Abstract
Purpose.
Exogenous vasoactive intestinal peptide (VIP) down-regulates pro-inflammatory but up-regulates anti-inflammatory cytokines, growth factors (GFs) and Toll-like receptors promoting healing in experimental Pseudomonas aeruginosa (P. aeruginosa) keratitis. Whether VIP is required for GF or GF receptor (R) expression in normal and infected corneas is unknown and is the purpose of this study.
Methods.
VIP knockout (−/−) and wild-type (WT) C57BL/6 (B6) mice were infected and tested using PCR array, real-time RT-PCR, ELISA, and immunostaining. VIP antagonist treatment studies also were done using B6 and BALB/c mice.
Results.
Infected corneas of VIP−/− versus WT B6 mice perforated earlier (2 vs. 5 days postinfection [p.i.]), and array data showed that GFs were differentially changed between groups. RT-PCR revealed that the infected cornea of VIP−/− versus WT mice expressed higher mRNA levels of epidermal growth factor (EGF) and hepatocyte growth factor (HGF), reduced FGF, EGFR, and HGFR, with no difference in FGFR; differences between groups were not seen in normal cornea. Immunostaining for GF and GFR in the normal cornea of VIP−/− versus WT mice was similar. However, at 1 day p.i., VIP−/− versus WT mice had more intense EGF and HGF, similar FGFR, and reduced FGF, EGFR, and HGFR staining. VIP antagonist treatment decreased protein levels for GFR at 5 days p.i. in both B6 and BALB/c mice, with no significant changes in normal cornea.
Conclusions.
The data showed that endogenous VIP is not requisite for GF or GFR expression in the normal cornea but, after infection, its absence or reduction is critical for their regulation.
In the cornea, endogenous absence of vasoactive intestinal peptide results in dysregulated growth factors and their receptors after bacterial infection, and the cornea perforates more quickly.
Introduction
Pseudomonas aeruginosa (P. aeruginosa), an opportunistic, gram-negative pathogen, is one of the most virulent organisms associated with microbial keratitis and often is associated with contact lens use.1 Infections progress rapidly and lead to inflammatory epithelial edema, stromal infiltration, corneal ulceration, and, oftentimes, vision loss.2 Experimental murine models of the disease have been established: Th1 responder mouse strains (e.g., C57BL/6 [B6]) are susceptible (i.e., cornea perforates), whereas Th2 responder strains (e.g., BALB/c) are resistant (i.e., cornea heals).3
In previous studies using these murine models, we provided evidence that an anti-inflammatory neuropeptide, vasoactive intestinal peptide (VIP) promotes resistance against P. aeruginosa corneal infection by regulation of cytokine production and subsequent alteration of the host inflammatory cell response.4 In addition, recent studies from this laboratory have provided mechanistic information that VIP treatment modulates keratitis through regulation of growth factors (GFs), angiogenic molecules, beta defensins,5 and Toll-like receptors (TLRs)6 in the infected cornea, contributing to healing. However, it remains untested whether endogenous VIP is required for production of GFs or growth factor receptors (GFRs) in the normal, uninfected cornea and after infection. This is of importance to determine, because it has been demonstrated in other experimental models7,8 that one of the ways that inflammation can be regulated is through GF binding to their receptors.
Thus, the current study investigated the expression of GFs and their receptors in the normal and infected cornea of VIP knockout (−/−) versus wild-type (WT) mice. Data from the studies provided evidence that the normal cornea of both groups of mice was similar morphologically and had a similar pattern of GF and GFR expression, suggesting that endogenous VIP is not requisite for their expression. However, after infection, corneal perforation occurred more rapidly (2 days postinfection [p.i.]) in VIP−/− versus WT mice, and real time RT-PCR and immunostaining studies showed disparate expression of GF and their receptors in the two groups. As the GFR appeared to be most consistently affected, B6 and BALB/c mice were treated with a VIP antagonist, infected and tested for GFR expression. In this experiment, GFR proteins were reduced for both murine strains, confirming the trend in the VIP−/− data. Overall, the current study provided evidence that endogenous VIP was not required for normal corneal expression of GF or GFR. However, if it was absent or reduced, GFs and their receptors were disregulated and the infected cornea perforated rapidly.
Materials and Methods
Infection
Eight-week-old female B6, VIP−/− (B6.129S4-Viptm1Clw/J) and BALB/c mice (Jackson Laboratory, Bar Harbor, ME) were anesthetized with ethyl ether and placed beneath a stereoscopic microscope at ×40 magnification. The cornea of the left eye was wounded9 and a 5-μL aliquot containing 1.0 × 106 CFU/μL of P. aeruginosa (strain 19660, American Type Culture Collection, Manassas, VA) delivered topically. Animals were treated in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
Ocular Response to Bacterial Infection
Corneal disease was graded10: 0 = clear or slight opacity, partially or fully covering the pupil; +1 = slight opacity, fully covering the anterior segment; +2 = dense opacity, partially or fully covering the pupil; +3 = dense opacity, covering the entire anterior segment; and +4 = corneal perforation or phthisis. After infection, a clinical score was recorded (days 1 and 2) for each group of mice (n = 5 per group per treatment).
VIP Antagonist Treatment
BALB/c and B6 mice (n = 5 per group per time per assay) were injected intraperitoneally with 100 μL PBS containing 10 μg VIP antagonist11 (Bachem, San Carlos, CA) on days −1, 0 (day of infection), and daily through 5 days p.i. Control mice were similarly injected with PBS. Normal, uninfected, and infected corneas were collected at 5 days p.i. for real-time RT-PCR mRNA detection and ELISA assay.
Real-Time RT-PCR
Total RNA was isolated from an individual cornea using RNA STAT-60 (Tel-Test, Friendsville, TX) per the manufacturer's recommendations and were quantitated spectrophotometrically (260 nm). One microgram of total RNA was reverse transcribed using Moloney murine leukemia virus (MMLV) reverse transcriptase. The 20 μL reaction mixture containing 200 U of MMLV-reverse transcriptase, 10 U of RNase inhibitor, 500 ng of oligo (dT) primers, 10 mM deoxyribonucleotide triphosphate, 100 mM dityhiothreitol, and MMLV reaction buffer (M-MLV RT; Invitrogen, Carlsbad, CA). After, cDNA was amplified using SYBR Green Master Mix (Bio-Rad, Hercules, CA) per the manufacture's recommendation. Briefly, the 20 μL reaction system contained: 10 μL SYBR Green PCR Master Mix, 0.5 μM primers, 2 μL cDNA (diluted 1:25), and diethyl pyrocarbonate water. All primer sets for the PCR array were purchased as a 96-well plate (RT2 Profiler Mouse Wound Healing PCR Array; SABiosciences Corporation, Frederick, MD). The individual primer sets were designed using primer quest tools (PrimerQuest; Integrated DNA Technologies, Cambridge, MA). Sequences of primers for β-actin, EGF, EGFR, FGF, FGFR, HGF, and HGFR are shown (Table 1).
Table 1. .
Nucleotide Sequence of the Primers Used in PCR Amplification
|
Gene |
GenBank No. |
Primer Sequence (5′–3′) |
Size (bp) |
| β-actin | NM_007393.3 | F - GAT TAC TGC TCT GGC TCC TAG C | 147 |
| R - GAC TCA TCG TAC TCC TGC TTG C | |||
| EGF | NM_010113.3 | F - ACG GTT TGC CTC TTT TCC TT | 130 |
| R - GTT CCA AGC GTT CCT GAG AG | |||
| EFGR | NM_007912.4 | F - GTG GAG GGA CAT CGT CCA AA | 100 |
| R - ATT GGG ACA GCT TGG ATC ACA T | |||
| FGF | NM_008008.4 | F - AAC AGC TAC AAC ATC ATG GAA ATC AG | 153 |
| R - AAT CAG TTC TTT GAA GTT GCA ATC CT | |||
| FGFR | NM_010207.2 | F - GTG CTT ATT GGG GAG TAT CTC CA | 102 |
| R - GAT CCA AGT TTC ACT GTC TAC CG | |||
| HGF | NM_010427.4 | F - ACT TCT GCC GGT CCT GTT G | 66 |
| R - GGG ATG GCG ACA TGA AGC A | |||
| HGFR | NM_008591.2 | F - GTG AAC ATG AAG TAT CAG CTC CC | 100 |
| R - TGT AGT TTG TGG CTC CGA GAT |
Quantitative real-time RT-PCR was performed using the MyiQ single color real-time RT-PCR detection system (MyiQ; Bio-Rad). Optimal conditions for PCR amplification of cDNA were established using routine methods.12 Relative mRNA levels were calculated after normalization to β-actin.
Immunofluorescent Staining
Normal, uninfected, and infected eyes were enucleated (n = 3 per group per time) at 1 day p.i. from WT and VIP−/− mice, immersed in 1× phosphate buffered saline (Dulbecco's PBS; Mediatech, Inc., Herndon, VA), embedded in OCT compound (Tissue-Tek; Miles, Elkhart, IN), and frozen in liquid nitrogen. Ten-micrometer sections were cut, mounted to poly-L-lysine-coated glass slides, and stored at 37°C overnight. After a 2-minute fixation in acetone, slides were blocked with 10 mM sodium phosphate buffer containing 2.5% BSA and donkey IgG (1:100) for 30 minutes at room temperature. Then, sections were incubated for 1 hour with 15 μg/mL goat anti-mouse EGF (R&D Systems, Minneapolis, MN) or 15 μg/mL goat anti-mouse HGF (R&D Systems) antibody. Antibodies for EGF and HGF were incubated simultaneously with antibodies for their receptors, 15 μg/mL goat anti-mouse EGFR (R&D Systems) or 15 μg/mL goat anti-mouse HGFR (R&D Systems). This was followed by secondary antibodies, X-conjugated donkey anti-goat antibody (1:1500) and Alexa Fluor 633-conjugated donkey anti-goat antibody (1:1500) (for EGFR and HGFR, respectively (Invitrogen) for another hour.
Staining for FGF and its receptor, FGFR, was done sequentially. After incubating the sections with 1:100 goat anti-mouse FGF antibody (Santa Cruz Biotechnology, Santa Cruz, CA) for 1 hour followed by the secondary Alexa Fluor 633-conjugated donkey anti-goat antibody (1:2000) (Invitrogen) for another hour, sections were incubated with rabbit anti-mouse FGFR antibody (1:100) (Santa Cruz) for 1 hour and Alexa Fluor 546-conjugated donkey anti-rabbit secondary antibody (1:1500) (Invitrogen) for another hour. Controls were similarly treated but the primary antibodies were replaced with the same host IgG (1:1000), goat or rabbit IgG (ChromPure; Jackson ImmunoResearch Laboratories, West Grove, PA). Finally, sections were visualized and digital images captured with a confocal laser scanning microscope (Leica TCS SP2; Leica Microsystems, Buffalo Grove, IL).
Bacterial Plate Counts
Corneas from PBS or VIP antagonist treated B6 mice were collected (n = 5 per group per time) at 1 and 5 days p.i., and the number of viable bacteria were quantitated. Individual corneas were homogenized in sterile water containing 0.85% (weight per volume) NaCl containing 0.25% BSA. Serial 10-fold dilutions of the samples were plated on Pseudomonas isolation agar (Difco Laboratories, Sparks, MD) in triplicate, and plates were incubated overnight at 37°C and bacteria counted. Results are reported as log10 number of CFU per cornea ± SEM.
Histopathology
For histopathology, whole infected eyes (n = 3 per group) were enucleated from PBS and VIP antagonist-treated B6 mice at 5 days p.i., immersed in PBS, rinsed, and fixed in 1% osmium tetroxide, 2.5% glutaraldehyde, and 0.2 M Sorenson's phosphate buffer (pH 7.4) (1:1:1) at 4°C for 3 hours. Eyes were rinsed with 0.1 M phosphate buffer, dehydrated in graded ethanol and propylene oxide, and then infiltrated and embedded in Epon-araldite. Thick sections (1.5 μm) were cut, stained, observed, and photographed with a photomicroscope (Leica DM4000B, Leica Microsystems, Inc.), as described before.9
ELISA
Protein levels for GFR were tested using ELISA kits. Corneas from PBS and VIP antagonist-treated mice were individually collected (n = 5 per group per time) from normal, uninfected, and infected corneas at 5 days p.i. For EGFR (EIAab Science Co., Ltd., Wuhan, China), for HGFR (R&D Systems) and for FGFR (MyBioSource, San Diego, CA), corneas were homogenized in 0.5 mL of PBS with 0.1% aqueous solution and protease inhibitor (1 tablet per 10 mL) (Tween 20; Roche Mannheim, Germany). All samples were centrifuged at 13,000 rpm for 5 minutes and an aliquot of each supernatant was assayed in duplicate per the manufacturer's instructions. Sensitivities of the ELISA assays were <0.08 ng/mL for EGFR, <10.0 μg/mL for HGFR, and <0.1 ng/mL for FGFR.
Statistics
The difference in clinical scores between two groups was tested by the Mann-Whitney U test. For all other experiments, an unpaired, two-tailed Student's t-test (for comparisons between two groups) was used. P < 0.05 was considered to be statistically significant.
Results
Ocular Response in VIP−/− Mice
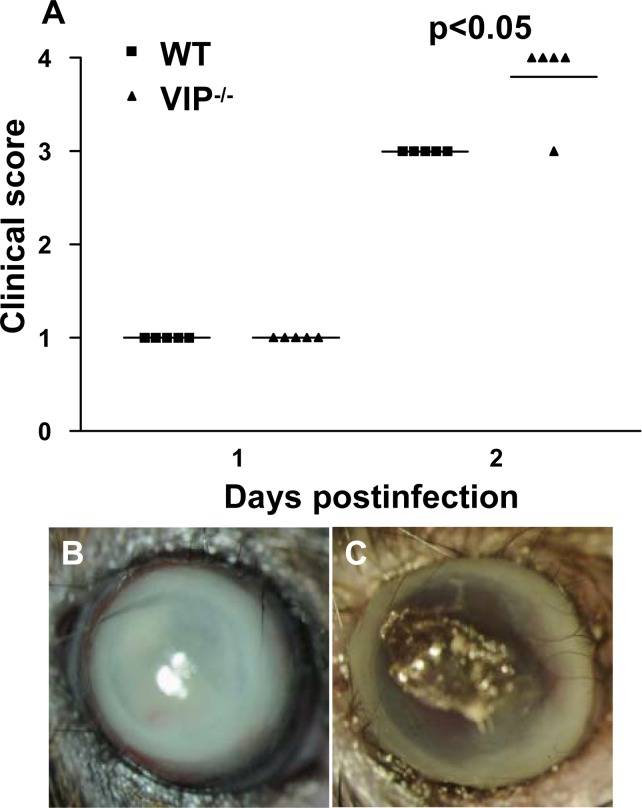
VIP−/− versus WT mice showed similar disease scores at 1 day p.i. At 2 days p.i., a significantly higher mean clinical score (P < 0.05) (Fig. 1), indicative of worsened disease and/or perforation, was recorded for the VIP−/−mice. Typical responses for each of the two groups was documented by photography using a slit lamp and showed that WT (Fig. 1B) mice had opacity over the anterior segment, while VIP−/− (Fig. 1C) mice exhibited corneal perforation, with the remainder of the eye heavily opaque due to the presence of an inflammatory infiltrate.
Figure 1. .
VIP−/− and WT B6 mice. Clinical scores (A) indicated statistically significant differences at 2 days p.i. between groups. Photographs taken with a slit lamp at 2 days p.i. showed no perforation in WT (B) versus early perforation in VIP−/− (C) mice. Magnification ×7.
PCR Array and Real-Time RT-PCR
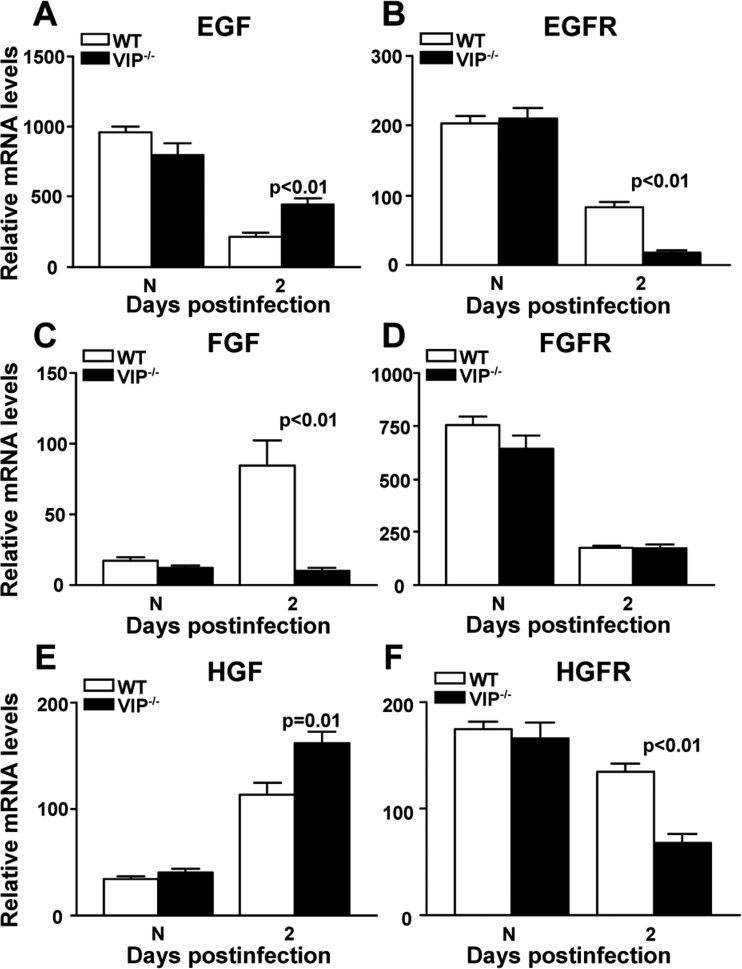
To determine whether endogenous VIP is required for GF production, mRNA levels of 84 wound healing related genes were profiled by PCR array at 2 days p.i. (Table 2). Eighteen of these GF and related molecules (e.g., EGF, EGFR, FGF, and HGF) were disparately changed in VIP−/− compared with WT B6 mice and are listed in Table 2. RT-PCR was used to selectively confirm the array data and to test for FGFR and HGFR, which are receptors for FGF and HGF but are not included on the array. In the normal, uninfected cornea, we found no significant difference at the mRNA level between the two mouse groups for GF or GFR expression (Figs. 2A–F). However, at 2 days p.i., VIP−/− versus WT mice showed significantly increased mRNA levels for EGF (P < 0.01) (Fig. 2A) and HGF (P = 0.01) (Fig. 2E), but lower levels of FGF (P < 0.01) (Fig. 2C). Growth factor receptors, EGFR (P < 0.01) (Fig. 2B) and HGFR (P < 0.01) (Fig. 2F) were significantly decreased in VIP−/− versus WT mice, with no significant changes to FGFR (Fig. 2D).
Table 2. .
Selected GFs from the Mouse Wound Healing RT2 Profiler PCR Array Comparing VIP−/− with WT mice
|
Genes |
Fold Up- or Down-Regulation |
| Angpt | −6.45 |
| Csf2 | −1.79 |
| Csf3 | 1.03 |
| Ctgf | −2.06 |
| Egf | 1.47 |
| Egfr | −1.83 |
| Fgf10 | 4.69 |
| Fgf2 | −1.41 |
| Fgf7 | −5.03 |
| Hbegf | −2.11 |
| Hgf | 1.25 |
| Igf1 | −3.63 |
| Mif | 1.21 |
| Pdgfa | 1.16 |
| Tgfa | −2.03 |
| Tgfb1 | 1.45 |
| Tnf | 1.92 |
| Vegfa | 1.27 |
Figure 2. .
GF and GFR mRNA expression. At 2 days p.i., EGF (A) and HGF (E) were significantly increased, while FGF (C) mRNA levels were significantly decreased in VIP−/− versus WT mice. EGFR (B) and HGFR (F) were decreased significantly, while FGFR (D) mRNA was similar in the two groups. No significant changes between the two groups were seen in normal cornea.
Immunostaining
Immunostaining confirmed the mRNA data above and showed no differences between the two groups of mice in the normal cornea for either the GFs (see Supplementary Material and Supplementary Fig. S1, http://www.iovs.org/content/53/12/7560/suppl/DC1) or for the GFRs (see Supplementary Material and Supplementary Fig. S2, http://www.iovs.org/content/53/12/7560/suppl/DC1). When comparing the two groups at 1 day p.i. (Fig. 3, red), immunostaining of the cornea of VIP−/− versus WT mice revealed increased EGF and HGF while FGF staining was decreased. For the GFRs, comparison in immunostaining between the two groups at 1 day p.i. (Fig. 4, blue) revealed that VIP−/− mice had decreased EGFR and HGFR staining, while staining for FGFR was similar. All controls (see Supplementary Material and Supplementary Figs. S1, S2, http://www.iovs.org/content/53/12/7560/suppl/DC1, and Figs. 3, 4), in which the primary antibody was replaced with same species IgG, were negative for immunostaining.
Figure 3. .
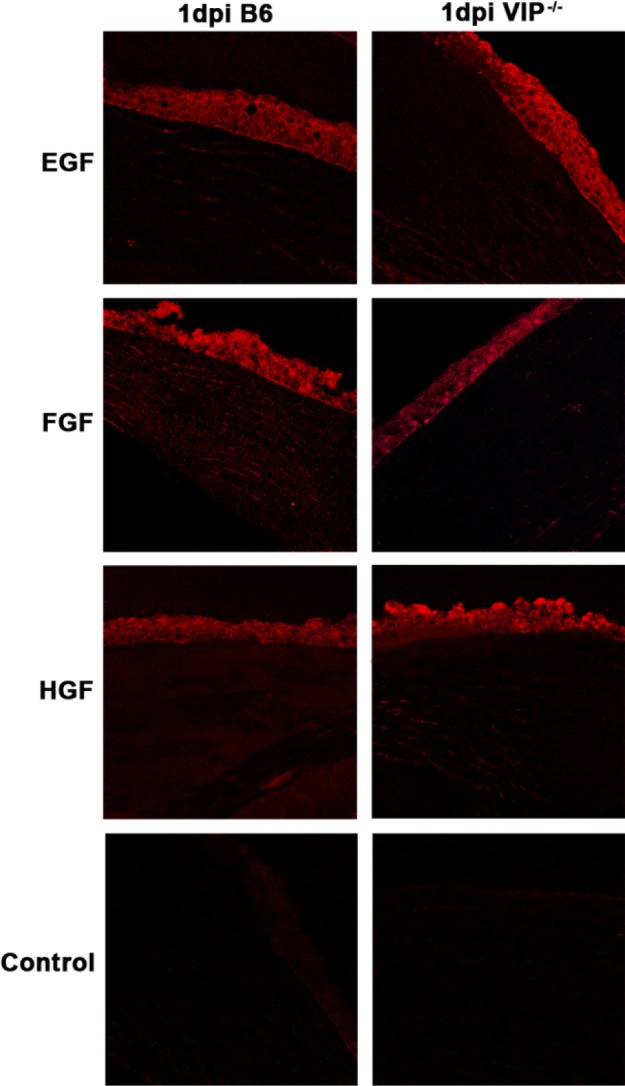
GF immunostaining (red) at 1 day p.i. Greater staining intensity for EGF and HGF, with reduced intensity for FGF was seen in VIP−/− compared with WT B6 mice. Negative controls, in which the primary antibody was replaced with a species-specific IgG showed no positive staining. Magnification ×170.
Figure 4. .
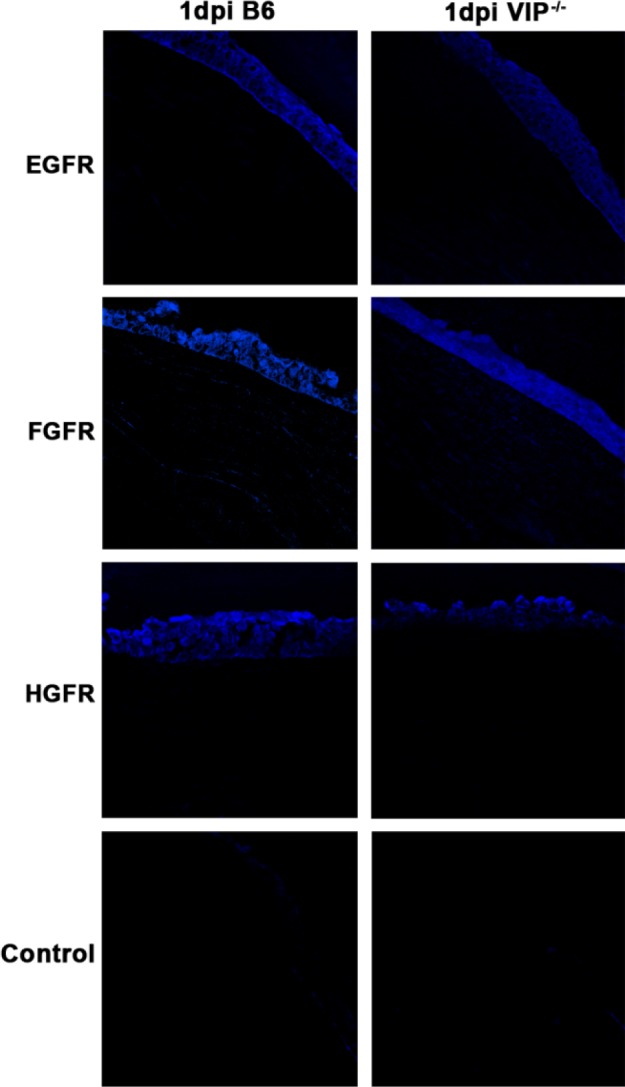
GFR immunostaining (blue) at 1 day p.i. Staining for GFRs was less intense for EGFR and HGFR in VIP−/− compared with WT B6 mice. No difference for FGFR was noted between groups. Negative controls, in which the primary antibody was replaced with a species-specific IgG, showed no positive staining. Magnification ×170.
Bacterial Counts
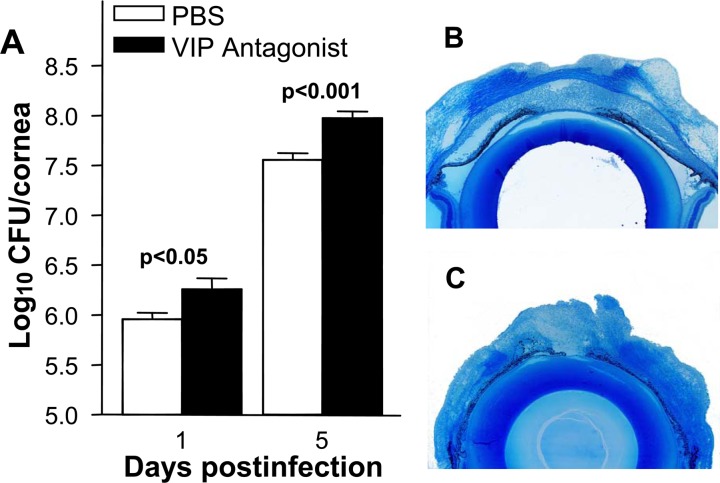
To determine whether VIP antagonist treatment correlated with increased bacterial numbers in the cornea, bacterial plate counts (Fig. 5A) were used to detect viable bacteria in the infected cornea of mice treated with VIP antagonist versus PBS at 1 and 5 days p.i. When compared with controls, VIP antagonist treatment led to increased bacterial plate counts at 1 (P < 0.05) and 5 days p.i. (P < 0.001).
Figure 5. .
VIP antagonist versus PBS treatment significantly increased bacterial plate counts at 1 and 5 days p.i. (A). Histopathology at 5 days p.i. showed that the cornea of PBS (B) versus VIP antagonist treated B6 mice (C) was thinned, but with a more intact stroma and was not perforated. Magnification ×9.5.
Histopathology
PBS (Fig. 5B) versus VIP antagonist (Fig. 5C) treated B6 mice exhibited corneal thinning in contrast to denudation of the corneal epithelium, stromal degeneration, severe edema, anterior chamber inflammation, and perforation.
Real-Time RT-PCR
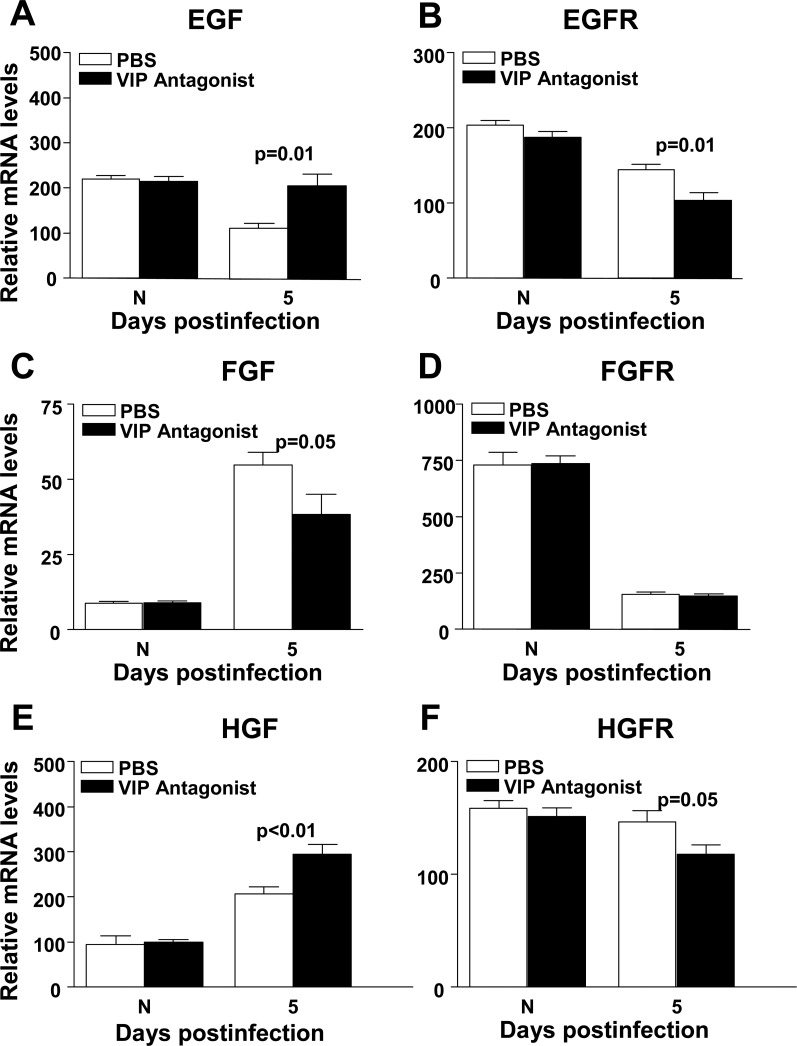
Next, to further test the effects of VIP on GF and GFR expression, real-time RT-PCR was done using B6 mice treated with a VIP antagonist versus PBS. Similar to the VIP−/− experiments, there was no significant difference between the two groups of mice at the mRNA level in either GFs or GFRs in the normal, uninfected cornea (Figs. 6A–F). However, at 5 days p.i., VIP antagonist versus PBS treated mice showed significantly increased mRNA levels for EGF (P = 0.01) (Fig. 6A,) and HGF (P < 0.01) (Fig. 6E), with decreased levels for FGF (P = 0.05) (Fig. 6C). GFRs, EGFR (P = 0.01) (Fig. 6B) and HGFR (P = 0.05) (Fig. 6F), were significantly decreased in VIP antagonist versus PBS treated mice, while there was no significant change for FGFR (Fig. 6D).
Figure 6. .
GF and GFR mRNA expression. EGF (A) and HGF (E) were significantly increased, while FGF (C) was decreased in VIP antagonist treated B6 mice at 5 days p.i. EGFR (B) and HGFR (F) were significantly decreased with no difference in FGFR (D) after VIP antagonist treatment. No difference was detected between groups in normal, uninfected cornea.
ELISA
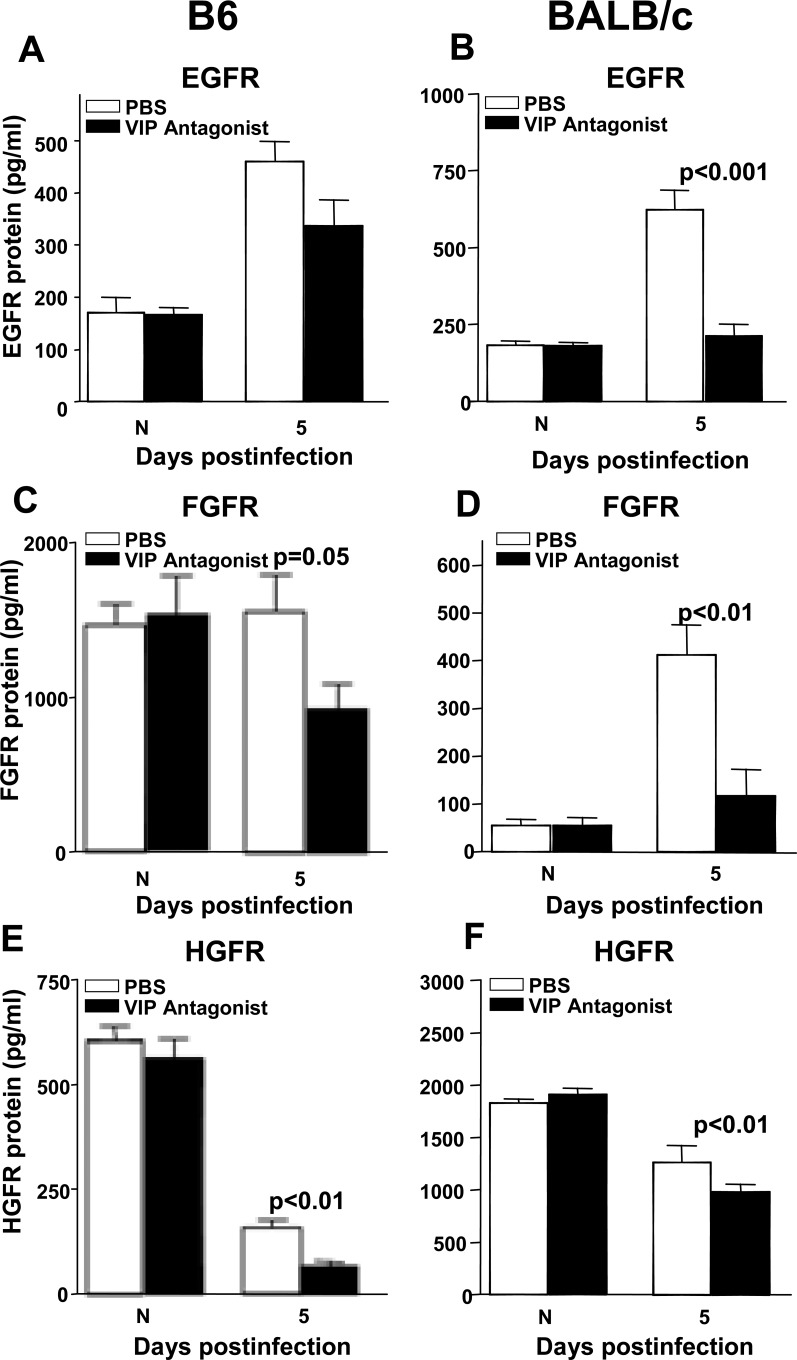
GFRs were tested at 5 days p.i. in B6 (Figs. 7A, C, E) and BALB/c (Figs. 7B, D, F) mice after treatment with VIP antagonist. For B6 mice, treatment with the VIP antagonist resulted in reduced levels of EGFR (Fig. 7A), although not significantly, while reduction in both FGFR (P = 0.05) (Fig. 7C) and HGFR (P < 0.01) (Fig. 7E) were significant. For BALB/c mice, VIP antagonist treatment significantly reduced all three GFR, EGFR (P < 0.001) (Fig. 7B), FGFR (P < 0.01) (Fig. 7D), and HGFR (P < 0.01) (Fig. 7F). No difference in GFRs between groups was detected in normal cornea.
Figure 7. .
GFR ELISA analysis. VIP antagonist versus PBS treatment decreased GFR (A–F) in both B6 and BALB/c mice at 5 days p.i. All were significant except for EGFR (A). No significant changes were seen in the normal cornea between the two groups.
Discussion
The neuropeptide VIP is a multifunctional molecule that regulates immune reactions and also participates in the maintenance and restoration of immune homeostasis.13 In addition, it has been considered in itself to be a growth factor14 as well as a type 2 cytokine.15
In prior work, this laboratory provided evidence that treatment with synthetic VIP regulated expression of pro- and anti-inflammatory cytokines,4 growth factors,5 and TLR6 in P. aeruginosa induced keratitis. This leads to decreased stromal destruction in a B6 mouse model in which the untreated, infected cornea perforates within approximately 1 week after experimental infection.4 The present study, using VIP−/− mice, tested whether endogenous absence of VIP or its reduction using an antagonist contributed to modulation of growth factors and their receptors. No detectable differences in either GFs or GFRs were detected in the normal cornea by RT-PCR or by immunostaining. Neither were there detectable differences in corneal morphology between the two groups. In contrast, in other models, the absence of VIP has been shown to lead to overall altered intestinal morphology, as well as thickening of smooth muscle and increased villi length in the bowel.16 In whole cultured (day 9.5) mouse embryos, VIP regulated insulin growth factor-I and embryonic development and when pregnant mice were treated with a VIP antagonist, inhibition of embryonic growth occurred.17
After infection, in the absence of the neuropeptide, GFs and GFRs were disregluated when tested by PCR array, RT-PCR, and immunostaining, and the cornea perforated more rapidly (within 2 days p.i.) for the majority of the VIP−/− versus WT mice. Furthermore, either the endogenous absence of VIP or antagonist treatment of B6 mice led to this disregulation. Specifically, VIP antagonist treatment of B6 mice reduced all three GFRs at the mRNA level (significantly, except for FGFR). When either B6 or BALB/c mice were treated with a VIP antagonist, GFR protein levels were decreased in both strains of mice when compared with controls. In this regard, others previously have shown interaction of VIP with the EGFR. In this regard, in vitro studies have shown that Gq protein-coupled receptor agonists such as VIP can directly activate (phosphorylate) the EGFR in T84 colonic epithelial cells by signaling pathways involving cAMP and protein kinase A and thereby regulate Cl− secretion.18 Previous studies also have shown that VIP receptors are Gs (VPAC1/2)19 and Gq coupled (VPAC2)20 (PAC1).21 cAMP, the downstream molecule of Gs coupled signaling, has been shown to increase nerve growth factor triggered signaling and differentiation in rat adrenal pheochromocytoma PC12 cells.22 On the other hand, phospholipase C-ε, the downstream molecule of Gq signaling, augments EGF-dependent COS-7 cell growth by inhibiting EGFR downregulation.23 Therefore, in the current study, we could not exclude the possibility that VIP binds to VPAC1/2 (Gs coupled) to activate cAMP, and/or binds PAC1 and VPAC2 (Gq coupled) to activate phospholipase C. The activated cAMP and/or phospholipase C, consequently, could regulate EGF and EGFR expression. In another study, it was reported that during infection of human alveolar epithelial cells with Pseudomonas fluorescens, a gram-negative rod blocking the EGFR increased epithelial susceptibility to pathogen-induced epithelial cell death24 consistent with, although not tested, in our keratitis model. Another growth factor, KGF/FGF-7 (FGF), and its receptor (FGFR) were both found to reduce Pseudomonas infection in a lung model through reducing bacterial load because intratracheal FGF was found to increase clearance of P. aeruginosa.25 Another study using an experimental burn model in human keratinocytes, also found that when FGF was added to P. aeruginosa in the presence of keratinocytes, bacterial growth was inhibited, and the same was observed when genetically modified keratinocytes were used.26 In addition, P. aeruginosa infection in a bioengineered skin model, in which FGF7 was expressed in diploid human keratinocytes, led to increased levels of antimicrobials β-defensin-2 and cathelicidin (LL-37) production and reduced bacterial load when compared with controls.27 The current study essentially agreed with these past studies in that absence of VIP or treatment with a VIP antagonist that disregulates GF and GFR contributed to earlier corneal perforation and/or increased bacterial load (tested only in antagonist treated mice). Previous studies from this laboratory also found that treatment using a GF mixture composed of EGF, FGF, and HGF increases antimicrobial peptides including murine beta-defensin-2 (mBD-2) and mBD3 expression, as well as decreases bacterial plate counts, resulting in less disease severity in the infected cornea.5 FGF also has been shown to promote wound healing in skin epithelium28 and to participate in lung epithelial repair.8 Because many cases of bacterial keratitis result directly or indirectly from disruption of the corneal epithelium,29 it also is possible that earlier perforation observed in VIP−/− mice may be due to reduced FGF levels with subsequent epithelial defect. HGFR mRNA and protein (immunostaining) also are decreased in the VIP−/− mice. HGFR recognizes HGF, which has anti-inflammatory properties, in that it can target vascular endothelial cells and disrupt nuclear factor-kappa B (NF-κB) signaling in these cells,30 critical to regulating inflammatory cytokines.
Overall, this study provided evidence that VIP is not required for GF or GFR expression in the normal cornea. However if the neuropeptide is endogenously absent or reduced through treatment, GF and GFR are disregulated, bacterial load is increased, and disease progression is enhanced.
Supplementary Material
Footnotes
Supported by NIH Grants R01EY016058, R01EY002986, and P30EY004068.
Disclosure: X. Jiang, None; S.A. McClellan, None; R.P. Barrett, None; Y. Zhang, None; M.E. Foldenauer, None; L.D. Hazlett, None
References
- 1.Wilhelmus KR. Review of clinical experience with microbial keratitis associated with contact lenses. CLAO J. 1987;13:211–214 [PubMed] [Google Scholar]
- 2.Hazlett LD. Corneal response to Pseudomonas aeruginosa infection. Prog Retin Eye Res. 2004;23:1–30 [DOI] [PubMed] [Google Scholar]
- 3.Hazlett LD, McClellan S, Kwon B, Barrett R. Increased severity of Pseudomonas aeruginosa corneal infection in strains of mice designated as Th1 versus Th2 responsive. Invest Ophthalmol Vis Sci. 2000;41:805–810 [PubMed] [Google Scholar]
- 4.Szliter EA, Lighvani S, Barrett RP, Hazlett LD. Vasoactive intestinal peptide balances pro- and anti-inflammatory cytokines in the Pseudomonas aeruginosa-infected cornea and protects against corneal perforation. J Immunol. 2007;178:1105–1114 [DOI] [PubMed] [Google Scholar]
- 5.Jiang X, McClellan SA, Barrett RP, Berger EA, Zhang Y, Hazlett LD. VIP and growth factors in the infected cornea. Invest Ophthalmol Vis Sci. 2011;52:6154–6161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jiang X, McClellan SA, Barrett RP, Zhang Y, Hazlett LD. Vasoactive intestinal peptide downregulates proinflammatory TLRs while upregulating anti-inflammatory TLRs in the infected cornea. J Immunol. 2012. In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Woodworth CD, Michael E, Marker D, Allen S, Smith L, Nees M. Inhibition of the epidermal growth factor receptor increases expression of genes that stimulate inflammation, apoptosis, and cell attachment. Mol Cancer Ther. 2005;4:650–658 [DOI] [PubMed] [Google Scholar]
- 8.Ware LB, Matthay MA. Keratinocyte and hepatocyte growth factors in the lung: roles in lung development, inflammation, and repair. Am J Physiol Lung Cell Mol Physiol. 2002;282:L924–L940 [DOI] [PubMed] [Google Scholar]
- 9.Huang X, Du W, McClellan SA, Barrett RP, Hazlett LD. TLR4 is required for host resistance in Pseudomonas aeruginosa keratitis. Invest Ophthalmol Vis Sci. 2006;47:4910–4916 [DOI] [PubMed] [Google Scholar]
- 10.Hazlett LD, Moon MM, Strejc M, Berk RS. Evidence for N-acetylmannosamine as an ocular receptor for P. aeruginosa adherence to scarified cornea. Invest Ophthalmol Vis Sci. 1987;28:1978–1985 [PubMed] [Google Scholar]
- 11.McClellan SA, Zhang Y, Barrett RP, Hazlett LD. Substance P promotes susceptibility to Pseudomonas aeruginosa keratitis in resistant mice: anti-inflammatory mediators downregulated. Invest Ophthalmol Vis Sci. 2008;49:1502–1511 [DOI] [PubMed] [Google Scholar]
- 12.Heid CA, Stevens J, Livak KJ, Williams PM. Real time quantitative PCR. Genome Res. 1996;6:986–994 [DOI] [PubMed] [Google Scholar]
- 13.Souza-Moreira L, Campos-Salinas J, Caro M, Gonzalez-Rey E. Neuropeptides as pleiotropic modulators of the immune response. Neuroendocrinology. 2011;94:89–100 [DOI] [PubMed] [Google Scholar]
- 14.Gressens P, Paindaveine B, Hill JM, Brenneman DE, Evrard P. Growth factor properties of VIP during early brain development. Whole embryo culture and in vivo studies. Ann N Y Acad Sci. 1997;814:152–160 [DOI] [PubMed] [Google Scholar]
- 15.Pozo D, Delgado M. The many faces of VIP in neuroimmunology: a cytokine rather a neuropeptide? FASEB J. 2004;18:1325–1334 [DOI] [PubMed] [Google Scholar]
- 16.Lelievre V, Favrais G, Abad C, et al. Gastrointestinal dysfunction in mice with a targeted mutation in the gene encoding vasoactive intestinal polypeptide: a model for the study of intestinal ileus and Hirschsprung's disease. Peptides. 2007;28:1688–1699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Servoss SJ, Lee SJ, Gibney G, Gozes I, Brenneman DE, Hill JM. IGF-I as a mediator of VIP/activity-dependent neurotrophic factor-stimulated embryonic growth. Endocrinology. 2001;142:3348–3353 [DOI] [PubMed] [Google Scholar]
- 18.Bertelsen LS, Barrett KE, Keely SJ. Gs protein-coupled receptor agonists induce transactivation of the epidermal growth factor receptor in T84 cells: implications for epithelial secretory responses. J Biol Chem. 2004;279:6271–6279 [DOI] [PubMed] [Google Scholar]
- 19.Couvineau A, Laburthe M. VPAC receptors: structure, molecular pharmacology and interaction with accessory proteins. Br J Pharmacol. 2012;166:42–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.MacKenzie CJ, Lutz EM, Johnson MS, Robertson DN, Holland PJ, Mitchell R. Mechanisms of phospholipase C activation by the vasoactive intestinal polypeptide/pituitary adenylate cyclase-activating polypeptide type 2 receptor. Endocrinology. 2001;142:1209–1217 [DOI] [PubMed] [Google Scholar]
- 21.Nishimoto M, Furuta A, Aoki S, Kudo Y, Miyakawa H, Wada K. PACAP/PAC1 autocrine system promotes proliferation and astrogenesis in neural progenitor cells. Glia. 2007;55:317–327 [DOI] [PubMed] [Google Scholar]
- 22.Chen MC, Lin H, Hsu FN, Huang PH, Lee GS, Wang PS. Involvement of cAMP in nerve growth factor-triggered p35/Cdk5 activation and differentiation in PC12 cells. Am J Physiol Cell Physiol. 2010;299:C516–527 [DOI] [PubMed] [Google Scholar]
- 23.Yun S, Hong WP, Choi JH, et al. Phospholipase C-epsilon augments epidermal growth factor-dependent cell growth by inhibiting epidermal growth factor receptor down-regulation. J Biol Chem. 2008;283:341–349 [DOI] [PubMed] [Google Scholar]
- 24.Choi HJ, Seo CH, Park SH, et al. Involvement of epidermal growth factor receptor-linked signaling responses in Pseudomonas fluorescens-infected alveolar epithelial cells. Infect Immun. 2011;79:1998–2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Viget NB, Guery BP, Ader F, et al. Keratinocyte growth factor protects against Pseudomonas aeruginosa-induced lung injury. Am J Physiol Lung Cell Mol Physiol. 2000;279:L1199–L1209 [DOI] [PubMed] [Google Scholar]
- 26.Sobral CS, Gragnani A, Morgan J, Ferreira LM. Inhibition of proliferation of Pseudomonas aeruginosa by KGF in an experimental burn model using human cultured keratinocytes. Burns. 2007;33:613–620 [DOI] [PubMed] [Google Scholar]
- 27.Erdag G, Medalie DA, Rakhorst H, Krueger GG, Morgan JR. FGF-7 expression enhances the performance of bioengineered skin. Mol Ther. 2004;10:76–85 [DOI] [PubMed] [Google Scholar]
- 28.Marchese C, Chedid M, Dirsch OR, et al. Modulation of keratinocyte growth factor and its receptor in reepithelializing human skin. J Exp Med. 1995;182:1369–1376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.O'Brien TP. Management of bacterial keratitis: beyond exorcism towards consideration of organism and host factors. Eye (Lond). 2003;17:957–974 [DOI] [PubMed] [Google Scholar]
- 30.Gong R, Rifai A, Dworkin LD. Anti-inflammatory effect of hepatocyte growth factor in chronic kidney disease: targeting the inflamed vascular endothelium. J Am Soc Nephrol. 2006;17:2464–2473 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.