Abstract
Purpose.
The role of VEGF-A in the normal ciliary body is largely unexplored. The ciliary body is similar in many respects to the choroid plexus of the brain, and we demonstrated previously the importance of VEGF-A in maintenance of choroid plexus vasculature and ependymal cells. Therefore, the role of VEGF-A in ciliary body homeostasis was explored.
Methods.
Swiss-Webster mice (VEGF-LacZ) were used to determine VEGF-A expression during ciliary body development and in the adult. VEGFR2 expression was determined in adult wild type C56BL/6J mice. Systemic VEGF-A neutralization in vivo was achieved with adenovirus-mediated overexpression of soluble VEGFR1 (sFlt1). Following VEGF-A neutralization, the ciliary epithelium was analyzed by light microscopy and transmission electron microscopy (TEM). The effect of VEGF-A blockade on ciliary body function also was assessed by measuring intraocular pressure.
Results.
VEGF-A expression was detected at embryonic day 18.5 (E18.5), the onset of ciliary process formation. In the adult ciliary body, VEGF-A was expressed by the pigmented epithelium, whereas VEGFR2 was localized primarily to the capillary endothelium and nonpigmented epithelium. Systemic VEGF-A neutralization led to a thinning of the nonpigmented epithelium, vacuolization of the pigmented epithelium, loss of capillary fenestrations, and thrombosis. These changes were associated with impaired ciliary body function, as evidenced by decreased intraocular pressure in sFlt1-overexpressing animals (15.31 ± 2.06 mm Hg) relative to controls (18.69 ± 1.49 mm Hg).
Conclusions.
VEGF-A has an important role in ciliary body homeostasis. Potential for undesired off-target effects should be considered with the chronic use of anti–VEGF-A therapies.
Systemic neutralization of VEGF-A in mouse resulted in a thinning of the nonpigmented ciliary epithelium, loss of capillary fenestrations, and thrombosis that was also correlated with impaired function, as evidenced by decreased intraocular pressure.
Introduction
The ciliary body, which is located in the anterior segment of the eye, mediates the critical functions of lens accommodation and aqueous humor secretion, and is comprised of smooth muscle fibers (ciliary muscles) and ciliary processes. The inner core of fenestrated capillaries of each ciliary process is covered by a double-layered epithelium, which consists of pigmented and nonpigmented layers. The epithelial layers are connected at their apical membranes through gap junctions. Tight junctions at the apical borders of the nonpigmented epithelium form the blood–aqueous barrier.1 The pigmented epithelium faces the ciliary stroma, whereas the nonpigmented epithelium is in closest proximity to the lens. Ciliary processes secrete aqueous humor through fenestrated capillaries; the balance between aqueous humor inflow and outflow determines intraocular pressure (IOP).
VEGF-A, a potent angiogenic factor, is a prime target for the treatment of ocular pathologies that involve neovascularization and vascular permeability, such as age-related macular degeneration (AMD), macular edema, and proliferative diabetic retinopathy. However, VEGF-A also has a role in the stability of quiescent vasculature in adult tissues, illustrated by the VEGF-A–dependent plasticity of fenestrated capillaries2 as well as the role of retinal-pigment-epithelium-(RPE)-derived soluble VEGF-A in maintenance of the choriocapillaris.3 There also is increasing evidence for a role for VEGF-A in nonvascular cells, as evidenced by significant retinal ganglion cell death associated with the inhibition VEGF-A function,4 and the impaired retinal function and increased apoptosis in photoreceptors and Müller cells observed following VEGF-A neutralization in the adult mouse.5 However, virtually nothing is known about the role of VEGF-A in the normal ciliary body. Given that intravitreal anti–VEGF-A agents display rapid penetration into the ciliary body,6 it is important to understand the role of VEGF-A in ciliary body homeostasis.
The ciliary body is similar to the choroid plexus of the brain in many respects. First, both are derived from the neuroepithelium and are comprised of a central core of fenestrated capillaries overlaid by epithelial cells. Second, members of the bone morphogenetic protein family have been shown to be involved in the development of both structures.7,8 Third, both are secretory structures responsible for the production of aqueous humor and cerebrospinal fluid, respectively. Finally, each constitutes a “blood barrier”; the choroid plexus forms the blood–cerebrospinal fluid barrier and the ciliary body is the site of the blood–aqueous humor barrier. We previously demonstrated the importance of VEGF-A in the maintenance of vascular and nonvascular cells of the choroid plexus. Our observation that systemic VEGF-A neutralization causes thrombosis and decreased choroid plexus vascular perfusion9 led us to investigate the effect of VEGF-A blockade on the ciliary body structure and function.
Materials and Methods
Animals
Timed-pregnant Swiss-Webster VEGF-LacZ mice10 were used to determine the time course of VEGF-A expression during ciliary body development. Pregnant mothers were euthanized by CO2 inhalation and embryos were collected at embryonic day 18.5 (E18.5). Adult mice 8 weeks old were euthanized by CO2 inhalation and the eyes collected. Embryos and adult eyes were fixed overnight at 4°C in 4% paraformaldehyde in PBS.
VEGFR2 expression in adult mice was determined in 8-week-old wild type C56BL/6J mice. After fixation and extensive washing with PBS, eyes and embryos were subjected to a sucrose gradient of 5% to 20% sucrose for 4 hours, followed by embedding in OCT compound (Sakura Finetechnical, Torrance, CA). Samples were cryosectioned (10 μm) and stored at −20°C until use.
Adenoviral-Mediated soluble VEGFR1 (sFlt1) Overexpression
To achieve systemic VEGF-A neutralization, 6- to 8-week-old CD-1 mice (Charles River Laboratories, Inc., Wilmington, MA) were injected via the tail vein with adenovirus expressing either soluble VEGFR1 (Ad-sFlt1) or empty vector (Ad-null) as follows: 2.5 × 109 viral particles (VP) for Ad-null and 2.5 × 109 VP for Ad-sFlt1 as described previously.9 Circulating sFlt1 plasma levels were determined by ELISA (R&D Systems, Minneapolis, MN) at day 12 post-injection and before animal sacrifice at days 14 or 28. Mice with plasma sFlt1 >200 ng/mL were included in the study. Ad-null–infected mice had no detectable sFlt1. Circulating levels are sustained at >200 ng/mL for at least 21 days post-infection.5,9 All animal experiments were conducted according to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research under protocols approved by the Schepens Eye Research Institute Institutional Animal Care and Use Committee.
β-Galactosidase Histochemistry
LacZ, as a reporter of VEGF-A expression, was visualized in cryosections of whole embryos (E18.5) and adult eyes of VEGF-LacZ mice. Tissue sections were stained for LacZ using the in situ β-galactosidase staining kit (Stratagene, La Jolla, CA), according to the manufacturer's instructions.
Ultrastructural Analysis
At day 14 following adenovirus injections, mice were deeply anesthetized by injection with ketamine (73 mg/kg) and xylazine (1.8 mg/kg). Animals were perfused slowly with 10 mL of sodium cacodylate buffer (0.2 M, pH 7.4), followed by 10 mL of half-strength Karnovsky's fixative (Electron Microscopy Sciences, Hatfield, PA) via a 21-gauge cannula inserted into the aorta via the left ventricle. Fluid was drained through an incision in the right atrium, and animal death was immediate upon perfusion. Eyes were enucleated, fixed in half-strength Karnovsky's fixative, and dissected to remove a quadrant containing the anterior segment for analysis of the ciliary body. A secondary fixation in 2% osmium tetroxide was performed, followed by dehydration and embedding. Ultrathin sections were treated with uranyl acetate and visualized by transmission electron microscopy (TEM) using a transmission electron microscope (Tecnai G2 Spirit BioTwin; FEI Company, Hillsboro, OR).
Immunohistochemistry
VEGFR2 was localized by fluorescent immunohistochemistry. Tissue sections were air-dried, then washed in PBS containing 0.2% Tween (PBST), blocked for one hour at room temperature with 5% goat serum, 1.5% BSA in PBST, and then incubated overnight at 4°C with a rabbit polyclonal antibody directed against murine VEGFR2 (5 μg/ml; a generous gift from Rolf Brekken, University of Texas Southwestern Medical Center, Dallas, TX) or in blocking buffer only as a negative control. Samples were washed in PBS, and a mixture containing goat anti-rabbit cy3 antibody (1:300; Jackson ImmunoResearch Laboratories, West Grove, PA) as well as 4′, 6-diamidino-2-phenylindole (DAPI; 1:100) was added for one hour at room temperature. Tissue sections were washed in PBS, slides were mounted, and images were taken with the Axioscope microscope (Axioscope Mot 2; Carl Zeiss Meditec, Inc., Dublin, CA).
Measurement of IOP
Mice were anesthetized by isoflurane inhalation (2%–4%) using a precision vaporizer, delivered in 100% O2. IOP measurements were taken on the right eye using a TonoLab Tonometer (Colonial Medical Supply, Franconia, NH), which allows noninvasive, accurate, and rapid measurement based on the rebound method.11 IOP measurements were taken immediately after the animal lost consciousness by placing the tip of the pressure sensor approximately 1/8 inch from the central cornea. The average IOP was displayed automatically after six measurements. The mean IOP of three measurements for each animal was then averaged for the entire group (n = 10). IOP measurements were taken at the same time of day at the indicated time points, and data are represented as the mean ± SD.
Statistical Analysis
Values are expressed as the mean ± SD, unless otherwise indicated. Statistical analysis was performed using an unpaired Student's t-test (***P < 0.001, **P < 0.01, *P < 0.05, not significant P > 0.05).
Results
VEGF-A Expression during Ciliary Body Development
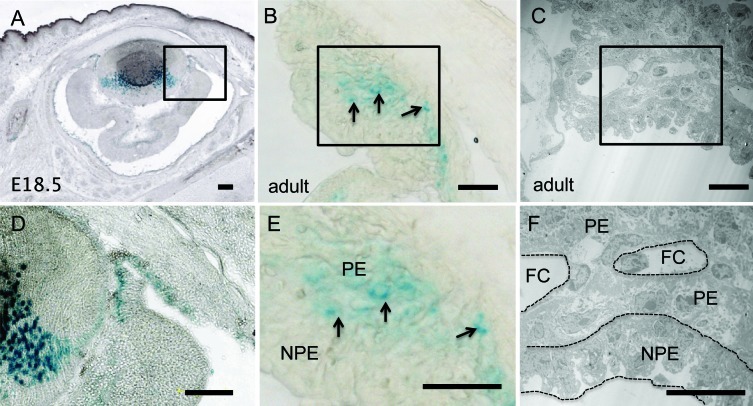
We first determined the pattern of VEGF-A expression during ciliary body development. Between E14.5 and E15.5 of mouse development, the rim of the optic cup begins to differentiate into the presumptive iris and ciliary body. The ciliary epithelium is derived from the two neuroepithelial layers, retina and RPE, at the optic cup rim; the nonpigmented epithelium is continuous with the neural retina, and the pigmented epithelium is juxtaposed between the RPE and outer iris.12 Before the initiation of ciliary infolding at E18.5, radial capillaries appear in the mesenchyme on the outer surface of the ciliary epithelium.12,13 In the VEGF-LacZ reporter mice,10 VEGF-A expression was detected in the ciliary body epithelium at E18.5, the time at which ciliary process infoldings first appear (Figs. 1A, 1D). This finding is consistent with our previous observations that VEGF-A is expressed at this developmental stage in the RPE and neural retina, the neuroepithelium from which the ciliary body arises.14
Figure 1. .
VEGF-A expression during ciliary body development and in adult. Cryosections (10 μm) of nonpigmented (A, D) E18.5 and (B, E) adult VEGF-LacZ/+ eyes were stained for β-galactosidase (blue). (A, D) VEGF-A was detected in the primitive ciliary body at E18.5, the stage at which ciliary process infolding begins. (B, E) In the adult, VEGF-A expression (black arrows) was localized primarily to the presumptive pigmented epithelium. (C, F) TEM micrograph of adult ciliary body indicates location of pigmented epithelium, nonpigmented epithelium, and capillaries. Scale bar: 160 μm (A, D) and 20 μm (B, C, E, F). NPE, nonpigmented epithelium; PE, pigmented epithelium; FC, fenestrated capillary.
Localization of VEGF-A and VEGFR2 in Adult Ciliary Epithelium
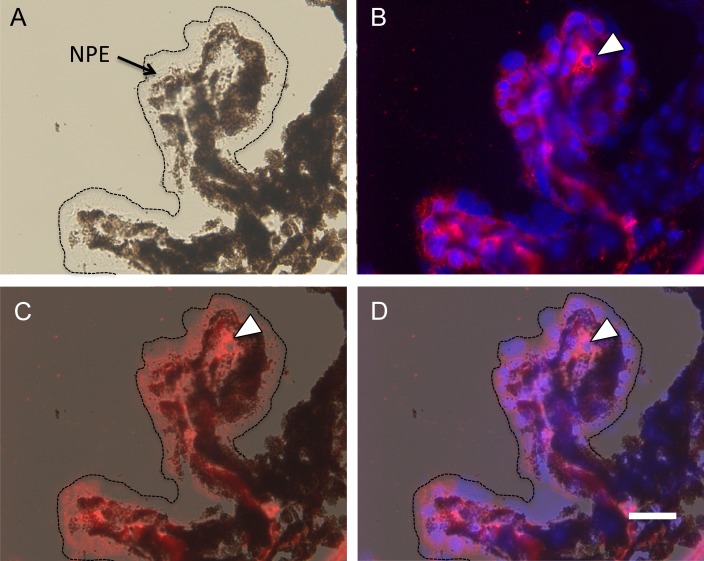
The next step was to determine the localization of VEGF-A and VEGFR2 in the adult ciliary body. Bright field microscopy revealed the extensive pigmentation of the pigmented epithelium, and its absence in the nonpigmented epithelium (Fig. 2A). Immunohistochemical localization revealed that VEGFR2 was expressed primarily by the nonpigmented epithelium and the capillary endothelial cells (Fig. 2B); this was confirmed by merging the fluorescent and bright field images (Figs. 2C, 2D). VEGF-A expression was examined in the VEGF-LacZ mouse, which is in a genetic background that is nonpigmented. Based on ultrastructural localization of pigmented and nonpigmented epithelial layers (Figs. 1C, 1F), VEGF-A expression, detected by β-galactosidase activity, was limited to the pigmented epithelium and virtually was absent from the nonpigmented epithelium (Figs. 1B, 1E). The finding that VEGF-A is expressed by the epithelium in closest proximity to the capillary core is consistent with our observation of VEGF-A expression by the RPE14 and its function in the maintenance of the capillary fenestrations.2,9
Figure 2. .
VEGFR2 expression by the nonpigmented ciliary epithelium. VEGFR2 expression in the adult ciliary body was localized by immunofluorescent microscopy using a rabbit polyclonal anti-mouse VEGFR2 antibody. (A) Bright field microscopy demonstrates the extensive pigmentation of the pigmented epithelial layer, and the location of the nonpigmented layer (dotted line and arrow). (B) DAPI staining of nuclei along with immunofluorescent labeling reveals VEGFR2 primarily in the nonpigmented epithelium and capillaries (white arrowhead). (C, D) Merging of the bright field and fluorescent images confirms localization of VEGFR2 expression in nonpigmented epithelial cells. Scale bar: 20 μm.
Effect of VEGF-A Neutralization on Ciliary Body Structure
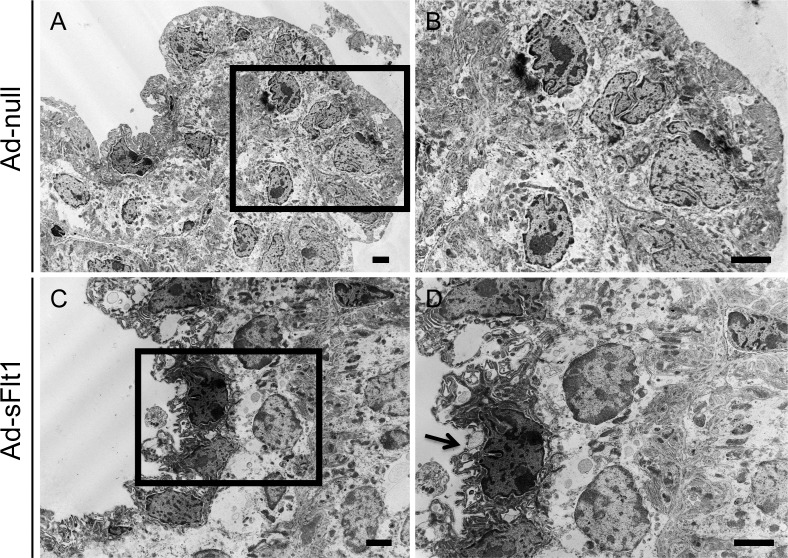
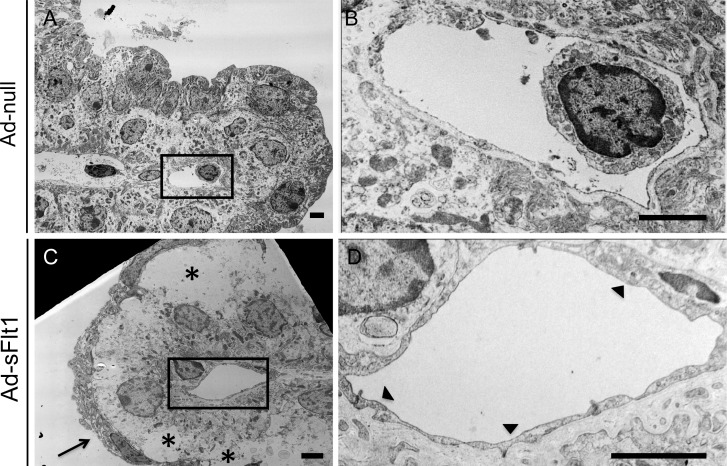
To determine if VEGF-A has a role in the adult ciliary body, VEGF-A was neutralized systemically by adenoviral expression of sFlt1 (Ad-sFlt1). Histologic analysis of mice expressing Ad-sFlt1 at 28 days post-infection revealed microthrombi in ciliary capillaries. There was also an intermittent breakdown of nonpigmented epithelium, as indicated by the marked thinning (compare Figs. 3C, 3D to Figs. 3A, 3B). Ultrastructural examination of the ciliary body at day 14 post-infection confirmed the degeneration and revealed shrunken cytoplasm of nonpigmented epithelial cells (compare Figs. 4C, 4D to Figs. 4A, 4B). The changes in the pigmented epithelial layer of the Ad-sFlt1 expressing mice were not uniform, with some normal regions (data not shown) and other highly vacuolated areas in comparison to that of the Ad-null-infected control group (compare Figs. 5C and 5A). The ciliary capillary endothelium was also affected by VEGF-A neutralization; the capillary endothelial wall of Ad-sFlt1–expressing mice displayed a complete loss of fenestrations and appeared significantly thickened (compare Figs. 5B and 5D). These observations indicated that VEGF-A neutralization leads to dramatic loss of ciliary body integrity.
Figure 3. .
VEGF-A neutralization leads to ciliary capillary thrombosis. Richardson stain of epoxy embedded ultrathin sections of eyes from (A, B) Ad-null and (C, D) Ad-sFlt1–expressing mice at day 28 post-infection revealed numerous microthrombi (white arrowheads) in the capillaries of Ad-sFlt1–expressing mice, but not in the capillaries of Ad-null animals. Regions of the nonpigmented epithelium appeared markedly thinner (black arrow), indicating degeneration. Scale bar: 20 μm.
Figure 4. .
Alterations in ciliary body ultrastructure following VEGF-A neutralization. Ultrastructural analysis of the ciliary body 14 days post-infection illustrates the normal ultrastructure of (A, B) Ad-null animals in comparison to the degeneration of the nonpigmented epithelial layer observed in (C, D) Ad-sFlt1–expressing mice. Arrow indicates the shrunken and nearly nonexistent cytoplasm of a nonpigmented epithelial cell. Scale bar: 2 μm.
Figure 5. .
VEGF-A neutralization leads to loss of capillary fenestrations. TEM micrographs of the ciliary body from (A, B) Ad-null and (C, D) Ad-sFlt1–expressing mice at day 14 post-infection revealed a thickening of the capillary endothelial wall and associated loss of fenestrations (black arrowheads) in (D) Ad-sFlt1 eyes, whereas (B) Ad-null capillaries retained their normal appearance. The normal organization of the ciliary epithelia was observed in (A) Ad-null animals whereas (C) Ad-sFlt1–expressing animals displayed degenerated nonpigmented epithelium (black arrow) and vacuolization of the pigmented epithelium (black asterisks). Scale bar: 2 μm.
Effect of VEGF-A Blockade on IOP
In light of the changes in the ciliary body structure in mice with systemic VEGF-A neutralization, the next step was to assess effects on ciliary body function. One important role of the ciliary body is the production of aqueous humor, which provides nutrients to the anterior segment and contributes to the maintenance of IOP. IOP is determined by the balance between aqueous humor production and drainage through the trabecular meshwork, juxtacanalicular tissue, and Schlemm's canal.15,16 Aqueous humor secretion across the ciliary epithelium is mediated by the transfer of solute, primarily NaCl, from the ciliary muscles to the posterior chamber, coupled with passive diffusion of water.17
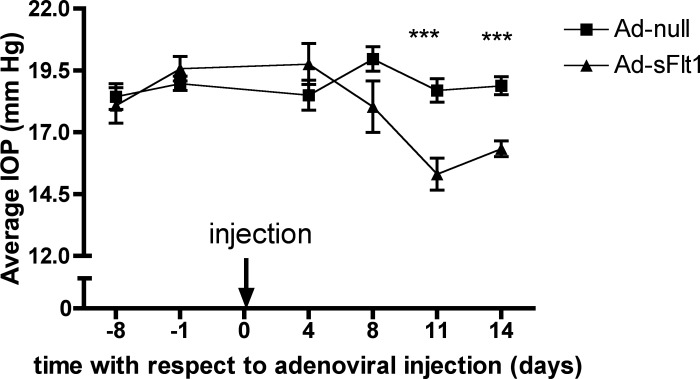
IOP of Ad-sFlt1 and Ad-null animals was measured over the course of 14 days post-infection. Measurements were taken in the same eye and at approximately the same time of day to avoid any variation due to circadian rhythm.18 There was a statistically significant reduction in IOP in the Ad-sFlt1 animals as early as day 11 post-infection. The mean IOP of the Ad-sFlt1–expressing mice was 15.31 ± 2.06 mm Hg versus 18.69 ± 1.49 mm Hg for the Ad-null–infected mice (Fig. 6), an observation that is consistent with impaired aqueous humor production.
Figure 6. .
VEGF-A neutralization leads to decreased IOP. The IOP of CD-1 mice was measured with a TonoLab tonometer before and at various time points following injection of the Ad-null or Ad-sFlt1. By day 11, there was a statistically significant reduction in IOP in the Ad-sFlt1–expressing animals. The values are expressed as the mean IOP ± SD of the animals in each group (n = 10). ***P < 0.001
Discussion
The expression of VEGF-A and its receptors, VEGFR1 and VEGFR2, is regulated spatially and temporally during eye development. We demonstrated that VEGF-A is expressed in the developing ciliary body at E18.5, the time point at which the ciliary infoldings first appear. We reported previously that VEGF-A expression is detected first in the developing optic vesicle at E9.5, before the formation of specialized eye structures.19 One proposed function of this early VEGF-A expression is to support choroidal development, which commences around optic cup formation at E10.5. VEGF-A is observed in the posterior fibers of the lens at E12.5,14,20 and increases in lens fiber and epithelial cells with age, reaching its maximum expression in the adult.20 VEGF-A secretion by the lens is believed to be involved in the regulation of vasculogenesis, likely by stimulating the proliferation and migration of angioblasts, which are at the origin of the future tunica vasculosa lentis.21 VEGF-A functions not only in lens differentiation,22 but also in the promotion of lens growth during fetal maturation.23 In the posterior segment, VEGF-A is detected in the primitive RPE and inner nuclear layer (INL) of the retina at E10.5; by E13.5, its expression in the outer retina is restricted to the RPE where it persists throughout adulthood.14 Postnatal expression of VEGF and its receptors not only supports retinal vascularization, but also is critical to proper neural retina development.24
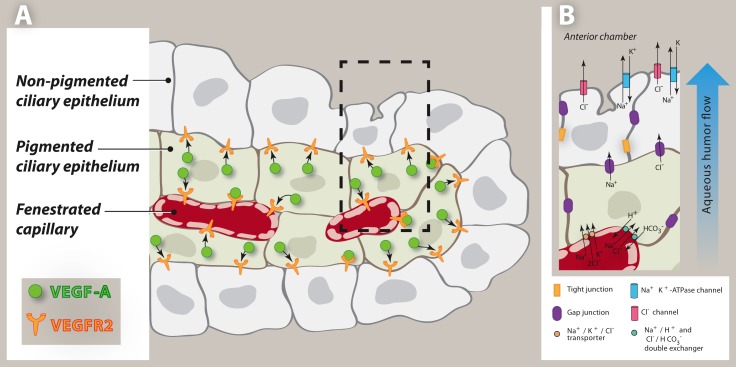
In the adult ciliary body, VEGFR2 is expressed primarily by the nonpigmented epithelium and by capillary endothelium, whereas VEGF-A is expressed by the pigmented epithelium, the epithelium that lies in closest proximity to the capillaries. Our finding contradicts a previous report that VEGF-A was detected in both epithelial layers25; however, as VEGF-A is a secreted protein, immunolocalization is not a reliable method to determine the site of its production. Since the nonpigmented epithelium expresses VEGFR2, it is most likely that the VEGF-A detected in the nonpigmented epithelium of the latter study was produced in the pigmented epithelium and was taken up by the nonpigmented cells (Fig. 7A).
Figure 7. .
Schematic of VEGF-A and VEGFR2 localization, and aqueous humor secretion in ciliary epithelia. (A) A representation of a ciliary process depicting ciliary epithelial layers and fenestrated capillaries. VEGFR2 is expressed primarily by the nonpigmented epithelium and by capillary endothelium, whereas VEGF-A is expressed by the pigmented epithelium, the epithelium that lies in close proximity to the capillaries. Pigmented ciliary epithelium–derived VEGF-A is proposed to support the nonpigmented ciliary epithelium as well as maintain capillary fenestrations. (B) Aqueous humor secretion is driven by the transepithelial transport of ions, primarily Na+, Cl−, and to a lesser degree HCO3−, across the ciliary epithelium, generating an osmotic gradient for water movement. Ions are taken up by the pigmented epithelial cells from the stroma by Na+, 2Cl− , K+ symports, and parallel Na+/ H+ and Cl− / HCO3− antiports. The ions then diffuse from the pigmented epithelium to the nonpigmented epithelium through gap junctions. Lastly, Na+ is released from the nonpigmented epithelium into the posterior chamber through Na+ K+-activated ATPase and Cl− is released through Cl− channels.
The expression of VEGF-A by the pigmented epithelium and VEGFR2 by the nonpigmented epithelium points to a role for VEGF-A in maintaining the integrity of the nonpigmented cells. Furthermore, the expression of VEGF-A by the pigmented epithelium and VEGFR2 by the capillary endothelium also suggests that it functions in the maintenance of capillary fenestrations. The role of VEGF-A in capillary survival, and in the formation and maintenance of fenestrations has been well documented.3,9,26 VEGF-A has been shown to induce the formation of fenestrations in vitro.26,27 Fenestrated endothelium is a characteristic of tissues that perform filtration, such as the kidney glomerulus, or secretion as in the choroid plexus of the brain and the ciliary body of the eye. VEGF-A–dependence of fenestrated capillaries was demonstrated by neutralizing VEGF-A with small molecule VEGF receptor tyrosine kinase inhibitors as well as soluble VEGF-A receptors.2,9 VEGF-A neutralization for one to three weeks led to vessel regression in the pancreas and thyroid, for instance, and capillaries recovered once VEGF-A neutralization was terminated.2
Maintenance of capillary fenestrations by pigmented ciliary epithelium–derived VEGF-A is critical to normal eye physiology. Aqueous humor secretion through the capillary fenestrations provides the oxygen and nutrients to the avascular anterior chamber, and IOP is determined by the balance between aqueous humor inflow and outflow.17,28,29 Aqueous humor secretion is driven primarily by the transepithelial transport of ions, primarily Na+, Cl−, and to a lesser degree HCO3−, across the ciliary epithelium, which generates an osmotic gradient for water movement.30 First, ion uptake by pigmented epithelial cells is facilitated by the fenestrations of the ciliary capillaries. Next, the ions diffuse from the pigmented epithelium to the nonpigmented epithelium through the gap junctions. Lastly, ions are released from the nonpigmented epithelium into the posterior chamber through Na+ K+-activated ATPase and Cl−channels.31,32 Carbonic anhydrase, either directly or indirectly, mediates the transport of HCO3− across the ciliary epithelium33 (Fig. 7B).
The defects in regions of the pigmented epithelial layer following VEGF-A neutralization could be secondary to damage of the neighboring nonpigmented cells and/or to the reduced function of the ciliary body vasculature. These notions are corroborated by the ultrastructural analysis, which revealed ciliary body epithelium degeneration, loss of capillary fenestrations and the formation of microthrombi following VEGF-A blockade. The loss of fenestrations from the ciliary body capillaries in Ad-sFlt1 mice was associated with an apparent impairment of ciliary body function, as evidenced by reduced IOP. Given that the secretion of aqueous humor is facilitated by capillary fenestrations, a loss of fenestrations might be expected to compromise aqueous humor secretion, thereby lowering IOP. This observation is consistent with a previous study in which selective destruction of the pigmented ciliary epithelial cells in a primate model led to a loss of capillary fenestrations and dramatically lowered IOP.34 We demonstrated previously the reformation of fenestrations in the choriocapillaris, a capillary bed located in the back of the eye, in spite of continued VEGF-A neutralization (either local or systemic).5 Subsequent analysis revealed that there was an apparent compensatory upregulation of VEGF-A by RPE cells.19 However, unlike the RPE, the ciliary body epithelium either lacks the compensatory mechanism exhibited by the RPE or the recovery takes longer than the 28-day period we assessed.
A study in nonhuman primates showed that bevacizumab, a VEGF-A neutralizing agent, penetrates quickly into the ciliary body following intravitreal injection, with the most intense staining detected at day four and remaining prominent through day 14.6 Nevertheless, reduced IOP is not a common complication of anti–VEGF-A treatment, most likely due to the fact that, unlike our experimental model of adenoviral expression of sFlt1, VEGF-A neutralization in the clinical setting neither is continuous nor complete. Rather, anti-VEGF-A delivered intravitreally has been shown to be cleared rapidly, as the half-life of the clinical dose of bevicizumab (1.25 mg) in the vitreous is 6.7 days.35
The name “vascular endothelial growth factor” now is rather misleading because of the increasing list of “nonvascular” functions of VEGF-A. VEGF-A and its receptors have been immunolocalized to neurons and astrocytes, and VEGF-A has been reported to induce neurite outgrowth as well as provide neuroprotection.20–22 Other evidence of nonvascular functions of VEGF-A include the observation of increased apoptosis in photoreceptors and Müller cells, and associated impaired retinal function following VEGF-A neutralization in adult mice,5 as well as significant retinal ganglion cell death upon VEGF-A neutralization in a model of ischemia preconditioning.4 In addition, we demonstrated that VEGF-A has an autocrine role in RPE survival in vitro,19 and others have reported that VEGF-A signaling enhances RPE cell survival under oxidative stress.36 For these reasons, however, current efforts to develop methods to achieve more sustained VEGF-A neutralization, either via slow release mechanisms or through the use of reagents with longer half-lives and/or higher VEGF-A affinities, may prove to be problematic.
Acknowledgments
Pat Pearson provided TEM sample preparation/images and Peter Mallen produced the ciliary process schematic.
Footnotes
Supported by Grants RO1EY015435 (PAD) and P30EY003790 (Core Grant for Vision Research, J. Zieske).
Disclosure: K.M. Ford, None; M. Saint-Geniez, None; T.E. Walshe, None; P.A. D'Amore, None
References
- 1.Raviola G, Raviola E. Intercellular junctions in the ciliary epithelium. Invest Ophthalmol Vis Sci. 1978;17:958–981 [PubMed] [Google Scholar]
- 2.Kamba T, Tam BY, Hashizume H, et al. VEGF-dependent plasticity of fenestrated capillaries in the normal adult microvasculature. Am J Physiol Heart Circ Physiol. 2006;290:H560–H576 [DOI] [PubMed] [Google Scholar]
- 3.Saint-Geniez M, Kurihara T, Sekiyama E, Maldonado AE, D'Amore PA. An essential role for RPE-derived soluble VEGF in the maintenance of the choriocapillaris. Proc Natl Acad Sci U S A. 2009;106:18751–18756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nishijima K, Ng YS, Zhong L, et al. VEGF-A is a survival factor for retinal neurons and a critical neuroprotectant during the adaptive response to ischemic injury. Am J Path. 2007;171:53–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saint-Geniez M, Maharaj AS, Walshe TE, et al. Endogenous VEGF is required for visual function: evidence for a survival role on Müller cells and photoreceptors. PLoS One. 2008;3:e3554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peters S, Heiduschka P, Julien S, Bartz-Schmidt KU, Schraermeyer U. Immunohistochemical localisation of intravitreally injected bevacizumab in the anterior chamber angle, iris and ciliary body of the primate eye. Br J Ophthalmol. 2008;92:541–544 [DOI] [PubMed] [Google Scholar]
- 7.Zhao S, Chen Q, Hung FC, Overbeek PA. BMP signaling is required for development of the ciliary body. Development. 2002;129:4435–4442 [DOI] [PubMed] [Google Scholar]
- 8.Furuta Y, Piston DW, Hogan BL. Bone morphogenetic proteins (BMPs) as regulators of dorsal forebrain development. Development. 1997;124:2203–2212 [DOI] [PubMed] [Google Scholar]
- 9.Maharaj AS, Walshe TE, Saint-Geniez M, et al. VEGF and TGF-beta are required for the maintenance of the choroid plexus and ependyma. J Exp Med. 2008;205:491–501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miquerol L, Gertsenstein M, Harpal K, Rossant J, Nagy A. Multiple developmental roles of VEGF suggested by a LacZ-tagged allele. Dev Biol. 1999;212:307–322 [DOI] [PubMed] [Google Scholar]
- 11.Cervino A. Rebound tonometry: new opportunities and limitations of non-invasive determination of intraocular pressure. Br J Ophthalmol. 2006;90:1444–1446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beebe DC. Development of the ciliary body: a brief review. Trans Ophthalmol Soc U K. 1986;105:123–130 [PubMed] [Google Scholar]
- 13.Napier HR, Kidson SH. Proliferation and cell shape changes during ciliary body morphogenesis in the mouse. Dev Dyn. 2005;233:213–223 [DOI] [PubMed] [Google Scholar]
- 14.Saint-Geniez M, Maldonado AE, D'Amore PA. VEGF expression and receptor activation in the choroid during development and in the adult. Invest Ophthalmol Vis Sci. 2006;47:3135–3142 [DOI] [PubMed] [Google Scholar]
- 15.Bill A, Phillips CI. Uveoscleral drainage of aqueous humour in human eyes. Exp Eye Res. 1971;12:275–281 [DOI] [PubMed] [Google Scholar]
- 16.Johnson DH, Matsumoto Y. Schlemm's canal becomes smaller after successful filtration surgery. Arch Ophthalmol. 2000;118:1251–1256 [DOI] [PubMed] [Google Scholar]
- 17.Civan MM, Macknight AD. The ins and outs of aqueous humour secretion. Exp Eye Res. 2004;78:625–631 [DOI] [PubMed] [Google Scholar]
- 18.Krishna R, Mermoud A, Baerveldt G, Minckler DS. Circadian rhythm of intraocular pressure: a rat model. Ophthalmic Res. 1995;27:163–167 [DOI] [PubMed] [Google Scholar]
- 19.Ford KM, Saint-Geniez M, Walshe T, Zahr A, D'Amore PA. Expression and role of VEGF in the adult retinal pigment epithelium. Invest Ophthalmol Vis Sci. 2011;52:9478–9487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shui YB, Wang X, Hu JS, et al. Vascular endothelial growth factor expression and signaling in the lens. Invest Ophthalmol Vis Sci. 2003;44:3911–3919 [DOI] [PubMed] [Google Scholar]
- 21.Gogat K, Le Gat L, Van Den Berghe L, et al. VEGF and KDR gene expression during human embryonic and fetal eye development. Invest Ophthalmol Vis Sci. 2004;45:7–14 [DOI] [PubMed] [Google Scholar]
- 22.Saint-Geniez M, Kurihara T, D'Amore PA. Role of cell and matrix-bound VEGF isoforms in lens development. Invest Ophthalmol Vis Sci. 2009;50:311–321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Garcia CM, Shui YB, Kamath M, et al. The function of VEGF-A in lens development: formation of the hyaloid capillary network and protection against transient nuclear cataracts. Exp Eye Res. 2009;88:270–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Robinson GS, Ju M, Shih SC, et al. Nonvascular role for vascular endothelial growth factor (VEGF): VEGFR-1 and VEGFR-2 activity is critical for neural retinal development. Faseb J. 2001;15:1215–1217 [DOI] [PubMed] [Google Scholar]
- 25.Ueda H, Kashiwagi K, Iizuka Y. Vascular endothelial growth factor and its receptors expression in the rat eye. Acta Histochem Cytochem. 2001;34:329–335 [Google Scholar]
- 26.Roberts WG, Palade GE. Increased microvascular permeability and endothelial fenestration induced by vascular endothelial growth factor. J Cell Sci. 1995;108:2369–2379 [DOI] [PubMed] [Google Scholar]
- 27.Esser S, Wolburg K, Wolburg H, Breier G, Kurzchalia T, Risau W. Vascular endothelial growth factor induces endothelial fenestrations in vitro. J Cell Biol. 1998;140:947–959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Do CW, Civan MM. Species variation in biology and physiology of the ciliary epithelium: similarities and differences. Exp Eye Res. 2009;88:631–640 [DOI] [PubMed] [Google Scholar]
- 29.Krupin T, Rosenberg LF, Sandridge AL, Bock CJ Jr, Berman A, Ruderman JM. Effects of topical k-strophanthin on aqueous humor and corneal dynamics. J Glaucoma. 1995;4:327–333 [PubMed] [Google Scholar]
- 30.Jacob TJ, Civan MM. Role of ion channels in aqueous humor formation. Am J Physiol. 1996;271:C703–C720 [DOI] [PubMed] [Google Scholar]
- 31.Do CW, Civan MM. Basis of chloride transport in ciliary epithelium. J Membr Biol. 2004;200:1–13 [DOI] [PubMed] [Google Scholar]
- 32.Do CW, Peterson-Yantorno K, Mitchell CH, Civan MM. cAMP-activated maxi-Cl(-) channels in native bovine pigmented ciliary epithelial cells. Am J Physiol Cell Physiol. 2004;287:C1003–C1011 [DOI] [PubMed] [Google Scholar]
- 33.Friedenwald JS, Herrmann H, Moses R. The distribution of certain oxidative enzymes in the ciliary body. Trans Am Ophthalmol Soc. 1943;41:141–156 [PMC free article] [PubMed] [Google Scholar]
- 34.Okisaka S, Kuwabara T. Selective destruction of the pigmented epithelium in the ciliary body of the eye. Science. 1974;184:1298–1299 [DOI] [PubMed] [Google Scholar]
- 35.Zhu Q, Ziemssen F, Henke-Fahle S, et al. Vitreous levels of bevacizumab and vascular endothelial growth factor-A in patients with choroidal neovascularization. Ophthalmology. 2008;115:1750–1755 [DOI] [PubMed] [Google Scholar]
- 36.Byeon SH, Lee SC, Choi SH, et al. Vascular endothelial growth factor as an autocrine survival factor for retinal pigment epithelial cells under oxidative stress via the VEGF-R2/PI3K/Akt. Invest Ophthalmol Vis Sci. 2010;51:1190–1197 [DOI] [PubMed] [Google Scholar]