Abstract
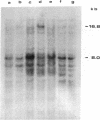
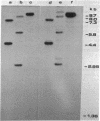
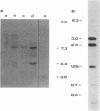
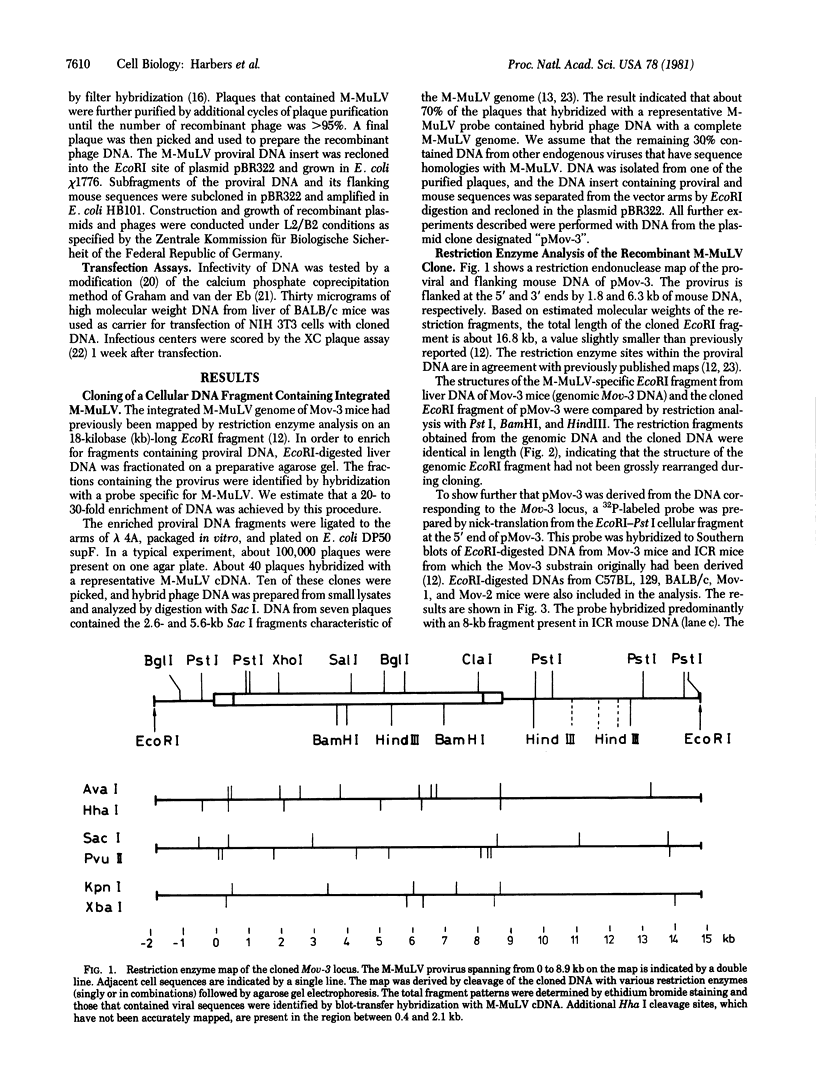
The Mov-3 substrain of mice carries Moloney murine leukemia virus as a Mendelian gene in its germ line. All mice segregating the Mov-3 locus activate virus and develop viremia and leukemia. The integrated provirus (i.e., Mov-3 locus) was molecularly cloned from Mov-3 liver DNA as a 16.8 kilobase long EcoRI fragment. Comparison of the cloned and genomic Mov-3 specific EcoRI fragment by restriction enzyme analysis showed no differences in the size of the fragments, indicating that no major sequence rearrangements occurred during cloning. The genomic and cloned Mov-3 DNAs were compared for methylation and infectivity. Analysis with Hha I showed that the genomic proviral and the flanking mouse sequences were methylated at cytosine residues, in contrast to the cloned Mov-3 locus. The cloned Mov-3 locus, however, was highly infectious in a transfection assay (1 x 10(-3) plaque-forming unit per viral genome) in contrast to the genomic Mov-3 DNA (less than 10(-7) per viral genome). Our results suggest that genes containing 5-methylcytosine are not expressed after transfection into susceptible cells and that removal of the methyl groups by molecular cloning in prokaryotes leads to expression generating infectious proviral DNA. If gene expression of transfected DNA is controlled by mechanisms that are relevant for gene expression in the animal, this suggests that DNA methylation may play a causative role in eukaryotic gene regulation.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benton W. D., Davis R. W. Screening lambdagt recombinant clones by hybridization to single plaques in situ. Science. 1977 Apr 8;196(4286):180–182. doi: 10.1126/science.322279. [DOI] [PubMed] [Google Scholar]
- Berns A. J., Lai M. H., Bosselman R. A., McKennett M. A., Bacheler L. T., Fan H., Maandag E. C., van der Putten H. V., Verma I. M. Molecular cloning of unintegrated and a portion of integrated moloney murine leukemia viral DNA in bacteriophage lambda. J Virol. 1980 Oct;36(1):254–263. doi: 10.1128/jvi.36.1.254-263.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird A. P., Southern E. M. Use of restriction enzymes to study eukaryotic DNA methylation: I. The methylation pattern in ribosomal DNA from Xenopus laevis. J Mol Biol. 1978 Jan 5;118(1):27–47. doi: 10.1016/0022-2836(78)90242-5. [DOI] [PubMed] [Google Scholar]
- Brown D. D., Dawid I. B. Specific gene amplification in oocytes. Oocyte nuclei contain extrachromosomal replicas of the genes for ribosomal RNA. Science. 1968 Apr 19;160(3825):272–280. doi: 10.1126/science.160.3825.272. [DOI] [PubMed] [Google Scholar]
- Brown D. D. Gene expression in eukaryotes. Science. 1981 Feb 13;211(4483):667–674. doi: 10.1126/science.6256857. [DOI] [PubMed] [Google Scholar]
- Cohen J. C. Methylation of milk-borne and genetically transmitted mouse mammary tumor virus proviral DNA. Cell. 1980 Mar;19(3):653–662. doi: 10.1016/s0092-8674(80)80042-0. [DOI] [PubMed] [Google Scholar]
- Daniels D. L., de Wet J. R., Blattner F. R. New map of bacteriophage lambda DNA. J Virol. 1980 Jan;33(1):390–400. doi: 10.1128/jvi.33.1.390-400.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desrosiers R. C., Mulder C., Fleckenstein B. Methylation of Herpesvirus saimiri DNA in lymphoid tumor cell lines. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3839–3843. doi: 10.1073/pnas.76.8.3839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gall J. G. Differential synthesis of the genes for ribosomal RNA during amphibian oögenesis. Proc Natl Acad Sci U S A. 1968 Jun;60(2):553–560. doi: 10.1073/pnas.60.2.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautier F., Bünemann H., Grotjahn L. Analysis of calf-thymus satellite DNA: evidence for specific methylation of cytosine in C-G sequences. Eur J Biochem. 1977 Oct 17;80(1):175–183. doi: 10.1111/j.1432-1033.1977.tb11869.x. [DOI] [PubMed] [Google Scholar]
- Gilboa E., Goff S., Shields A., Yoshimura F., Mitra S., Baltimore D. In vitro synthesis of a 9 kbp terminally redundant DNA carrying the infectivity of Moloney murine leukemia virus. Cell. 1979 Apr;16(4):863–874. doi: 10.1016/0092-8674(79)90101-6. [DOI] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Guntaka R. V., Rao P. Y., Mitsialis S. A., Katz R. Modification of avian sarcoma proviral DNA sequences in nonpermissive XC cells but not in permissive chicken cells. J Virol. 1980 May;34(2):569–572. doi: 10.1128/jvi.34.2.569-572.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohn B., Murray K. Packaging recombinant DNA molecules into bacteriophage particles in vitro. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3259–3263. doi: 10.1073/pnas.74.8.3259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hozumi N., Tonegawa S. Evidence for somatic rearrangement of immunoglobulin genes coding for variable and constant regions. Proc Natl Acad Sci U S A. 1976 Oct;73(10):3628–3632. doi: 10.1073/pnas.73.10.3628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes N. E., Kennedy N., Rahmsdorf U., Groner B. Hormone-responsive expression of an endogenous proviral gene of mouse mammary tumor virus after molecular cloning and gene transfer into cultured cells. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2038–2042. doi: 10.1073/pnas.78.4.2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaenisch R. Germ line integration and Mendelian transmission of the exogenous Moloney leukemia virus. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1260–1264. doi: 10.1073/pnas.73.4.1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaenisch R., Jähner D., Nobis P., Simon I., Löhler J., Harbers K., Grotkopp D. Chromosomal position and activation of retroviral genomes inserted into the germ line of mice. Cell. 1981 May;24(2):519–529. doi: 10.1016/0092-8674(81)90343-3. [DOI] [PubMed] [Google Scholar]
- Jähner D., Jaenisch R. Integration of Moloney leukaemia virus into the germ line of mice: correlation between site of integration and virus activation. Nature. 1980 Oct 2;287(5781):456–458. doi: 10.1038/287456a0. [DOI] [PubMed] [Google Scholar]
- Jähner D., Stuhlmann H., Jaenisch R. Conformation of free and of integrated Moloney leukemia virus proviral DNA in preleukemic and leukemic BALB/Mo mice. Virology. 1980 Feb;101(1):111–123. doi: 10.1016/0042-6822(80)90488-2. [DOI] [PubMed] [Google Scholar]
- Mandel J. L., Chambon P. DNA methylation: organ specific variations in the methylation pattern within and around ovalbumin and other chicken genes. Nucleic Acids Res. 1979 Dec 20;7(8):2081–2103. doi: 10.1093/nar/7.8.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGhee J. D., Ginder G. D. Specific DNA methylation sites in the vicinity of the chicken beta-globin genes. Nature. 1979 Aug 2;280(5721):419–420. doi: 10.1038/280419a0. [DOI] [PubMed] [Google Scholar]
- O'Rear J. J., Mizutani S., Hoffman G., Fiandt M., Temin H. M. Infectious and noninfectious recombinant clones of the provirus of SNV differ in cellular DNA and are apparently the same in viral DNA. Cell. 1980 Jun;20(2):423–430. doi: 10.1016/0092-8674(80)90628-5. [DOI] [PubMed] [Google Scholar]
- Rowe W. P., Pugh W. E., Hartley J. W. Plaque assay techniques for murine leukemia viruses. Virology. 1970 Dec;42(4):1136–1139. doi: 10.1016/0042-6822(70)90362-4. [DOI] [PubMed] [Google Scholar]
- Shen C. K., Maniatis T. Tissue-specific DNA methylation in a cluster of rabbit beta-like globin genes. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6634–6638. doi: 10.1073/pnas.77.11.6634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skalka A., DeBona P., Hishinuma F., McClements W. Avian endogenous proviral DNA: analysis of integrated ev 1 and a related gs- chf- provirus purified by molecular cloning. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 2):1097–1104. doi: 10.1101/sqb.1980.044.01.118. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Spradling A. C., Mahowald A. P. Amplification of genes for chorion proteins during oogenesis in Drosophila melanogaster. Proc Natl Acad Sci U S A. 1980 Feb;77(2):1096–1100. doi: 10.1073/pnas.77.2.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuhlmann H., Jähner D., Jaenisch R. Infectivity and methylation of retroviral genomes is correlated with expression in the animal. Cell. 1981 Oct;26(2 Pt 2):221–232. doi: 10.1016/0092-8674(81)90305-6. [DOI] [PubMed] [Google Scholar]
- Sutter D., Doerfler W. Methylation of integrated adenovirus type 12 DNA sequences in transformed cells is inversely correlated with viral gene expression. Proc Natl Acad Sci U S A. 1980 Jan;77(1):253–256. doi: 10.1073/pnas.77.1.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabak H. F., Flavell R. A. A method for the recovery of DNA from agarose gels. Nucleic Acids Res. 1978 Jul;5(7):2321–2332. doi: 10.1093/nar/5.7.2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanyushin B. F., Tkacheva S. G., Belozersky A. N. Rare bases in animal DNA. Nature. 1970 Mar 7;225(5236):948–949. doi: 10.1038/225948a0. [DOI] [PubMed] [Google Scholar]
- Vardimon L., Neumann R., Kuhlmann I., Sutter D., Doerfler W. DNA methylation and viral gene expression in adenovirus-transformed and -infected cells. Nucleic Acids Res. 1980 Jun 11;8(11):2461–2473. doi: 10.1093/nar/8.11.2461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigler M., Levy D., Perucho M. The somatic replication of DNA methylation. Cell. 1981 Apr;24(1):33–40. doi: 10.1016/0092-8674(81)90498-0. [DOI] [PubMed] [Google Scholar]
- van der Ploeg L. H., Flavell R. A. DNA methylation in the human gamma delta beta-globin locus in erythroid and nonerythroid tissues. Cell. 1980 Apr;19(4):947–958. doi: 10.1016/0092-8674(80)90086-0. [DOI] [PubMed] [Google Scholar]