using room temperature crystallization. Crystal size ranged from 110 to 589 μm on blank glass slides and from 141 to 581 μm on SIFs after complete evaporation. Crystals were believed to have stopped growing after complete evaporation of the aqueous solution because of a decrease in super-saturation of the solution.8 Consistent with previous research, all α-crystals had the largest face zone and were elongated along what was believed to be the c-axis.6
Figure 1.
Optical images of L-alanine crystals formed on blank glass slides and SNFs from 2.70 M solution at room temperature and using the MA-MAEC technique. All images were taken with the same optical setup.
As described previously,7 these observations were attributed to the fact that SIFs serve as selective nucleation sites for L-alanine crystal growth and as microwave-transparent medium for the creation of thermal gradient between the warmer solution and the silver nanostructures that remain at room temperature after microwave heating. The microwave heating allows for the significant reduction in the time of crystallization process. It is well-known that amine groups have afinity toward plasmonic nanoparticles, such as silver in particular.9 Therefore, it is thought that the amine groups of L-alanine assemble onto silver nanostructures, becoming probable nucleation sites for the growth of crystals. This hypothesis was tested by comparison of crystal growth on blank glass slides and SIFs. Compared to L-alanine crystals formed on blank glass slides at room temperature, crystals grown on SIFs were more abundant and had fewer imperfections. They also appeared to be more homogeneous in size than crystals grown on glass slides, where larger variation in the size of the crystals was observed. In this regard, on glass slides (n = 5, minimum count of 100 crystals) the crystal size varied between 111 and 305 μm, while SIFs (n = 5, minimum count of 100 crystals) crystal sizes ranged from 162 to 294 μm, with the majority of crystal sizes concentrated at 200 μm or higher.
It is also important to note that the size distribution of the crystals grown on blank glass slides and SIFs using microwave power level 1 was homogeneous as compared to the heterogeneous size distribution observed using microwave power levels 5 and 10. This is attributed to the excess microwave heating of the solution and the crystals formed during microwave heating (at power level 5 and 10). It is thought that excess microwave heating affects the crystal nucleation and growth by further increasing the rate of these processes.
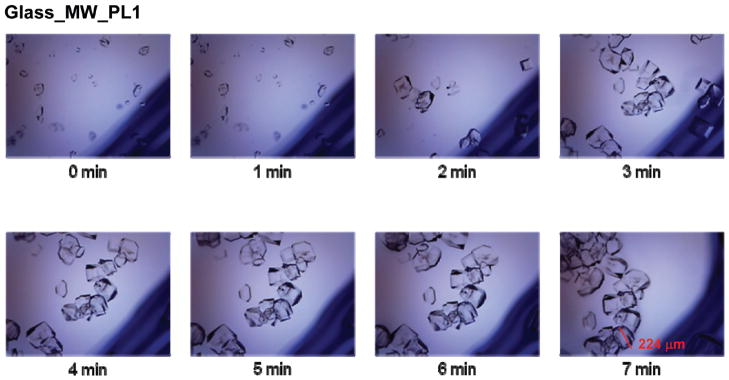
In order to better understand the crystallization process during room temperature and microwave heating evaporation, optical images of the solution and the growing crystals on blank glass slides and SIFs were taken at time intervals as indicated in the figures (2, 3 and Supporting Information, Figures S1–S7). In all these experiments, microwave heating was stopped for a brief period of time (~10 s) to collect optical images. Figure 2 (Glass_MW_PL1) shows the timed crystal growth progression on glass slides at microwave power level 1. Smaller crystals appeared by the time the first image (t = 0 min) was taken. The crystal growth is clearly seen in the subsequent images, where the crystals seemed to grow to their final size at 4–7 min. These images also show the crystal movement (t = 0 to t = 6 min) in solution, after which they rest in their final places after the complete evaporation of the solvent (at t = 7 min). Similar observations were also made for crystals grown on glass slides using microwave power level 5 and 10 (Supporting Information, Figures S1–S3).
Figure 2.
Time progression of the growth of L-alanine crystals on blank glass slides using the MAEC technique at microwave power level 1.
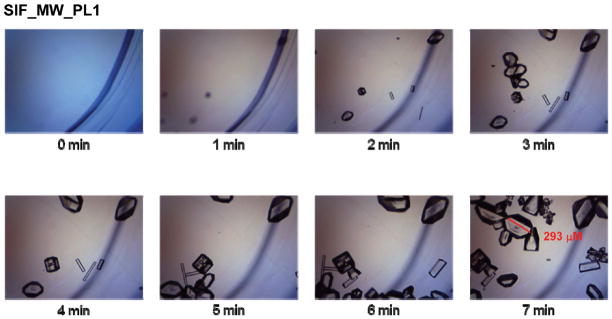
Figure 3 (SIF_MW_PL1) shows the timed crystal growth progression on SIFs using microwave power level 1. Crystals first started to appear on SIFs around 2 min of microwave heating, after which significant growth was observed until complete evaporation at t = 7 min. At microwave power levels 1 and 5, significant improvement of the growth of crystals was observed on SIFs compared to glass slides. These crystals were much more abundant and of better quality than those grown using the MAEC technique on glass, which were imperfect and scarce in quantity. Crystal growth occurred on all SIFs samples of each microwave heating condition and took only 22 s to 7 min for complete evaporation (microwave power level 1 and 10, respectively), proving that the same crystals can be grown using the MA-MAEC technique over 10-fold faster than the traditional evaporative crystallization method. The abundance of L-alanine crystals formed using the MA-MAEC method can be explained by the presence of silver nanoparticles on the surface. SIFs served as nucleation sites that afforded for the growth of crystals in large quantities.7 In comparison, the nucleation and growth of L-alanine crystals were random in nature due to the lack of functional surface groups on glass slides.
Figure 3.
Time progression of the growth of L-alanine crystals on SIFs using the MA-MAEC technique at microwave power level 1.
It is also important to note that when applying microwave heating to the L-alanine solution on both glass slides and SIFs, crystal organization improved when the microwave was stopped and started multiple times for imaging purposes, as compared to uninterrupted microwave heating of the same amount of time. This might be explained by the high amount of microwave energy being absorbed by the L-alanine solution in a short period of time. The amount of energy present may have been higher than required for crystal growth and thus may have prevented the crystals from their normal growth. In addition, the rate growth of L-alanine crystals on glass and SIFs using microwave power level 1 was slowest between 0 and 2 min (nucleation), fastest between 2 and 3 min (growth) and slow > 3 min (growth). These observations were also attributed to the evaporation of the solvent during intermittent microwave heating of the samples. It is thought that nucleation (and slow growth) of L-alanine crystals occurred in the first 2 min as the extent of solvent was the largest. As the nucleation sites were formed and solvent started to evaporate, the fast growth of L-alanine crystals occurred between 2 and 3 min of microwave heating. After >3 min, the rate of growth of L-alanine crystals slowed significantly due to the decrease in the number of L-alanine molecules in the solvent. Complete evaporation of solvent occurred at 7 min of microwave heating.
Figures 4 and 5 shows the Raman spectra of L-alanine crystals grown on glass slides and SIFs at room temperature and using the MA-MAEC technique. Observable peaks appear in the same locations as those in previously published results4 for L-alanine grown on both glass and SIFs. This indicates that the crystals produced in this study possess vibrational properties similar to other L-alanine crystals and thus can be deemed the type of L-alanine crystals typically formed through room temperature evaporation from an aqueous L-alanine solution. Furthermore, since the Raman peaks are observed in identical locations on glass slides and SIFs, it can be concluded that the use of microwave heating and SIFs accelerates the crystallization process without altering the structural and vibrational properties of the crystals grown on them.
Figure 4.
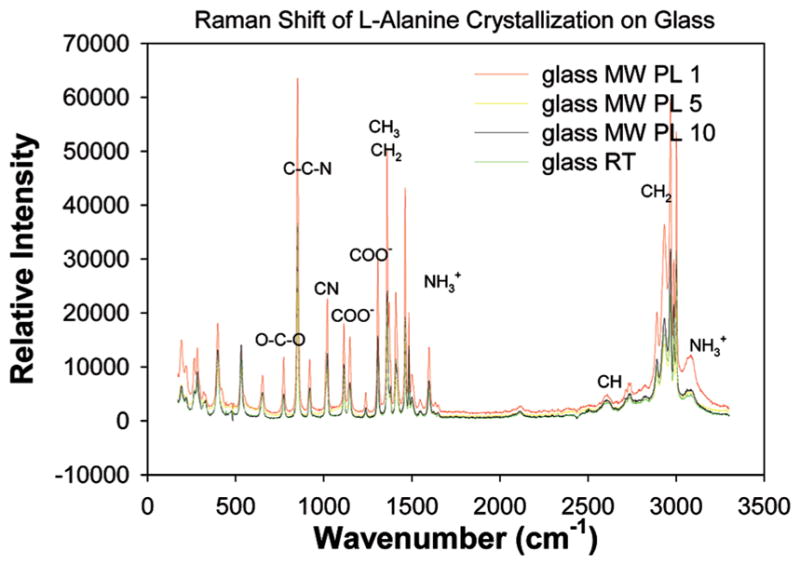
Raman spectrum of L-alanine crystallized on blank glass slides at room temperature and using the MA-MAEC technique. Notation of functional groups at peaks signifies the presence of a functional group at the indicated wavelength.
Figure 5.
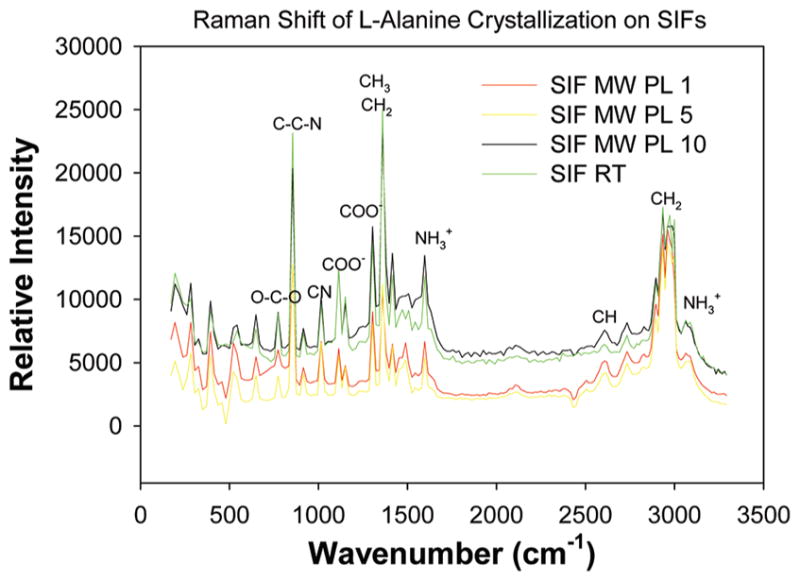
Raman spectrum of L-alanine crystallized on SIFs at room temperature and using the MA-MAEC technique. Notation of functional groups at peaks signifies the presence of a functional group at the indicated wavelength.
In summary, the presented results prove that the MA-MAEC technique is a highly effective method for rapid crystallization of L-alanine. Crystals produced using microwave-heating were larger in size than those grown at room temperature for both SIFs and glass slides, and were produced at a rate over 10-fold faster than that of the room temperature method. The presence of silver nanostructures on surfaces afforded selective nucleation sites compared to on blank glass slides, and therefore the simultaneous growth of more crystals was able to occur. Furthermore, the majority of crystals grown on SIFs was of better quality and appeared with fewer imperfections than those grown on glass. The use of the MA-MAEC technique increases the efficiency of the crystallization of amino acids and may be able to aid in crystallization of other organic molecules (i.e., drug compounds) at much faster rates than they are currently produced at room temperature evaporation. Work is currently underway for the demonstration of the MA-MAEC technique to other amino acids, small molecules, and proteins. The results of these studies will be published in due course.
Supplementary Material
Acknowledgments
The project described was supported by Award Number 5-K25EB007565-05 from the National Institute of Biomedical Imaging and Bioengineering. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Biomedical Imaging and Bioengineering or the National Institutes of Health. The authors also acknowledge Department of Homeland Security, Office of University Programs, Science & Technology Directorate: DHS 2008-ST-062-000011 for support.
Footnotes
Supporting Information. Additional optical images of L-alanine crystals, experimental details, and powder X-ray diffraction images are provided. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Ito L, Kobayashi T, Shiraki K, Yamaguchi H. Effect of amino acids and amino acid derivatives on crystallization of hemoglobin and ribonuclease A. J Synchrotron Radiat. 2008;15:316–318. doi: 10.1107/S0909049507068598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ng KM, Harjo B, Wibowo C. Development of amino acid crystallization processes: L-glutamic acid. Ind Eng Chem Res. 2007;46(9):2814–2822. [Google Scholar]
- 3.Yamada K, Sato A, Shimizu T, Yamazaki T, Yokoyama S. L-Alanine hydrochloride monohydrate. Acta Crystallogr Sect E. 2008;64:O806–U1439. doi: 10.1107/S1600536808008751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mohan R, Kumar KS, Raghavalu T, Mathivanan V, Kovendhan M, Sivakumar B, Kumar GR, Raj SG. Structural, optical, spectral and thermal studies of nonlinear optical pure and deuterated L-alanine single crystals. J Cryst Growth. 2008;310(6):1182–1186. [Google Scholar]
- 5.Machida KKA, Saito Y, Uno T. Polarized Raman spectra and intermolecular potential of L-alanine crystal. Spectrochim Acta, Part A. 1978;34:909–914. [Google Scholar]
- 6.Lechuga-Ballesteros DRHN. Effects of molecular structure and growth kinetics on the morphology of L-alanine crystals. Int J Pharm. 1995;115:151–160. [Google Scholar]
- 7.Pinard MA, Aslan K. Metal-assisted and microwave-accelerated evaporative crystallization. Cryst Growth Des. 2010;10(11):4706–4709. doi: 10.1021/cg101059c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koyama M, Shiraishi M, Sasaki K, Kon-no K. Preparation of L-alanine crystals containing gold nanoparticles. J Dispersion Sci Technol. 2008;29(9):1266–1271. [Google Scholar]
- 9.Myerson AS, Lee AY, Lee IS, Dettet SS, Boerner J. Crystallization on confined engineered surfaces: A method to control crystal size and generate different polymorphs. J Am Chem Soc. 2005;127(43):14982–14983. doi: 10.1021/ja055416x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.