Abstract
Purpose.
To compare ocular axial elongation in infants after unilateral cataract surgery corrected with a contact lens (CL) or primary intraocular lens (IOL) implantation.
Methods.
Baseline axial length (AL) was measured at the time of cataract surgery (1–6 months) and at age 1 year. AL at baseline and age 1 year and the change in length/mo were analyzed in relation to treatment modality, cataractous versus fellow eye, and age at surgery using linear mixed models.
Results.
Mean baseline AL did not differ between the CL and IOL groups for either cataractous or fellow eyes. Eyes with cataracts were shorter than fellow eyes by an average of 0.6 mm (95% confidence interval [CI], 0.4–0.8 mm; P < 0.0001). For the operated eyes, the mean change in AL/mo was smaller in the CL group (0.17 mm/mo) than in the IOL group (0.24 mm/mo) (P = 0.0006) and was independent of age at surgery (P = 0.19). In contrast, the change in AL/mo for fellow eyes decreased with older age at surgery (P < 0.0001). At age 1 year, operated eyes treated with a CL were 0.6 mm shorter on average than operated eyes treated with an IOL (P = 0.009).
Conclusions.
At baseline, eyes with cataracts were shorter than fellow eyes. The change in AL/mo was smaller in operated eyes treated with a CL than in operated eyes treated with an IOL, but was not significantly related to age at surgery. (ClinicalTrials.gov number, NCT00212134.)
We compared ocular axial elongation in infants after unilateral cataract surgery corrected with a contact lens or intraocular lens implantation. Aphakic eyes were on average 0.6 mm shorter than pseudophakic eyes at age 12 months.
Introduction
One of the most challenging facets of implanting intraocular lenses (IOLs) during infancy is predicting the growth of the eye so that the desired refractive error can be achieved when the eye is fully grown. The infant eye elongates rapidly during the first year of life.1,2 Both visual deprivation and optical defocus can alter ocular growth.3,4 In addition, glaucoma during early childhood can cause excessive axial elongation.5 Postoperative refractive errors are usually corrected by spectacles or contact lenses. However, if the eye grows more than expected after IOL implantation, a large myopic refractive error may develop that in extreme cases may necessitate an IOL exchange.
The effect of removing the crystalline lens on axial elongation during early childhood is poorly understood.6–12 In a newborn rabbit model, a unilateral lensectomy has been shown to significantly decrease axial elongation.13 A similar effect has also been observed using a nonhuman primate model.14,15 The effect is age dependent, with the greatest effect occurring in neonates.16 While some series have reported a reduction of axial elongation following cataract surgery and IOL implantation in children, others have reported increased axial elongation.6,17,18 Furthermore, unilateral cataract surgery during infancy has been reported to be associated with more axial elongation in the operated eye than bilateral cataract surgery.19,20 These effects have important clinical implications in determining the optimal IOL power to implant in a child to achieve the desired refractive correction later in life.
The Infant Aphakia Treatment Study (IATS) is a randomized clinical trial comparing the effect of primary IOL implantation versus aphakia corrected with a contact lens (CL) in infants 1 to 6 months of age following unilateral cataract surgery. We previously reported that visual acuity was not significantly different between the operated eyes of the two treatment groups at age 12 months.21,22 We now report the longitudinal changes in axial length (AL) from the time of cataract surgery until an examination under anesthesia (EUA) was performed between the ages of 11 and 12 months.
Methods
The study design, surgical technique, follow-up schedules, patching and optical correction regimens, evaluation methods, definitions used for glaucoma and glaucoma suspect, and patient characteristics at baseline have been reported in detail previously and are only summarized in this report.21,23 The study followed the tenets of the Declaration of Helsinki, was approved by the institutional review boards of the participating institutions, and was in compliance with the Health Insurance Portability and Accountability Act. The off-label research use of the AcrySof SN60AT and MA60AC IOLs (Alcon Laboratories, Fort Worth, TX) was covered by US Food and Drug Administration investigational device exemption No. G020021.
Study Design
The main inclusion criteria were a visually significant congenital cataract (≥3 mm central opacity) in one eye, a normal fellow eye, and an age of 28 days to <210 days at the time of cataract surgery. The main exclusion criteria were an acquired cataract, persistent fetal vasculature (PFV) causing stretching of the ciliary processes, a corneal diameter <9 mm, a medical condition that might interfere with recognition visual acuity testing at age 4.5 years, an intraocular pressure (IOP) ≥25 mm Hg, and prematurity (<36 gestational weeks). Patients were randomized either to have an IOL placed at the time of the initial surgery or to be left aphakic and optically corrected with a CL.
Axial Length Measurements
Baseline AL measurements on both eyes of all patients were obtained during an EUA performed prior to cataract surgery. A second EUA was performed 2 to 4 weeks prior to the grating acuity assessment at age 1 year. Thirty minutes prior to the EUA, both eyes were dilated with 1% cyclopentolate and 2.5% Neo-Synephrine. A-scan biometry was performed using immersion or applanation. Most A-scans were performed with an Eye Cubed (Innovative Imaging, Sacramento, CA; now owned by Ellex, Eden Prairie, MN) ultrasound unit, which has a clinical accuracy of ±0.1 mm. The A-scan with the best waveforms (e.g., the highest five spikes with a perpendicular retinal spike) was recorded on a case report form, and the tracing was printed. A-scan tracings were then submitted to the Data Coordinating Center.
A-scan tracings were graded by a masked certified echographer and graded as being valid, invalid, or unreadable. A-scans were judged as valid if the gates, mode, and eye type (phakic, aphakic, or pseudophakic acrylic) were set correctly; if corneal, lens (baseline and pseudophakic eyes only), and retinal spikes were visible and of sufficient gain to be measurable; and if the leading edge of the retinal spike was perpendicular to the baseline. If an error was detected that could cause the AL measurement to be inaccurate by >0.2 mm (e.g., inappropriate mode, improper gate or caliper placement, or poor spike quality), then the scan was classified as invalid. A-scans were judged to be unreadable if the quality of the printout was degraded so that the scan could not be adequately assessed. Measurements from invalid, unreadable, and missing A-scans were not included in the analyses. A-scans from patients with glaucoma or glaucoma suspect were also excluded from the analysis because of the excessive axial elongation that occurs in infantile eyes with glaucoma.5,24
Surgical Technique
Infants randomized to the CL group underwent a lensectomy and anterior vitrectomy. Infants randomized to the IOL group had their lens aspirated followed by the implantation of an AcrySof SN60AT (Alcon Laboratories) IOL into the capsular bag. In the event that both haptics could not be implanted into the capsular bag, an AcrySof MA60AC IOL was implanted into the ciliary sulcus. The IOL power was calculated based on the Holladay 1 formula targeting an 8-diopter (D) undercorrection for infants 4 to 6 weeks of age and a 6 D undercorrection for infants older than 6 weeks. Following IOL placement, a posterior capsulectomy and an anterior vitrectomy were performed through the pars plana/plicata.
Optical Correction
Within a week after cataract surgery, patients randomized to the CL group were fit with a Silsoft (Bausch and Lomb, Rochester, NY) or a rigid gas-permeable contact lens with a 2.0 D overcorrection to provide a near point focus. For patients randomized to the IOL group, spectacles were prescribed prior to the 1-month postoperative visit with an overcorrection of 2.0 D. The prescribed optical correction was to be worn at all times while the patient was awake. Spectacle, CL, and patching adherence was monitored by a 7-day diary completed 2 months after surgery and 48-hour recall interviews conducted 3 and 6 months after surgery.25
Statistical Considerations
Mean age at baseline was compared between treatment groups using an independent groups t-test. To assess whether there was a difference in age according to whether or not patients were included in the analyses, a one-way analysis of variance was used to compare mean age among three groups of patients: patients included in the analysis; patients excluded because of glaucoma; and patients excluded because of missing, unreadable, or invalid A-scans.
Mean AL at baseline was compared between treatment groups and eyes (cataractous versus fellow) accounting for age at surgery with a linear mixed model assuming compound symmetry that included treatment as a between-subjects factor, eye as a within-subject factor, age as a covariate, and all interactions. Adjusted (least squares) means and 95% confidence intervals for baseline AL were calculated from the model. Baseline AL in the cataractous eye was related to age with linear regression.
Since there were only two time points at which AL was measured, and since the age at which the first measurement was made varied among the patients while the second measurement was done at relatively similar ages, the longitudinal change in AL was quantified as the change in AL/mo. The calculation was made by subtracting the first measurement of AL from the second measurement and dividing by the time difference in months between the measurements. If either of the two measurements was missing for an eye, that eye was not included in the analysis. The change in AL (mm/mo) was related to treatment (IOL versus CL), eye (cataractous versus fellow eye), and age at surgery using a linear mixed model assuming compound symmetry with treatment as a between-subjects factor, eye as a within-subject factor, age at surgery as a covariate, and all interactions. Adjusted (least squares) means and 95% confidence intervals for the change in AL/mo were calculated from the model according to treatment, eye, and selected ages. Separate models were also run for the cataractous and fellow eyes.
Mean AL at 1 year of age was evaluated using the same linear mixed model as for AL at baseline. For all analyses, a P value < 0.05 was deemed statistically significant. All statistical analyses were done with SAS 9.2 (SAS Institute Inc., Cary, NC).
Results
Patients
Of the 114 patients enrolled (57 per treatment), 10 patients were subsequently diagnosed with glaucoma (CL = 3, IOL = 7) in their operated eye, and 4 were considered to be glaucoma suspects (CL = 2, IOL = 2) by the age 1 year EUA.26 Because elevation of intraocular pressure during infancy is known to cause ocular enlargement, these patients were excluded from the analyses (Table 1). We also excluded an eye from the analyses if either the baseline or age 1 year AL was not available because the A-scan tracing was missing, invalid, or unreadable. Of the 100 patients without glaucoma or glaucoma suspect, 21 (CL = 7, IOL = 14) were excluded because both eyes had certain AL data that were unavailable, leaving 79 patients (CL = 45, IOL = 34). Several of these 79 patients had either the operated or the fellow eye excluded because certain AL data were unavailable for that eye. Among the 79 patients, for 62 (CL = 37, IOL = 25), both eyes were included; for 9 (CL = 3, IOL = 6), only the operated eye was included; and for 8 (CL = 5, IOL = 3), only the fellow eye was included. The total number of eyes included in the analyses was 141 (operated = 71, fellow = 70). Of these 141 eyes, AL was measured by immersion at both exams for 95 (67%), by contact at both exams for 26 (18%), by immersion at one exam and contact at the other for 11 (8%), and by immersion at one exam and an unspecified technique at the other for 9 (6%).
Table 1. .
Number of Patients Included in the Analyses
|
Category |
Treatment |
Total, No. of Patients |
|
|
Contact Lens, No. of Patients |
IOL, No. of Patients |
||
| Randomized | 57 | 57 | 114 |
| Without glaucoma | 52 | 48 | 100 |
| Axial length available | |||
| Both eyes | 37 | 25 | 62 |
| Operated eye only | 3 | 6 | 9 |
| Fellow eye only | 5 | 3 | 8 |
| Total | 45 | 34 | 79 |
Baseline Characteristics
The mean (± standard deviation) age at surgery among the 79 patients included in the analysis was 2.6 ± 1.6 months (range, 0.9–6.8); 45 (57%) were female and 68 (86%) were white. The mean patient age was not significantly different between treatment groups (CL, 2.5 ± 1.7 months; IOL, 2.6 ± 1.7 months; P = 0.81). The mean age among the 14 patients excluded for glaucoma or glaucoma suspect was 1.7 months versus 2.6 months for both the 21 patients excluded because of missing, unreadable, or invalid A-scans and the 79 patients included in the analyses (P = 0.17).
P values for the linear mixed model analysis of baseline AL are shown in Table 2. As expected because of randomization, baseline AL was not significantly related to treatment (P = 0.62). This was demonstrated in an analysis of least squares means, which showed that mean AL adjusted for age was not significantly different between the treatment groups for either the cataractous eyes (CL, 18.1 mm; IOL, 17.9 mm; P = 0.46) or the fellow eyes (CL, 18.5 mm; IOL, 18.7 mm; P = 0.23). The treatment groups were compared for cataractous and fellow eyes separately since the linear mixed model analysis showed that baseline AL differed according to the type of eye (P < 0.0001, Table 2). Mean AL adjusted for age for fellow eyes (18.6 mm) was significantly greater than for cataractous eyes (18.0 mm) (mean difference, 0.6 mm; 95% confidence interval [CI], 0.4–0.8 mm; P < 0.0001).
Table 2. .
P Values for Linear Mixed Model Analyses
|
Effect |
P Values for Axial Length Analyses |
||
|
Baseline AL* |
Change in AL per Month |
Age 1 Year AL |
|
| Treatment (CL vs. IOL) | 0.62 | 0.91 | 0.23 |
| Eye (cataractous vs. fellow) | <0.0001 | 0.0002 | <0.0001 |
| Age at surgery | <0.0001 | 0.0002 | 0.0012 |
| Interaction: treatment × eye | 0.34 | 0.042 | 0.23 |
| Interaction: treatment × age | 0.67 | 0.32 | 0.64 |
| Interaction: eye × age | 0.049 | 0.0072 | 0.001 |
| Interaction: treatment × eye × age | 0.99 | 0.73 | 0.58 |
AL, axial length.
Baseline AL was strongly associated with age at surgery (P < 0.0001, Table 2). We examined this relationship in detail for the cataractous eyes. Since baseline AL was not related to treatment, we combined the cataractous eyes from the two treatment groups. Figure 1 demonstrates the strong association between baseline AL and age (n = 75, r = 0.69; P < 0.0001). On average, the AL increases 0.5 mm (95% CI, 0.4–0.6 mm) per month among patients in the age range studied (1–7 months of age). The estimated mean AL at 1 month of age was 17.2 mm (95% CI, 16.9–17.5) and increased to 19.9 mm at age 6 months (95% CI, 19.4–20.4).
Figure 1. .
Axial length versus age at surgery for the cataractous eyes.
Change in Axial Length
Longitudinal changes in AL for the operated and fellow eyes are shown in Figure 2. Age at the time of the first measurement varied among patients but was relatively similar for the second measurement. Table 2 shows the P values associated with the various terms in the linear mixed model. Figure 3 shows the mean change in AL/mo estimated from the model versus age at surgery according to treatment group (CL versus IOL) and eye (operated versus fellow). The notable results are the significant two-way interactions. One of these was eye × age (0.0072), indicating that the change in AL/mo decreased significantly with increasing age at surgery in the fellow eyes but not in the operated eyes. This is demonstrated in Figure 3, where the slopes of the lines are greater for the fellow eyes than for the operated eyes. The second significant two-way interaction was treatment × eye (0.042), suggesting that the difference between operated and fellow eyes was not the same for the two treatment groups. This can be seen in Figure 3, as the operated and fellow eyes are more similar in the IOL group than in the CL group.
Figure 2. .
Longitudinal changes in axial length for the aphakic and pseudophakic eyes and fellow eyes in the CL and IOL groups.
Figure 3. .
Mean change in axial length per month versus age at surgery according to treatment group (contact lens versus intraocular lens) and eye (operated versus fellow).
Separate models were fit for the operated and fellow eyes that included treatment, age at surgery, and the interaction of treatment and age. The interaction was not significant for either (operated: P = 0.47; fellow: P = 0.71). For the no-interaction model for operated eyes, age was not significant (P = 0.19), but the mean change in AL/mo was significantly different between treatments (estimated means: CL, 0.17 mm/mo; IOL, 0.24 mm/mo; P = 0.0006). For the no-interaction model for fellow eyes, treatment was not significant (P = 0.13), but age was significant (P < 0.0001). In a linear regression model with age as the only variable, the estimated mean change in AL/mo was 0.28 mm/mo at 1 month of age (95% CI, 0.26–0.30) and 0.14 mm/mo at 6 months of age (95% CI, 0.11–0.18).
Axial Length at 1 Year of Age
In the linear mixed model for AL at 1 year of age, there was a significant interaction for eye and age at surgery (Table 2), indicating that the effect of age at surgery on the AL at 1 year of age differed between operated eyes and fellow eyes (P = 0.0012). Separate models were fit for the operated and fellow eyes that included treatment, age at surgery, and the interaction of treatment and age.
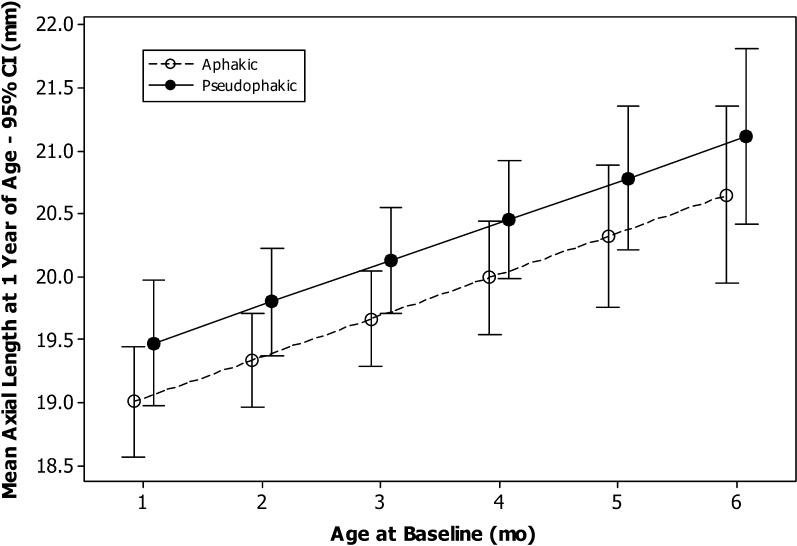
For the operated eyes, the interaction was not significant (P = 0.54). For the model without interaction, age at surgery was significant (P < 0.0002) with a trend for significance with treatment (P = 0.1). Therefore, we report the mean AL at 1 year of age estimated from the model for different values of age at surgery for the two treatment groups. The mean AL was 19.0 mm for aphakic and 19.5 mm for pseudophakic eyes having surgery at 1 month of age and 20.6 mm for aphakic and 21.1 mm for pseudophakic eyes having surgery at 6 months of age (Fig. 4).
Figure 4. .
Mean axial length at 1 year of age for aphakic and pseudophakic eyes as a function of age at the time of cataract surgery.
For fellow eyes, neither treatment (P = 0.63), age at surgery (P = 0.46), nor the interaction (P = 0.89) was statistically significant. Since none of these factors were significant, we report the overall mean AL at 1 year of age for fellow eyes: 20.7 ± 0.6 mm (95% CI for mean, 20.5–20.8 mm).
Discussion
Prior to cataract surgery, the mean AL was similar for the cataractous eyes in the two treatment groups. However, at 1 year of age, the mean AL of the pseudophakic eyes was 0.6 mm longer than the mean AL of the aphakic eyes. This difference is the result of a reduced rate of axial elongation in aphakic eyes compared to pseudophakic eyes, which was independent of the age of the infant at the time of cataract surgery. In contrast, the fellow eyes had nearly the same axial elongation in the two treatment groups, resulting in comparable ALs at age 12 months.
A number of retrospective case series have evaluated the effect of pediatric cataract surgery on axial elongation. Differing results have been reported, ranging from reduced12,27 to greater axial elongation in pseudophakic eyes or no effect.6,20,28 However, unlike the IATS, all of these studies were retrospective, with a wide range of inclusion criteria. In addition, most used applanation biometry whereas we primarily used immersion biometry.29 Finally, we analyzed only the data from patients with valid A-scan tracings as judged by a masked echographer. None of the publications on these other studies mentioned evaluation of the quality of the A-scans used in the analyses.
Studies using a nonhuman primate model have also reported a retardation of axial elongation following a lensectomy during infancy. Lambert et al.15 simulated a unilateral congenital cataract by placing a translucent contact lens on the right eye of a cohort of infant Rhesus monkeys. When the monkeys were 11 to 16 days of age, a lensectomy was performed on their right eyes. Some of the eyes had an IOL implanted while others were left aphakic and corrected with a contact lens. At 5 weeks of age, both the aphakic and the pseudophakic eyes were significantly shorter than their fellow eyes. At 12 months of age, the mean ALs of the pseudophakic eyes were 2.0 ± 0.2 mm shorter than their fellow eyes whereas the aphakic eyes were 2.3 ± 0.2 mm shorter than their fellow eyes. The retardation of axial elongation that occurs following a lensectomy has been shown to be age dependent. While a large reduction in axial elongation was noted in monkeys undergoing a lensectomy at 4 days and 2 weeks of age, only a small effect was noted after a lensectomy at 7.5 months and 1 year of age.16 We did not find the retardation of AL to be age dependent in our study. However, we studied only infants 1 to 6 months of age. Bradley and coworkers30 have also reported that axial elongation in the untreated eye can be affected by the treatment of the fellow eye in a nonhuman primate model. In our study, we did not find that the type of treatment of the operated eyes had an effect on axial elongation of the untreated fellow eyes.
It is uncertain why aphakic eyes in our study experienced less ocular growth than pseudophakic eyes. One possibility is that the pseudophakic eyes had an increased number of additional intraocular surgeries, which we have reported previously.21,22 Most of these surgeries were performed to clear visual axis opacities. These additional intraocular surgeries may have elevated the level of chemical mediators in these eyes, such as prostaglandins, which may have altered ocular growth.31,32 A second possibility is that axial elongation was altered for optical reasons. Defocusing with plus and minus lenses has been shown to alter axial elongation in a nonhuman primate model.4,33 However, this seems unlikely since the operated eye in both treatment groups was focused to a near point and since compliance with contact lens and spectacle use was quite good.21 Finally, there may have been a difference in the IOP between the operated eyes in the two treatment groups. However, this is unlikely since all eyes that were judged to have glaucoma or glaucoma suspect were excluded from the analysis.
As expected, we found that the mean change in AL/mo was greater in the fellow eyes of patients enrolled at a younger age. Others have reported a deceleration of axial elongation during the first year of life in normal eyes.1,2 Therefore an infant enrolled in the study at 1 month of age would be expected to have a greater mean change in AL/mo than an infant enrolled in the study at 6 months of age. A novel finding in our study was that the mean change in AL/mo for the operated eyes was not correlated with the age at cataract surgery.
This study has a number of limitations. First, the follow-up was variable, ranging from 6 to 11 months depending on the age of the child at the time of cataract surgery. In addition, the follow-up was only until approximately age 1 year; therefore our estimates of change per month apply only up to that age. We plan to repeat biometry on all of these patients when they are 5 years of age, which will allow us to assess axial elongation after these patients have completed the period of rapid axial elongation in the first 2 years of life.1,2 Second, the quality of the A-scans varied between clinical centers. While two-thirds of patients had biometry performed in both eyes using immersion, the other one-third had biometry performed in both eyes using applanation or in one eye using applanation and in one eye using immersion. A recent prospective study comparing AL measurements obtained by immersion and contact biometry found that AL measurements obtained using applanation were on average 0.27 mm shorter, presumably secondary to corneal compression during applanation.29 Third, of the 114 patients enrolled in the study, 35 (31%) were not included in the analyses. Fourteen patients were excluded because they developed glaucoma.34 In addition, 21 patients were not included because of missing or invalid A-scans. However, this group was similar in age to the patients who were included, which suggests that factors related to age, such as greater difficulty in measuring AL in younger patients, did not systematically eliminate patients from analysis. Finally, more of the patients in the CL (n = 45) versus the IOL group (n = 34) were included in the analysis. This was partially due to the increased number of eyes in the IOL group that had glaucoma or were glaucoma suspects (IOL, n = 9; CL, n = 5) and possibly the increased difficulty of obtaining a valid A-scan in a pseudophakic eye.
Our findings are clinically important because they suggest that cataract surgery coupled with IOL implantation is associated with increased axial elongation compared to cataract surgery without IOL implantation during infancy. In our study, we targeted an undercorrection in the operated eye for infants undergoing IOL implantation of 8 D for infants 6 weeks or younger and 6 D for infants older than 6 weeks, which was based on data from a pilot study.35 After these patients have been followed over a longer time period, we will be able to evaluate the long-term accuracy of our targeted undercorrection.
Acknowledgments
Cynthia Kendall graded the A-scan tracings and provided training to the IATS investigators on how to perform biometry.
Appendix
The Infant Aphakia Treatment Study Group
Administrative Units and Participating Clinical Centers:
Clinical Coordinating Center (Emory University): Scott R. Lambert, MD (Study Chair); Lindreth DuBois, MEd, MMSc (National Coordinator).
Data Coordinating Center (Emory University): Michael Lynn, MS (Director), Betsy Bridgman, BS; Marianne Celano, PhD; Julia Cleveland, MSPH; George Cotsonis, MS; Carey Drews-Botsch, PhD; Nana Freret, MSN; Lu Lu, MS; Seegar Swanson; Thandeka Tutu-Gxashe, MPH.
Visual Acuity Testing Center (University of Alabama, Birmingham): E. Eugenie Hartmann, PhD (Director); Clara Edwards; Claudio Busettini, PhD; Samuel Hayley, BS.
Steering Committee: Scott R. Lambert, MD; Edward G. Buckley, MD; David A. Plager, MD; M. Edward Wilson, MD; Michael Lynn, MS; Lindreth DuBois, MEd, MMSc; Carolyn Drews-Botsch, PhD; E. Eugenie Hartmann, PhD; Donald F. Everett, MA.
Contact Lens Committee: Buddy Russell, COMT; Michael Ward, MMSc.
Participating Clinical Centers (in order by the number of patients enrolled):
Medical University of South Carolina; Charleston, South Carolina (14): M. Edward Wilson, MD; Margaret Bozic, CCRC, COA.
Harvard University; Boston, Massachusetts (14): Deborah K. Vanderveen, MD; Theresa A. Mansfield, RN; Kathryn Bisceglia Miller, OD.
University of Minnesota; Minneapolis, Minnesota (13): Stephen P. Christiansen, MD; Erick D. Bothun, MD; Ann Holleschau, BA; Jason Jedlicka, OD; Patricia Winters, OD; Jacob Lang, OD.
Cleveland Clinic; Cleveland, Ohio (10): Elias I. Traboulsi, MD; Susan Crowe, BS, COT; Heather Hasley Cimino, OD.
Baylor College of Medicine; Houston, Texas (10): Kimberly G. Yen, MD; Maria Castanes, MPH; Alma Sanchez, COA; Shirley York.
Oregon Health and Science University; Portland, Oregon (9): David T Wheeler, MD; Ann U. Stout, MD; Paula Rauch, OT, CRC; Kimberly Beaudet, CO, COMT; Pam Berg, CO, COMT.
Emory University; Atlanta, Georgia (9): Scott R. Lambert, MD; Amy K. Hutchinson, MD; Lindreth DuBois, MEd, MMSc; Rachel Robb, MMSc; Marla J. Shainberg, CO.
Duke University; Durham, North Carolina (8): Edward G. Buckley, MD; Sharon F. Freedman, MD; Lois Duncan, BS; B.W. Phillips, FCLSA; John T. Petrowski, OD.
Vanderbilt University; Nashville, Tennessee (8): David Morrison, MD; Sandy Owings, COA, CCRP; Ron Biernacki, CO, COMT; Christine Franklin, COT.
Indiana University (7): David A. Plager, MD; Daniel E. Neely, MD; Michele Whitaker, COT; Donna Bates, COA; Dana Donaldson, OD.
Miami Children's Hospital (6): Stacey Kruger, MD; Charlotte Tibi, CO; Susan Vega.
University of Texas Southwestern; Dallas, Texas (6): David R. Weakley, MD; David R. Stager Jr, MD; Joost Felius, PhD; Clare Dias, CO; Debra L. Sager; Todd Brantley, OD.
Data and Safety Monitoring Committee: Robert Hardy, PhD (Chair); Eileen Birch, PhD; Ken Cheng, MD; Richard Hertle, MD; Craig Kollman, PhD; Marshalyn Yeargin-Allsopp, MD (resigned); Cyd McDowell; Donald F. Everett, MA.
Medical Safety Monitor: Allen Beck, MD.
Footnotes
See Appendix for a list of the members of the Infant Aphakia Treatment Study Group.
Supported by National Institutes of Health Grants U10 EY13272 and U10 EY013287 and in part by NIH Departmental Core Grant EY06360 and Research to Prevent Blindness, Inc., New York, New York.
Disclosure: S.R. Lambert, None; M.J. Lynn, None; L.G. DuBois, None; G.A. Cotsonis, None; E.E. Hartmann, None; M.E. Wilson, None
References
- 1.Gordon RA, Donzis PB. Refractive development of the human eye. Arch Ophthalmol. 1985;103:785–789 [DOI] [PubMed] [Google Scholar]
- 2.Manzitti E, Gamio S, Damel A, Benozzi J. Eye length in congenital cataracts. In: Cotlier E, Lambert S, Tayor D.eds Congenital Cataracts. Austin, TX: R.G. Landes Company; 1994:251–259 [Google Scholar]
- 3.Wiesel TN, Raviola E. Myopia and eye enlargement after neonatal lid fusion in monkeys. Nature. 1977;266:66–68 [DOI] [PubMed] [Google Scholar]
- 4.Hung LF, Crawford ML, Smith EL. Spectacle lenses alter eye growth and the refractive status of young monkeys. Nat Med. 1995;1:761–765 [DOI] [PubMed] [Google Scholar]
- 5.Sampaolesi R, Caruso R. Ocular echometry in the diagnosis of congenital glaucoma. Arch Ophthalmol. 1982;100:574–577 [DOI] [PubMed] [Google Scholar]
- 6.Hutchinson AK, Wilson ME, Saunders RA. Outcomes and ocular growth rates after intraocular lens implantation in the first 2 years of life. J Cataract Refract Surg. 1998;24:846–852 [DOI] [PubMed] [Google Scholar]
- 7.Flitcroft DI, Knight-Nanan D, Bowell R, Lanigan B, O'Keefe M. Intraocular lenses in children: changes in axial length, corneal curvature, and refraction. Br J Ophthalmol. 1999;83:265–269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Inatomi M, Kora Y, Kinohira Y, Yaguchi S. Long-term follow-up of eye growth in pediatric patients after unilateral cataract surgery with intraocular lens implantation. J AAPOS. 2004;8:50–55 [DOI] [PubMed] [Google Scholar]
- 9.Lal G, Trivedi RH, Wilson ME Jr, Scarlett LC, Peterseim MM. Interocular axial length difference in eyes with pediatric cataracts. J AAPOS. 2005;9:358–362 [DOI] [PubMed] [Google Scholar]
- 10.Trivedi RH, Wilson ME Jr. Changes in interocular axial length after pediatric cataract surgery. J AAPOS. 2007;11:225–229 [DOI] [PubMed] [Google Scholar]
- 11.McClatchey SK, Dahan E, Maselli E, et al. A comparison of the rate of refractive growth in pediatric aphakic and pseudophakic eyes. Ophthalmology. 2000;107:118–122 [DOI] [PubMed] [Google Scholar]
- 12.Sminia ML, de Faber JT, Doelwijt DJ, Wubbels RJ, Tjon-Fo-Sang M. Axial eye length growth and final refractive outcome after unilateral paediatric cataract surgery. Br J Ophthalmol. 2010;94:547–550 [DOI] [PubMed] [Google Scholar]
- 13.Kugelberg U, Zetterstrom C, Lundgren B, Syren-Nordqvist S. Eye growth in the aphakic newborn rabbit. J Cataract Refract Surg. 1996;22:337–341 [DOI] [PubMed] [Google Scholar]
- 14.Lambert SR, Fernandes A, Grossniklaus H, Drews-Botsch C, Eggers H, Boothe RG. Neonatal lensectomy and intraocular lens implantation: effects in rhesus monkeys. Invest Ophthalmol Vis Sci. 1995;36:300–310 [PubMed] [Google Scholar]
- 15.Lambert SR, Fernandes A, Drews-Botsch C, Tigges M. Pseudophakia retards axial elongation in neonatal monkey eyes. Invest Ophthalmol Vis Sci. 1996;37:451–458 [PubMed] [Google Scholar]
- 16.Lambert SR. The effect of age on the retardation of axial elongation following a lensectomy in infant monkeys. Arch Ophthalmol. 1998;116:781–784 [DOI] [PubMed] [Google Scholar]
- 17.Griener ED, Dahan E, Lambert SR. Effect of age at time of cataract surgery on subsequent axial length growth in infant eyes. J Cataract Refract Surg. 1999;25:1209–1213 [DOI] [PubMed] [Google Scholar]
- 18.Kora Y, Shimizu K, Inatomi M, Fukado Y, Ozawa T. Eye growth after cataract extraction and intraocular lens implantation in children. Ophthalmic Surg. 1993;24:467–475 [PubMed] [Google Scholar]
- 19.Lorenz B, Worle J, Friedl N, Hasenfratz G. Ocular growth in infant aphakia. Bilateral versus unilateral congenital cataracts. Ophthalmic Paediatr Genet. 1993;14:177–188 [DOI] [PubMed] [Google Scholar]
- 20.Vasavada AR, Raj SM, Nihalani B. Rate of axial growth after congenital cataract surgery. Am J Ophthalmol. 2004;138:915–924 [DOI] [PubMed] [Google Scholar]
- 21.Lambert SR, Buckley EG, Drews-Botsch C, et al. A randomized clinical trial comparing contact lens with intraocular lens correction of monocular aphakia during infancy: grating acuity and adverse events at age 1 year. Arch Ophthalmol. 2010;128:810–818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Plager DA, Lynn MJ, Buckley EG, Wilson ME, Lambert SR. Complications, adverse events, and additional intraocular surgery 1 year after cataract surgery in the infant aphakia treatment study. Ophthalmology. 2011;118:2330–2334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lambert SR, Buckley EG, Drews-Botsch C, et al. The infant aphakia treatment study: design and clinical measures at enrollment. Arch Ophthalmol. 2010;128:21–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kiskis AA, Markowitz SN, Morin JD. Corneal diameter and axial length in congenital glaucoma. Can J Ophthalmol. 1985;20:93–97 [PubMed] [Google Scholar]
- 25.Drews-Botsch CD, Celano M, Kruger S, Hartmann EE. Adherence to occlusion therapy in the first six months of follow-up and visual acuity among participants in the Infant Aphakia Treatment Study (IATS). Invest Ophthalmol Vis Sci. 2012;53:3368–3375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Beck AD, Freedman SF, Lynn MJ, Bothun E, Neely DE, Lambert SR. Glaucoma-related adverse events in the Infant Aphakia Treatment Study: 1-year results. Arch Ophthalmol. 2012;130:300–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Griener E, Lambert SR. Successful treatment of tractional corectopia using 2 mJ of energy with an Nd:YAG laser. J AAPOS. 1999;3:250–251 [DOI] [PubMed] [Google Scholar]
- 28.Hussin HM, Markham R. Changes in axial length growth after congenital cataract surgery and intraocular lens implantation in children younger than 5 years. J Cataract Refract Surg. 2009;35:1223–1228 [DOI] [PubMed] [Google Scholar]
- 29.Trivedi RH, Wilson ME. Axial length measurements by contact and immersion techniques in pediatric eyes with cataract. Ophthalmology. 2011;118:498–502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bradley DV, Fernandes A, Boothe RG. The refractive development of untreated eyes of rhesus monkeys varies according to the treatment received by their fellow eyes. Vision Res. 1999;39:1749–1757 [DOI] [PubMed] [Google Scholar]
- 31.Lenart TD, Drack AV, Tarnuzzer RW, Fernandes A, Lambert SR. Heterochromia after pediatric cataract surgery. J AAPOS. 2000;4:40–45 [DOI] [PubMed] [Google Scholar]
- 32.Tarnuzzer RW, Fernandes A, Iuvone PM, Lambert SR. Neonatal aphakia retards ocular growth and alters scleral gene expression in rhesus monkeys. Mol Vis. 2005;11:36–49 [PubMed] [Google Scholar]
- 33.Smith EL III, Hung LF. The role of optical defocus in regulating refractive development in infant monkeys. Vision Res. 1999;39:1415–1435 [DOI] [PubMed] [Google Scholar]
- 34.Beck AD, Freedman SF, Lynn MJ, Bothun E, Neely D, Lambert SR;Infant Aphakia Treatment Study Group Glaucoma-related adverse events in the Infant Aphakia Treatment Study (IATS): 1-year results. Arch Ophthalmol. 2012;130:300–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lambert SR, Buckley EG, Plager DA, Medow NB, Wilson ME. Unilateral intraocular lens implantation during the first six months of life. J AAPOS. 1999;3:344–349 [DOI] [PubMed] [Google Scholar]