Abstract
Cadmium is an environmental toxicant whose exposure is associated with multiple human pathologies. To prevent cadmium-induced toxicity, organisms produce a variety of detoxification molecules. In response to cadmium, the nematode Caenorhabditis elegans increases the steady-state levels of several hundred genes, including two metallothioneins, mtl-1 and mtl-2, and the cadmium-specific response gene, cdr-1. Despite the presumed importance in metal detoxification of mtl-1 and mtl-2, knockdown of their expression does not increase cadmium hypersensitivity, which suggests that these genes are not required for resistance to metal toxicity in C. elegans. To determine whether cdr-1 is critical in metal detoxification and compensates for the loss of mtl-1 and/or mtl-2, C. elegans strains were generated in which one, two, and all three genes were deleted, and the effects of cadmium on brood size, embryonic lethality, the Bag phenotype, and growth were determined. Growth at low cadmium concentrations was the only endpoint in which the triple mutant displayed more sensitivity than the single and double mutants. A lack of hypersensitivity in these strains suggests that other factors may be involved in the response to cadmium. Caenorhabditis elegans produces phytochelatins (PCs) that are critical in the defense against cadmium toxicity. PC levels in wild type, cdr-1 single, mtl-1, mtl-2 double, and triple mutants were measured. PC levels were constitutively higher in the mtl-1, mtl-2 double, and triple mutants compared with wild type. Following cadmium exposure, PC levels increased. The lack of cadmium hypersensitivity when these genes are deleted may be attributed to the compensatory effects of increases in PCs.
Key Words: Caenorhabditis elegans, metallothionein, cadmium, phytochelatin.
Humans are continuously exposed to the carcinogenic metal cadmium through various routes including diet and cigarette smoke. Cadmium enters cells and accumulates by binding to cytoplasmic and nuclear material (Beyersmann and Hechtenberg, 1997). At cytotoxic concentrations, cadmium exposure results in various cellular responses including cell death and cell transformation. At the molecular level, this metal inhibits DNA repair pathways, RNA synthesis, and protein synthesis. It also induces the expression of heat shock proteins and affects cell cycle progression, proliferation, and differentiation (Beyersmann and Hechtenberg, 1997; Sirover and Loeb, 1976).
The nematode Caenorhabditis elegans has proven to be a useful model to further understand the toxicological effects of cadmium exposure in vivo. In C. elegans, cadmium exposure results in a concentration-dependent decrease in growth and delay in egg laying (Popham and Webster, 1979). Cadmium also affects feeding and movement, known endpoints associated with neurotoxicity in C. elegans (Boyd et al., 2010; Haq et al., 2003). In addition, toxicogenomic analysis shows that a number of genes are upregulated in response to cadmium, including two metallothionein (MT) genes, mtl-1 and mtl-2, and the cadmium-responsive gene, cdr-1 (Cui et al., 2007; Leung et al., 2008).
MTs are small cysteine-rich metal-binding proteins that function in the detoxification of cadmium, as well as other metals, and metal homeostasis (Klaassen et al., 1999). In C. elegans, two MT genes, mtl-1 and mtl-2, were identified and found to be expressed in the intestines after exposure to various metals and heat shock (Cioci et al., 2000; Freedman et al., 1993; Slice et al., 1990). In contrast to mammalian cells and transgenic mice (Lazo et al., 1995; Masters et al., 1994), C. elegans strains null for MT were not hypersensitive to cadmium. Swain et al. (2004) reported that low concentrations of cadmium (< 50µM) did not affect C. elegans strains that were fed double-stranded RNA (dsRNA) for either mtl-1 or mtl-2 when testing for brood size, nematode length (a measure of growth), generation time, and lifespan; however, higher concentrations (> 75µM) induced metal hypersensitivity compared with the wild type strain. Knocking out both genes did not significantly affect brood size, volumetric growth, or lifespan when compared with the single mutants at low cadmium concentrations (Hughes and Sturzenbaum, 2007), and only a marked difference from wild type was observed at high cadmium concentrations (Swain et al., 2004). In various studies by other investigators, sensitivity to cadmium was not observed in single or double mtl-1 or mtl-2 mutants, which suggested that other genes or mechanisms, such as the transsulfuration pathway, may compensate for the loss of MT function to protect C. elegans against cadmium toxicity (Hughes and Sturzenbaum, 2007; Hughes et al., 2009).
In addition to MTs, C. elegans has another cadmium- responsive gene, cdr-1, which provides resistance to cadmium toxicity (Liao and Freedman, 1998). cdr-1 expression was observed in intestinal cells in response to cadmium, similar to MTs. Unlike the MTs, however, the only stressor found to induce cdr-1 transcription was cadmium (Liao et al., 2002). Exposure of nematodes fed cdr-1 dsRNA to cadmium resulted in small, sterile nematodes that failed to develop into adulthood, indicating the importance of CDR-1 in mediating resistance to cadmium toxicity (Cui et al., 2007; Liao et al., 2002).
The reported lack of hypersensitivity of the mtl-1, mtl-2 double mutant to cadmium suggests that other factors are important in protecting the nematode from cadmium toxicity. To determine whether CDR-1 is a component in ameliorating cadmium toxicity, single, double, and triple mutants were constructed and tested for hypersensitivity to cadmium exposure. Endpoints tested included brood size, embryonic lethality, percentage displaying the Bag (bag of worms) phenotype, and growth. These endpoints were monitored in response to cadmium and other stressors such as temperature. In addition to determining the roles of these three cadmium-inducible genes in ameliorating cadmium toxicity, the contribution of phytochelatins (PCs) was explored. PCs are sulfhydryl-rich polymers of two or more glutathione (GSH) (PC2–PCn). C. elegans PCs and their cognate biosynthesizing enzymes have been shown to be essential in the resistance to cadmium toxicity (Vatamaniuk et al., 2001).
MATERIALS AND METHODS
Strains. The following strains were used: N2 Bristol wild type, JF97 mtl-1(tm1770), VC128 mtl-2(gk125), JF27 cdr-1(tm723), and VF2 pcs-1(tm1748). Each mutant strain was outcrossed three times to the N2 Bristol wild type strain. Mutants were selected using genotyping PCR (see below). All strains were maintained on nematode growth medium (NGM) agar plates containing OP50 Escherichia coli at 20°C (Sulston and Hodgkin, 1988), unless otherwise indicated.
Construction of double and triple mutants. To create mtl-1, mtl-2, and cdr-1 double and triple mutants, recombination events were selected from crosses involving mtl-1(tm1770), mtl-2(gk125), and cdr-1(tm723). To create the double mutants, mtl-1 males were crossed with mtl-2 or cdr-1 hermaphrodites, and cdr-1 males were crossed with mtl-2 hermaphrodites. To create the triple mutant, mtl-2, cdr-1 hermaphrodites were crossed with mtl-1 males, and mtl-1, mtl-2 hermaphrodites were crossed with cdr-1 males. From each cross, one F2 L4 larva was placed on an NGM agar plate and 12–20 F2 lines were isolated for each cross. Genomic DNA was isolated from F3 progeny using single worm lysis buffer (50mM KCl, 10mM Tris-HCl, pH 8.3, 2.5mM MgCl2, 0.45% NP-40, 0.45% Tween 20, and 0.05 µg/µl proteinase K) and incubated for 1h at 65ºC followed by a 20-min incubation at 95ºC. PCR was performed to identify recombinants that were homozygous for the deletion mutations. Products generated using gene-specific primers are presented in Supplementary table 1. Three independent PCR reactions were performed for each line to confirm the presence of the deletion. This PCR assessment was repeated throughout the experiments to ensure that all lines had the correct genotype.
Brood size analysis assay. One L4 hermaphrodite was placed on an NGM agar plate. Adults were transferred every 24h for 3 days. Larva and dead embryos were counted on each plate 24h after removal of the adult. To test the effect of temperature on brood size, plates were placed at 20°C, 25°C, and 27°C for the duration of the experiment. For heat shock, L4 larvae were placed at 33°C for 2h, removed, and then placed at 20°C till the end of the experiment. For brood size analysis in response to cadmium, 100mM CdCl2 dissolved in K medium (32mM KCl and 51mM NaCl) (Williams and Dusenbery, 1990) was added to NGM agar plates to a final plate concentration of 50, 75, and 100µM. Preliminary studies showed no noticeable difference between exposures on K agar plates versus NGM agar plates. The cadmium was allowed to dry for 24h, and the plates were then seeded with OP50 E. coli. One L4 hermaphrodite was placed on a plate and allowed to grow at 20°C. Adults were transferred every 1.5 days due to the slower egg-laying rate in cadmium-exposed animals. Larva and dead embryos were counted on each plate 24h after removal of the adult. For all conditions, total brood size refers to the number of larvae counted over the length of the experiment. Percent embryonic lethality was calculated by dividing the number of dead embryos by the total brood size (dead embryos and larva) multiplied by 100. For all conditions, n = 12 (three independent experiments).
Statistical analysis of brood size was accomplished by first determining whether brood size and embryonic lethality were normally distributed. Brood size was normally distributed and was therefore analyzed using parametric statistical methods. Analysis of variance (ANOVA) followed by Dunnett’s test at α = 0.05 was used to compare the single, double, and triple mutant strains with the wild type. Two-sample t-tests were used to compare brood sizes of single mutants with double mutants, single mutants with the triple mutant, and double mutants with the triple mutant.
Percent embryonic lethality was not normally distributed and could not be normalized with logarithm, square root, or arcsine transformations; therefore, nonparametric statistical methods were used. Kruskal-Wallis ANOVA was used to test the equality of percent embryonic lethality across all mutants, followed by Mann-Whitney tests to compare pairs of mutants, including comparisons of each mutant with the wild type, single mutants with double mutants, single mutants with the triple mutant, and double mutants with the triple mutant. Separate analyses were conducted for each temperature and cadmium concentration.
Assay for the bag phenotype. The bag phenotype is characterized by the buildup of embryos in a hermaphrodite due to its inability to release embryos. Twenty-five L4 hermaphrodites were placed on an NGM agar plate containing cadmium at various concentrations and incubated at 20°C. After 48h, adults were transferred to fresh plates, to be removed from their progeny, and subsequently transferred every 24h for three more days. During each transfer, any nematodes displaying the Bag phenotype were noted and removed from the experiment. On day 6, the number of living adults was determined. For each condition, five plates were tested.
Growth analysis assay. The Complex Object Parametric Analyzer and Sorter (COPAS) Biosort was used to determine the effects of cadmium on growth as previously described (Boyd et al., 2010). Mutant strains were synchronized using the alkaline hypochlorite preparation as previously described (Khanna et al., 1997). Briefly, gravid adults were incubated in 1% Clorox containing 250µM NaOH. Embryos were collected and washed with K medium. They were then placed in K-plus medium (K medium plus 5 µg/ml cholesterol, 3mM CaCl2, and 3mM MgSO4) (Williams and Dusenbery, 1990) and allowed to hatch overnight to synchronize at the L1 larval stage.
Using the COPAS Biosort, 50 L1 larvae were placed in each well of a 96-well microtiter plate. Each well contained K-plus medium, OP50 E. coli, and cadmium at the indicated concentrations. Nematodes were then incubated at 20°C for 48h. Visual inspection of the animals was made, and the COPAS Biosort was then used to determine the time of flight, which is a measure of the length of the nematode, and extinction, which is a measure of the optical density of the nematode. Both these measurements correspond to the developmental stage of the nematode. Under control conditions, wild type L1 larvae developed to the L4 stage in 48h. Three independent experiments (n ~ 200 L1/experiment) were conducted at all concentrations.
Statistical analysis of growth was accomplished as previously described (Boyd et al., 2010). Dunn’s multiple testing procedure (Cardillo, 2006) was used to compare mutant strains with the wild type at each cadmium concentration. Dunn’s test is nonparametric, not requiring normally distributed data, and sets an overall significance level of 0.05 for all comparisons to a given reference group. After comparing all mutant strains with wild type, each double mutant and the triple mutant were compared with their component single mutants. For example, the double mutant, mtl-2, cdr-1, was compared with the single mutants mtl-2 and cdr-1.
Detection of PCs using liquid chromatography-mass spectrometry. Age-synchronized L1 nematodes were placed on NGM agar plates containing 0 or 12µM CdCl2 and incubated at 20°C for 48h. Nematodes were then collected by centrifugation and rinsed twice with K medium to remove all bacteria from the gut of the nematodes. Final nematode pellets (~400 µl) were transferred to 2.5-ml tubes, immediately frozen, and stored at −80°C until use.
To prepare extracts for liquid chromatography-mass spectrometry (LC/MS) analysis, frozen pellets were thawed at 4°C and 1ml of 0.6mM tris(2-carboxyethyl)phosphine hydrochloride in 100% methanol (Sigma-Aldrich, St Louis, MO) was added and mixtures were homogenized using a Mini-Bead Beater (Biospec Products, Bartlesville, OK). Homogenates were centrifuged and supernatants were then transferred to clean tubes containing 60 µl of freshly prepared 266mM N-ethylmaleimide (NEM) (Sigma-Aldrich). Supernatants and pellets were stored at −80°C until use.
Levels of GSH, PC2, and PC3 were determined in derivatized C. elegans supernatants using an Agilent Technologies 6224 TOF LC/MS equipped with an Ascentis 0.2 × 50mm 2.7µM C-18 column (Supelco, Sigma-Aldrich). Compounds were separated with a 0–90% linear, acetonitrile gradient over 26min. Data were analyzed using Agilent Mass Hunter Qualitative Analysis Software, Version B.03.01 (Agilent Technologies, Santa Clara, CA). To determine the exact masses and retention times of GSH-NEM, PC2-NEM, and PC3-NEM, NEM derivatives of GSH, PC2, and PC3 standards were prepared and analyzed as described earlier. Reduced and oxidized GSH treated with tris(2-carboxyethyl)phosphine hydrochloride or NEM gave identical chromatographic and spectral features. Extracted ion chromatograms for the exact masses of GSH-NEM, PC2-NEM, and PC3-NEM were integrated and compared with protein concentration (see below). Several independent experiments were analyzed by normalizing data for each experiment to PC2-NEM extracted ion chromatogram integrated peak area observed in wild type C. elegans.
Soluble protein was prepared by adding 0.9mM NaOH to the insoluble pellet and heating the mixture at 80°C for 1h with occasional shaking. The solution was then cooled and centrifuged, and the protein concentration was determined using the Bradford protein quantification assay following the manufacturer’s instructions (Bio-Rad Laboratories, Hercules, CA). To estimate a dilution factor from median measured protein absorbance for each strain and repetition of the experiment, second-degree polynomials were fit to the protein concentration data (Olson and Markwell, 2007). These dilutions were subsequently used as multiplicative scaling factors for median peak heights measured using LC/MS. For each strain and cadmium treatment, the base-10 logarithm of the product of the dilution factor and peak height was used for statistical analyses. These normalized data are referred to as the “scaled peak height.” To detect differences between median scaled peak heights for different strains and treatments for PC2 and PC3, Kruskal-Wallis rank sum tests were performed. Pairwise differences were then assessed using Mann-Whitney nonparametric two-sample tests. One-sided Mann-Whitney tests were used to assess cadmium treatment effects; two-sided tests were used to assess strain effects within untreated and within cadmium-treated nematodes.
RESULTS
Construction of mtl-1, mtl-2, and cdr-1 Doubleand Triple Mutants
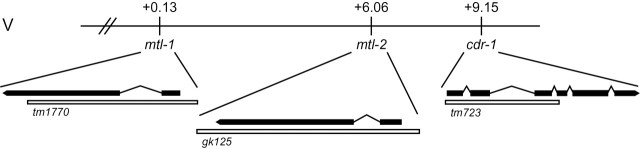
To test whether mtl-1, mtl-2, and cdr-1 have redundant functions in the nematode’s response to cadmium, a triple mutant was created. Individual C. elegans strains containing a deletion in one of each of the genes were obtained: mtl-1(tm1770), mtl-2(gk125), and cdr-1(tm723). Genetic crosses were performed to create double mutants and a triple mutant. Because the three genes were located on chromosome V, mtl-1 at 0.13 cM, mtl-2 at 6.06 cM, and cdr-1 at 9.15 cM, recombination events were selected (Fig. 1). PCR was used to genotype the strains to ensure that all strains were homozygous for their respective deletions. Mutant strains carrying deletions in two of each of the genes were generated for all three combinations: mtl-1(tm1770), mtl-2(gk125) [JF81]; mtl-1(tm1770), cdr-1(tm723) [JF82]; and mtl-2(gk125), cdr-1(tm723) [JF83]. In addition, a triple-mutant strain carrying a deletion in all three genes was generated, mtl-1(tm1770), mtl-2(gk125), cdr-1(tm723) [JF84].
FIG. 1.
Location of mtl-1, mtl-2, and cdr-1 near the center of chromosome V. A schematic diagram of the location of the three genes on the chromosome and their gene structure is presented. Solid rectangles and lines represent exons and introns, respectively. The locations of the deletions in each mutant line, namely tm1770 in mtl-1, gk125 in mtl-2, and tm723 in cdr-1, are represented by white rectangles.
Mutant Strains Displayed a Wild Type Phenotype at 20°C
When grown under control conditions at 20°C, wild type C. elegans had a brood size of 310.8±9.5. Single mtl-1, mtl-2, and cdr-1 mutants displayed a similar brood size as wild type, 299.8±12.7, 284.3±12.7, and 290.6±8, respectively (Supplementary table 2). Although mtl-1 had a brood size similar to that of wild type, it had a higher percentage of embryonic lethality: 3.4% ± 2 compared with 0.8% ± 0.2 for wild type (Supplementary table 2). The mtl-1, mtl-2 double mutant was the only strain that displayed a decrease in brood size, which was significantly different from that of wild type (237.5±24.3; p < 0.01). This double mutant also had a marked increase in embryonic lethality, 4.6% ± 2. The other double-mutant strains, mtl-1, cdr-1 and mtl-2, cdr-1, had brood sizes similar to those of wild type, but an increase in embryonic lethality similar to mtl-1 (Supplementary table 2). Brood size and embryonic lethality of the triple mutant were not significantly different from those of wild type, 274.9±7.7 and 1.3% ± 0.26, respectively.
Although all mutants, except the mtl-1, mtl-2 double mutant, displayed brood sizes similar to those of wild type, they displayed increases in embryonic lethality. A high incidence of males was not observed in any of the mutant strains, suggesting that the observed increase in embryonic lethality was not the result of unrepaired DNA damage or nondisjunction of the chromosomes. Interestingly, a high number and an earlier onset of unfertilized embryos (oocytes) were observed in all mutant strains compared with wild type (data not shown). These observations suggest some type of defect in the germ line of the mutants (McCarter et al., 1999).
Mutant Strains Were Affected by High Temperatures
In C. elegans, MTL-1 and MTL-2 expression is induced by multiple stressors including heat shock (Cioci et al., 2000; Freedman et al., 1993), whereas CDR-1 is only induced by cadmium (Liao et al., 2002). Based on these observations, the effects of heat on brood size and embryonic lethality were tested to determine whether the mutant strains were hypersensitive to this stressor. After heat shock, none of the mutants displayed a significant change in brood size compared with wild type. There was, however, an increase in embryonic lethality for all mutant strains (data not shown). Because heat shock at 33°C occurred for a short period of time and nematodes were allowed to recover, it is not surprising that a difference in brood size was not observed. To better test whether heat affected the mutants, brood size and embryonic lethality were assayed when strains were maintained at 25°C and 27°C. For all strains, including wild type, there was a slight drop (12% on average) in brood size and an increase (1.6% on average) in embryonic lethality at 25°C and a larger drop (76% on average) in brood size and a large increase (35.6% on average) in embryonic lethality at 27°C (Fig. 2). The only significant decreases in brood size, compared with the wild type strain, were seen in the mtl-1, mtl-2 double mutant at 25°C and cdr-1 at 27°C (p < 0.01 and p < 0.05, respectively) (Fig. 2A). In addition, the mtl-1, mtl-2 double mutant had a significantly smaller brood size compared with mtl-1 at both 25°C and 27°C and mtl-2 at 25°C (p < 0.05, Supplementary table 3). mtl-1 and the mtl-1, mtl-2 and mtl-1, cdr-1 double mutants displayed a significant increase in embryonic lethality compared with wild type at 25°C (Fig. 2B). At 27°C all mutant lines, except mtl-1, displayed an increase in embryonic lethality compared with wild type (Fig. 2B). The triple mutant had a brood size similar to that of the other mutants and wild type at both 25°C and 27°C. Because MTL-1 and MTL-2 are expressed in response to heat shock and CDR-1 is not expressed in response to temperature (Liao et al., 2002), it is not surprising that the mtl-1, mtl-2 double mutant is most affected by temperature.
FIG. 2.
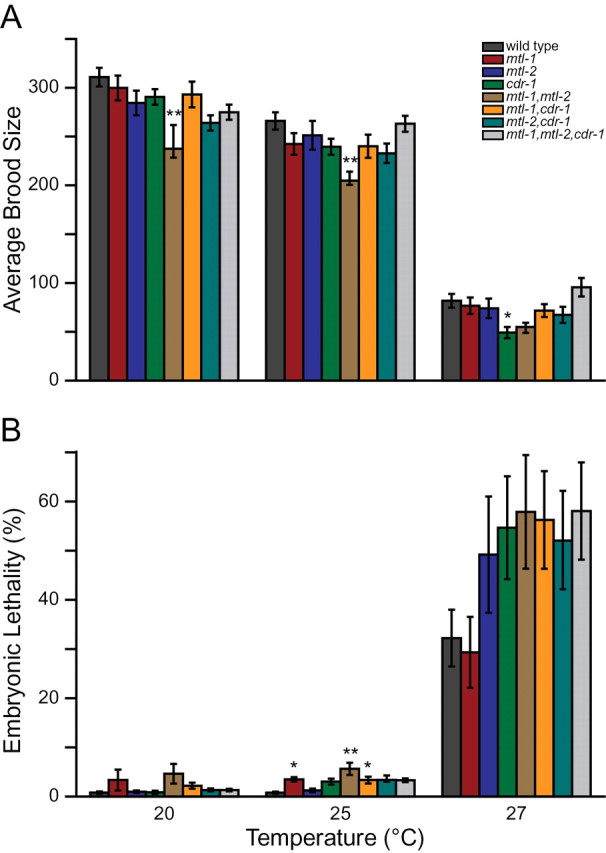
Effect of temperature on brood size and embryonic lethality. Brood size (A) and embryonic lethality (B) were analyzed at 20°C, 25°C, and 27°C. Means ± standard error for 12 replicates are represented. p value comparisons between mutant strains and wild type were determined using variance and Dunnett’s test. * and ** indicate p values of p < 0.05 and p < 0.01 compared with wild type, respectively.
Effects of Cadmium Exposure on Brood Size and Embryonic Lethality
Brood size and embryonic lethality were analyzed in response to cadmium exposure in the mutant strains. A slower egg-laying rate has been observed in hermaphrodites exposed to cadmium, which may be due to the neurotoxic effects of cadmium (Popham and Webster, 1979; Swain et al., 2004). To compensate for the slower egg-laying rate and ensure all progeny were counted, brood size measurements were made over a longer period of time than that used in the temperature assay. Brood sizes at 50 and 75µM cadmium were reduced at a similar level in all strains, including wild type (Fig. 3A). At 100µM cadmium, all mutants displayed a decrease in brood size greater than wild type, but only mtl-1 was significantly different from wild type (p < 0.05). In addition, there was no difference among the mutant strains (Supplementary table 3). Embryonic lethality was not affected by cadmium and, at all concentrations tested, was similar to that of untreated nematodes (Fig. 3B, Supplementary table 4).
FIG. 3.
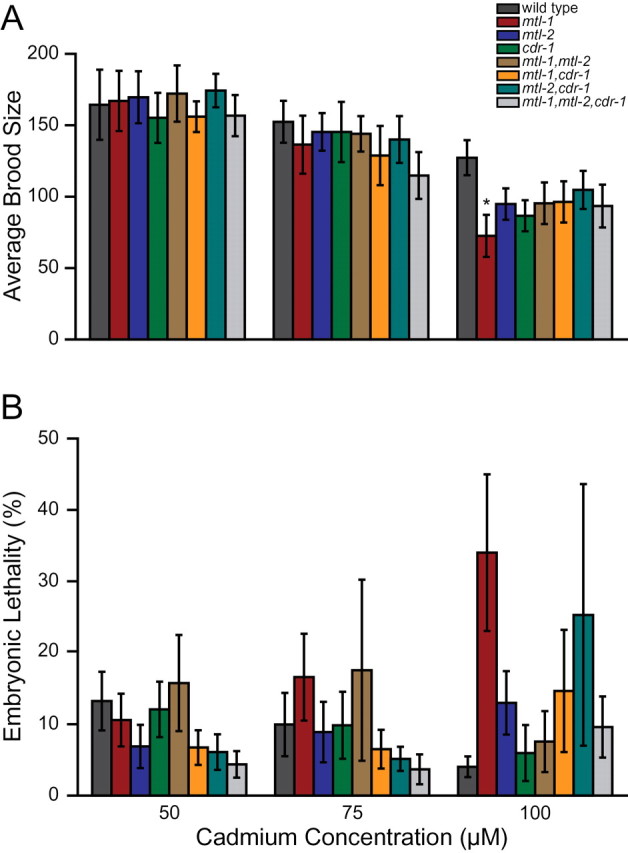
Effect of cadmium on brood size and embryonic lethality. Brood size (A) and embryonic lethality (B) were analyzed at 50, 75, and 100µM cadmium. Means ± standard error for 12 replicates is represented. p value comparisons between mutants and wild type were determined using variance and Dunnett’s test. * indicates a p value of p < 0.05 compared with wild type.
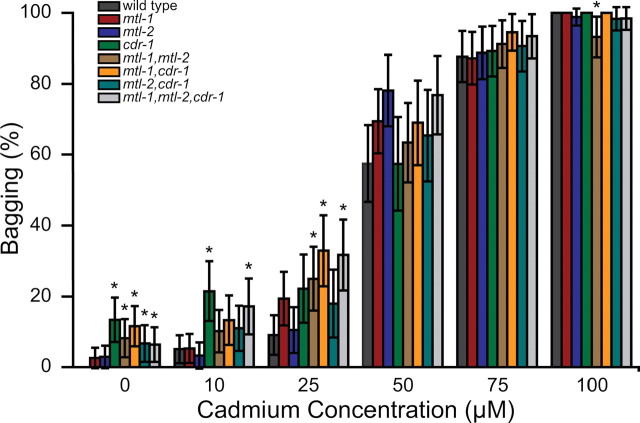
During a continual exposure to cadmium, hermaphrodites begin to develop a lethal phenotype known as Bag (Bagof worms). This phenotype occurs when the vulva muscles do not function properly, resulting in the inability of the hermaphrodite to lay embryos. The hermaphrodite will continue to produce fertilized embryos that accumulate and eventually hatch inside the adult. Over half of the wild type hermaphrodites displayed a Bag phenotype when exposed to 50µM cadmium and 100% when exposed to 100µM cadmium (Fig. 4). At 10µM cadmium, 5.1% of wild type hermaphrodites had the Bag phenotype. Similar responses were observed for mtl-1 and mtl-2 (5.3 and 3.3%, respectively). At this concentration, cdr-1 and the triple mutant had a Bag phenotype percentage of 21.5 and 17.2%, respectively, which were significantly different from wild type (p < 0.05, Fig. 4). At 25µM cadmium, the triple mutant as well as the mtl-1, mtl-2 and mtl-1, cdr-1 double mutants (31.7, 25 and 32.9%, respectively) were significantly different compared with wild type (9.1%). At concentrations above 50µM cadmium, wild type nematodes were significantly affected and there were no differences between the wild type and mutants strains (Fig. 4). The Bag phenotype was observed at a higher percentage at lower cadmium concentrations in the double and triple mutants, suggesting this phenotype is more sensitive to cadmium toxicity.
FIG. 4.
Effect of cadmium on egg laying. Graph represents the percentage of nematodes with the Bag phenotype in response to various cadmium concentrations. Mean ± 95% confidence interval. n ~ 100 for each strain and condition. * indicates a p value of p < 0.05 compared with wild type.
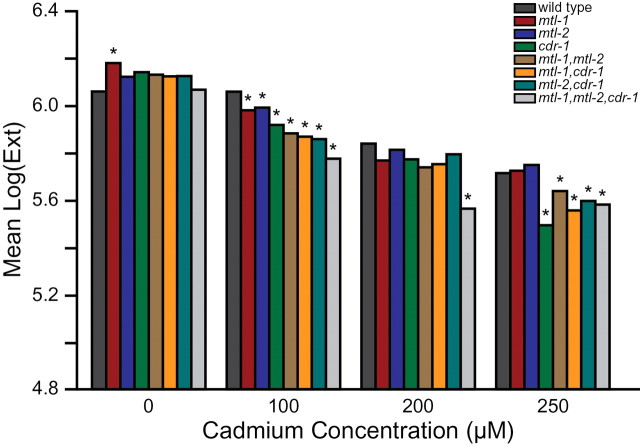
Effects of Cadmium Exposure on Growth Rates
To measure growth rate, L1 larvae were exposed to cadmium for 48h after which the effect of the metal was visually determined, and the size distribution of the nematode population was measured using the COPAS Biosort. Growth of wild type larvae was inhibited in a concentration-dependent manner in response to cadmium (Fig. 5). Wild type larvae began to display effects of cadmium at 200µM, and young L4 larvae were paralyzed (observation, data not shown). At 250µM cadmium, nematode growth was arrested at the L3 larval stage, and paralysis was observed. At 300µM cadmium, growth of wild type nematodes was arrested at the L1/L2 larval stage and were paralyzed or dead (data not shown). The single, double, and triple mutants began to display significant growth effects due to cadmium exposure at 100µM compared with wild type (p < 0.05, Fig. 5). The triple mutant was the most affected and significantly different compared with the single mutants (p < 0.05, Supplementary table 5). The mtl-1, mtl-2 double mutant was also significantly different from the single mutants (p < 0.05, Supplementary table 5). At 200µM cadmium, wild type and mutant nematodes were affected compared with untreated; only the triple mutant was significantly different from wild type (p < 0.05, Fig. 5) and was also different from the single mutants (p < 0.05, Supplementary table 5). At 250µM cadmium, all the mutants, except mtl-1 and mtl-2, were significantly different from the wild type strain, and the double and triple mutants were significantly different from mtl-1 and mtl-2. In response to cadmium, growth of the triple mutant was more affected compared with that of wild type and the single and double mutants.
FIG. 5.
Effect of cadmium on growth. Growth in response to cadmium was determined using the COPAS Biosort. Graph displays medians for one representative experiment, which was in agreement with two other replicates.* indicates a p value of p < 0.05 compared with wild type.
Effects of Cadmium Exposure on PC Levels
The lack of hypersensitivity of the single, double, and triple mutants suggests other mechanisms and/or pathways may be key players in the cadmium response. To test whether the transsulfuration pathway resulting in PC synthesis could compensate for the absence of the MT genes and cdr-1, PC concentrations in wild type and mutant strains in the presence or absence of cadmium were determined using high-resolution LC/MS. In the absence of cadmium, GSH and PC2, but not PC3, were detected in wild type nematodes. Cadmium-exposed wild type nematodes showed a significant increase in PC2 (p < 0.05, Supplementary table 6) and detectable levels of PC3 (Fig. 6), but no significant changes in GSH levels (results not shown). In the presence of cadmium, the levels (i.e., median scaled peak heights) for PC2 and PC3 were similar for the wild type, double, and triple mutants, as did the triple mutant and the other three strains. However, cdr-1 showed a statistically significant (p < 0.05) decrease in scaled peak height (approximately 0.5) for both PC2 and PC3 in response to cadmium relative to the other stains (Fig. 6 and Supplementary tables 6 and 7). Similar to wild type, cdr-1 did not contain detectable levels of PC3 in the absence of metal. In contrast, the mtl-1, mtl-2 double mutant and the triple mutant contained measurable levels of PC3 in the absence of cadmium (Fig. 6). The higher constitutive levels of PC3 in these strains suggest that they have excess metal, which could result in higher phytochelatin synthase (PCS) activity. Interestingly, PC levels in cdr-1 were significantly lower than those of wild type, the mtl-1, mtl-2 double mutant after cadmium treatment, and the double and triple mutant under control conditions (p < 0.05, Supplementary table 6). The triple mutant displayed levels of PC2 and PC3 similar to that in the double mutant under all conditions and cdr-1 after cadmium treatment, suggesting that CDR-1 does not compensate for the loss of MTL-1 and MTL-2 in response to metal exposure. It should be noted that E. coli do not produce PCs and therefore changes in PC2 and PC3 levels are limited to effects in C. elegans.
FIG. 6.
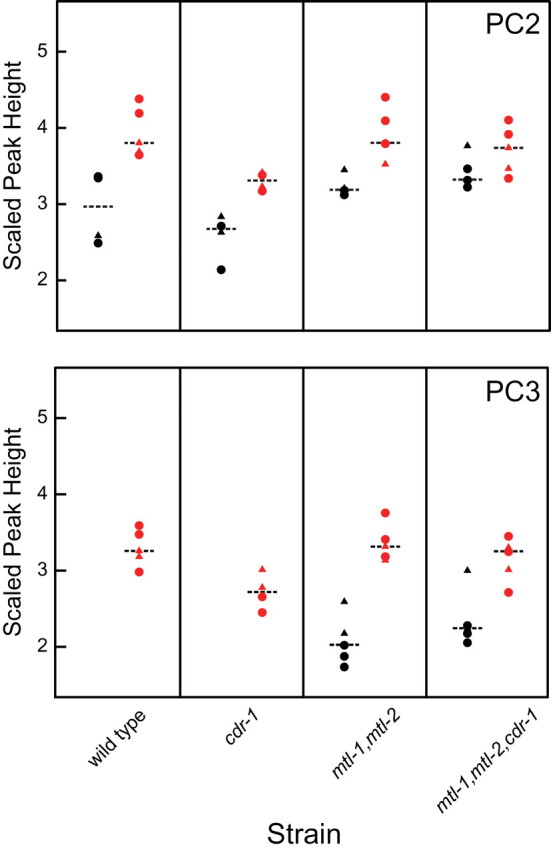
Levels of PCs in response to cadmium treatment. Scaled median peak heights for PC2 (upper panel) and PC3 (lower panel) in wild type, cdr-1, mtl-1, mtl-2 double mutant, and the triple mutant with (gray symbols) and without (black symbols) cadmium. Triangle and circles represent measurements made on different days; horizontal dotted line indicates median of measurements over the 2 days.
DISCUSSION
To determine the roles of MTL-1, MTL-2, and CDR-1 in mediating resistance to cadmium toxicity, several toxicological endpoints were assessed using single, double, and triple mutants. Although MTL-1 and MTL-2 may have redundant functions, the mtl-1, mtl-2 double mutant was not more sensitive to cadmium than the single mutants when testing brood size and embryonic lethality, percentage with a Bag phenotype, and growth (Figs. 3, 4, and 5, respectively). These findings contrast with what has been reported for mammalian MTs, where mammalian cells lacking MT and MT null mice are hypersensitive to cadmium (Lazo et al., 1995; Masters et al., 1994). However, this is consistent with earlier C. elegans studies showing a lack of hypersensitivity when knocking out MTs by either RNAi or mutation (Hughes and Sturzenbaum, 2007; Swain et al., 2004).
CDR-1 may be involved in the detoxification of cadmium through a pathway involving MT, but the data presented in this report suggest that other factors compensate for this loss. It was reported that cdr-1 knockdown using RNAi resulted in severe growth retardation and sick nematodes in response to cadmium (Liao et al., 2002). The results in this current study show that the deletion mutant, tm723, although sensitive, does not display the previously reported level of sensitivity to cadmium. When measuring brood size and embryonic lethality, the cdr-1 mutant was not hypersensitive (Fig. 3); however, the mutant displayed cadmium hypersensitivity when the Bag phenotype and growth were assayed (Figs. 4 and 5). This difference in sensitivity between RNAi-treated nematodes and the deletion mutant may be a result of cdr-1 RNAi affecting other cdr transcripts. C. elegans expresses six cdr-1 homologues. The nucleotide and amino acid sequence of cdr-1 are highly similar to those of the other cdr genes (Dong et al., 2005, 2008). Similar to cdr-1, the steady-state mRNA levels of cdr-2, cdr-3, and cdr-4 increase in response to cadmium, thus knocking down multiple CDR isoforms may be necessary to produce cadmium hypersensitivity. In this current study, a cdr-1-specific deletion mutant was used and was found to be affected by cadmium, but not significantly different from that of wild type nematodes.
The limited hypersensitivity in the triple mutant suggests that CDR-1 does not compensate for the loss of MTs and that other stress response genes may be involved or are more essential to this response. Various stress response genes are also induced by cadmium, and it may be the case that these other genes are upregulated in the double and triple mutants, compensating for the loss of MTL-1, MTL-2, and/or CDR-1. Microarray analysis identified 37 genes that were significantly upregulated following a 4-h exposure to cadmium in wild type C. elegans (Cui et al., 2007). Most significantly upregulated were cdr-1, mtl-2, and mtl-1, but various cytochrome P450 genes, glutathione S-transferases, and a number of genes with unknown functions were also significantly upregulated. Following a 24-h cadmium exposure, the expression of heat shock proteins and many other genes was upregulated. Roh et al. also examined genes that were upregulated after 24-h exposure to cadmium and found various stress response genes including cytochrome P450, glutathione S-transferases, an inhibitory p53-interacting protein, copper/zinc superoxide dismutase, and a catalase involved in the protection of reactive oxygen species (Roh et al., 2006). When tested by RNAi, many of these upregulated genes also conferred hypersensitivity to cadmium when measuring growth (Cui et al., 2007). Interestingly, RNAi of the stress-responsive genes, age-1 and daf-2, involved in the insulin signaling pathway, resulted in resistance to cadmium (Barsyte et al., 2001). Analysis of transcriptional levels in response to cadmium of various stress response genes in the single, double, and triple mutants could provide insights as to why these lines are not as hypersensitive to cadmium as one might expect.
GSH and PCs are sulfhydryl-containing cellular components whose expression is affected by cadmium exposure (Hughes et al., 2009; Vatamaniuk et al., 2001). Although PCs are normally found in plants and yeast, a homolog for PCS, pcs-1, the enzyme required to convert GSH to PCs, was identified in C. elegans (Vatamaniuk et al., 2002). C. elegans are hypersensitive to low concentrations of cadmium when pcs-1 is knocked down by RNAi (Vatamaniuk et al., 2001). The pcs-1(tm1748) deletion mutant produces no viable offspring when exposed to 12µM cadmium (Hughes et al., 2009), a much lower concentration than that needed to affect brood size in the mtl-1 and mtl-2 single and double mutants. At 5µM cadmium, RNAi of pcs-1 results in a Bag phenotype (Vatamaniuk et al., 2001). This phenotype occurs at a lower concentration of cadmium than those found for the mtl-1, mtl-2, and cdr-1 single, double, and triple mutants, for which the Bag phenotype is prevalent at 25µM (Fig. 4). In addition, a mtl-1(tm1770), mtl-2(gk125), pcs-1(tm1748) triple mutant was more sensitive to cadmium and did not produce any viable offspring at 0.5µM, suggesting that the MTs do play a role, most likely different from PCS-1, in cadmium detoxification (Hughes et al., 2009). In the current study, PC levels were assessed in the cdr-1 single mutant, mtl-1, mtl-2 double mutant, and mtl-1, mtl-2, cdr-1 triple mutant in both the presence and absence of cadmium. In the absence of added metal, PC3 was at an undetectable level in wild type and cdr-1, but constitutive levels of PC3 were observed in the mtl-1, mtl-2 double mutant and the mtl-1, mtl-2, cdr-1 triple mutant (Fig. 6). These results suggest that PCs compensate for the loss of mtl-1 and mtl-2 in metal detoxification. This observation is similar to that previously observed by Hughes et al. who monitored metabolomics changes in C. elegans associated with cadmium exposure (Hughes et al., 2009). In addition, endogenous PC levels in cdr-1 are similar to those of wild type, and levels in the triple mutant are the same as the mtl-1, mtl-2 double mutant in both the absence and presence of cadmium, suggesting that CDR-1 acts independently of PCS-1.
Analysis of the mtl-1, mtl-2, and cdr-1 single, double, and triple mutants suggests that the cellular response to cadmium exposure involves more than transcriptional upregulation of these cadmium-responsive genes. These results support a model where MTs are important for metal homeostasis, but not critical for the defense against cadmium toxicity. PCs, however, play a major role in the defense against cadmium toxicity in vivo. The role of sulfhydryl-containing compounds in the defense against cadmium toxicity has been suggested in studies using mammalian cells. In several cell lines (A549-T27, V79) and rodents, depletion of GSH made the organisms hypersensitive to cadmium (Kang and Enger, 1988; Ochi et al., 1988; Singhal et al., 1987). It has been suggested that GSH acts as the initial intracellular metal chelator that then shuttles metals to MT for long-term storage (Freedman et al., 1989). Thus, low-molecular-weight sulfhydryl-containing molecules may serve roles as the major defenders against cadmium toxicity.
Here we report that the three genes, mtl-1, mtl-2, and cdr-1, which yield the greatest increases in their steady-state mRNA levels following cadmium exposure, are not essential for resistance to cadmium toxicity. The lack of a large response to cadmium exposure when one or more of these genes is deleted may attribute to the compensatory effects of increased levels of PCs.
SUPPLEMENTARY DATA
Supplementary data are available online at http://toxsci.oxfordjournals.org/.
FUNDING
Nematode strains used in this work were provided by the Caenorhabditis Genetics Center, which is funded by the National Institutes of Health National Center for Research Resources. This research was supported (in part) by the Intramural Research Program of the National Institutes of Health, National Institute of Environmental Health Sciences (Z01ES102045 and Z01ES102046).
ACKNOWLEDGMENTS
The authors would like to thank the following people for their assistance with this manuscript: Julie Rice and Windy Boyd (Biomolecular Screening Branch NTP) for growth experiments using the COPAS Biosort; Marjolein Smith and Sandra McBride (SRA International) and Grace Kissling (Biostatistics Branch, NIEHS) for the statistical analysis; Jake Bundy (Imperial College, London, England) on C. elegans sample preparation for the LC/MS; and George R. Dubay (Duke University, Durham, NC) with the LC/MS.
References
- Barsyte D.,, Lovejoy D. A.,, Lithgow G. J. (2001). Longevity and heavy metal resistance in daf-2 and age-1 long-lived mutants of Caenorhabditis elegans FASEB J 15 627–634 [DOI] [PubMed] [Google Scholar]
- Beyersmann D., Hechtenberg S. (1997). Cadmium, gene regulation, and cellular signalling in mammalian cells Toxicol. Appl. Pharmacol 144 247–261 [DOI] [PubMed] [Google Scholar]
- Boyd W. A.,, Smith M. V.,, Kissling G. E.,, Freedman J. H. (2010). Medium- and high-throughput screening of neurotoxicants using C. elegans Neurotoxicol. Teratol 32 68–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardillo G. (2006). Dunn’s Test: A Procedure for Multiple, Non Parametric, Comparisons MATLAB Central, MathWorks, Natick, MA. [Google Scholar]
- Cioci L. K., Qiu L., Freedman J. H. (2000). Transgenic strains of the nematode Caenorhabditis elegans as biomonitors of metal contamination Environ. Toxicol. Chem 19 2122–2129 [Google Scholar]
- Cui Y.,, McBride S. J.,, Boyd W. A.,, Alper S.,, Freedman J. H. Toxicogenomic analysis of Caenorhabditis elegans reveals novel genes and pathways involved in the resistance to cadmium toxicity. Genome Biol. (2007);8:R122. doi: 10.1186/gb-2007-8-6-r122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong J.,, Boyd W. A.,, Freedman J. H. (2008). Molecular characterization of two homologs of the Caenorhabditis elegans cadmium-responsive gene cdr-1: cdr-4 and cdr-6 J. Mol. Biol 376 621–633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong J.,, Song M. O.,, Freedman J. H. (2005). Identification and characterization of a family of Caenorhabditis elegans genes that is homologous to the cadmium-responsive gene cdr-1 Biochim. Biophys. Acta 1727 16–26 [DOI] [PubMed] [Google Scholar]
- Freedman J. H.,, Ciriolo M. R.,, Peisach J. (1989). The role of glutathione in copper metabolism and toxicity J. Biol. Chem 264 5598–5605 [PubMed] [Google Scholar]
- Freedman J. H.,, Slice L. W.,, Dixon D.,, Fire A.,, Rubin C. S. (1993). The novel metallothionein genes of Caenorhabditis elegans. Structural organization and inducible, cell-specific expression J. Biol. Chem 268 2554–2564 [PubMed] [Google Scholar]
- Haq F.,, Mahoney M.,, Koropatnick J. (2003). Signaling events for metallothionein induction Mutat. Res 533 211–226 [DOI] [PubMed] [Google Scholar]
- Hughes S., Stürzenbaum S. R. (2007). Single and double metallothionein knockout in the nematode C. elegans reveals cadmium dependent and independent toxic effects on life history traits Environ. Pollut 145 395–400 [DOI] [PubMed] [Google Scholar]
- Hughes S. L.,, Bundy J. G.,, Want E. J.,, Kille P.,, Stürzenbaum S. R. (2009). The metabolomic responses of Caenorhabditis elegans to cadmium are largely independent of metallothionein status, but dominated by changes in cystathionine and phytochelatins J. Proteome Res 8 3512–3519 [DOI] [PubMed] [Google Scholar]
- Kang Y. J., Enger M. D. (1988). Glutathione is involved in the early cadmium cytotoxic response in human lung carcinoma cells Toxicology 48 93–101 [DOI] [PubMed] [Google Scholar]
- Khanna N.,, Cressman C. P., III, Tatara C. P.,, Williams P. L. (1997). Tolerance of the nematode Caenorhabditis elegans to pH, salinity, and hardness in aquatic media Arch. Environ. Contam. Toxicol 32 110–114 [DOI] [PubMed] [Google Scholar]
- Klaassen C. D.,, Liu J.,, Choudhuri S. (1999). Metallothionein: An intracellular protein to protect against cadmium toxicity Annu. Rev. Pharmacol. Toxicol 39 267–294 [DOI] [PubMed] [Google Scholar]
- Lazo J. S.,, Kondo Y.,, Dellapiazza D.,, Michalska A. E.,, Choo K. H.,, Pitt B. R. (1995). Enhanced sensitivity to oxidative stress in cultured embryonic cells from transgenic mice deficient in metallothionein I and II genes J. Biol. Chem 270 5506–5510 [DOI] [PubMed] [Google Scholar]
- Leung M. C.,, Williams P. L.,, Benedetto A.,, Au C.,, Helmcke K. J.,, Aschner M.,, Meyer J. N. (2008). Caenorhabditis elegans: An emerging model in biomedical and environmental toxicology Toxicol. Sci 106 5–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao V. H.,, Dong J.,, Freedman J. H. (2002). Molecular characterization of a novel, cadmium-inducible gene from the nematode Caenorhabditis elegans. A new gene that contributes to the resistance to cadmium toxicity J. Biol. Chem 277 42049–42059 [DOI] [PubMed] [Google Scholar]
- Liao V. H.,, Freedman J. H. (1998). Cadmium-regulated genes from the nematode Caenorhabditis elegans. Identification and cloning of new cadmium-responsive genes by differential display J. Biol. Chem 273 31962–31970 [DOI] [PubMed] [Google Scholar]
- Masters B. A.,, Kelly E. J.,, Quaife C. J.,, Brinster R. L.,, Palmiter R. D. (1994). Targeted disruption of metallothionein I and II genes increases sensitivity to cadmium Proc. Natl. Acad. Sci. U.S.A 91 584–588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarter J.,, Bartlett B.,, Dang T.,, Schedl T. (1999). On the control of oocyte meiotic maturation and ovulation in Caenorhabditis elegans Dev. Biol 205 111–128 [DOI] [PubMed] [Google Scholar]
- Ochi T.,, Otsuka F.,, Takahashi K.,, Ohsawa M. (1988). Glutathione and metallothioneins as cellular defense against cadmium toxicity in cultured Chinese hamster cells Chem. Biol. Interact 65 1–14 [DOI] [PubMed] [Google Scholar]
- Olson B. J., Markwell J. (2007). Assays for determination of protein concentration Current Protocols in Protein Science 48, 3.4.1–3.4.29. [DOI] [PubMed] [Google Scholar]
- Popham J. D., Webster J. M. (1979). Cadmium toxicity in the free-living nematode, Caenorhabditis elegans Environ. Res 20 183–191 [DOI] [PubMed] [Google Scholar]
- Roh J. Y.,, Lee J.,, Choi J. (2006). Assessment of stress-related gene expression in the heavy metal-exposed nematode Caenorhabditis elegans: A potential biomarker for metal-induced toxicity monitoring and environmental risk assessment Environ. Toxicol. Chem 25 2946–2956 [DOI] [PubMed] [Google Scholar]
- Singhal R. K.,, Anderson M. E.,, Meister A. (1987). Glutathione, a first line of defense against cadmium toxicity FASEB J 1 220–223 [DOI] [PubMed] [Google Scholar]
- Sirover M. A., Loeb L. A. (1976). Infidelity of DNA synthesis in vitro: Screening for potential metal mutagens or carcinogens Science 194 1434–1436 [DOI] [PubMed] [Google Scholar]
- Slice L. W.,, Freedman J. H.,, Rubin C. S. (1990). Purification, characterization, and cDNA cloning of a novel metallothionein-like, cadmium-binding protein from Caenorhabditis elegans J. Biol. Chem 265 256–263 [PubMed] [Google Scholar]
- Sulston J., Hodgkin J. (1988). Methods. In The Nematode Caenorhabditis elegans (Wood W. B., Ed.), pp. 587–606 Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY: [Google Scholar]
- Swain S. C.,, Keusekotten K.,, Baumeister R.,, Stürzenbaum S. R. (2004). C. elegans metallothioneins: New insights into the phenotypic effects of cadmium toxicosis J. Mol. Biol 341 951–959 [DOI] [PubMed] [Google Scholar]
- Vatamaniuk O. K.,, Bucher E. A.,, Ward J. T.,, Rea P. A. (2001). A new pathway for heavy metal detoxification in animals. Phytochelatin synthase is required for cadmium tolerance in Caenorhabditis elegans J. Biol. Chem 276 20817–20820 [DOI] [PubMed] [Google Scholar]
- Vatamaniuk O. K.,, Bucher E. A.,, Ward J. T.,, Rea P. A. (2002). Worms take the ‘phyto’ out of ‘phytochelatins’ Trends Biotechnol 20 61–64 [DOI] [PubMed] [Google Scholar]
- Williams P. L., Dusenbery D. B. (1990). Aquatic toxicity testing using the nematode, Caenorhabditis elegans Environ. Toxicol. Chem 9 1285–1290 [PubMed] [Google Scholar]