Abstract
The authors examined whether peak expiratory flow (PEF) is a valid measure of health status in older adults. Survey and test data from the 2006 and 2008 cycles of the Health and Retirement Study, a longitudinal study of US adults over age 50 years (with biennial surveys initiated in 1992), were used to develop predicted PEF regression models and to examine relations between low PEF values and other clinical factors. Low PEF (<80% of predicted value) was prevalent among persons with chronic conditions, including frequent pain, obstructive lung disease, heart disease, diabetes, and psychological distress. Persons with higher physical disability scores had substantially higher adjusted odds of having low PEF, on par with those for conditions known to be associated with poor health (cancer, heart disease, and stroke). In a multivariate regression model for difficulty with mobility, PEF remained an independent factor (odds ratio (OR) = 1.69, 95% confidence interval (CI): 1.53, 1.86). Persons with low PEF in 2006 were more likely to be hospitalized (OR = 1.26, 95% CI: 1.10, 1.43) within the subsequent 2 years and to estimate their chances of surviving for 10 or more years at less than 50% (OR = 1.69, 95% CI: 1.24, 2.30). PEF is a valid measure of health status in older persons, and low PEF is an independent predictor of hospitalization and poor subjective mortality assessment.
Keywords: aged, health services, mortality, peak expiratory flow rate, respiratory function tests
Simple measurements of physiologic function are useful objective measures of health status in populations and may also have prognostic implications for individuals (1–3). Measures of limitation in lung function are among those physiologic measures that have been associated with decreased physical capacity, poorer health status, and reduced survival (4–10). Because of its reproducibility and utility in diagnosing lung disease, spirometry has been the preferred test of lung function (11). However, the forced expiratory maneuver required for accurate assessment of forced expiratory volume in 1 second (FEV1) and forced vital capacity (FVC), the key metrics for diagnosis and disease classification per American Thoracic Society/European Respiratory Society criteria, can be difficult for elderly persons to perform correctly, especially if they are physically debilitated or have cognitive impairment (12–15).
Peak expiratory flow (PEF), though not as well validated as FEV1 and FVC as a health status measure, can be a more practical physiologic measurement to obtain from elderly persons, especially in large surveys (16). Percentage of predicted PEF has been shown to be a predictor of survival among older adults in highly selected populations (6, 17, 18) and in adults with diabetes (19). Other investigators have also found significant correlations between percentage of predicted PEF and other measures of physical and cognitive function in the very elderly (20). However, PEF has not been well-validated in large studies representative of middle-aged and older adults in the general population, and it is not known whether poor PEF is a predictor of health-care utilization. The purpose of this project was to examine how well PEF performs as a measure of current health status among adults over age 50 years, how it compares with other measures of physical impairment and conditions associated with poor health, and whether it remains an independent predictor of poor health and increased future health-care utilization after adjustment for comorbidity.
MATERIALS AND METHODS
Data sources
Data were de-identified secondary data from the Health and Retirement Study, a longitudinal panel study designed to explain the antecedents and consequences of retirement, with biennial surveys initiated in 1992. The study is sponsored by the National Institute on Aging and conducted by investigators at the University of Michigan. It consists of biennial surveys of US adults over age 50 years. Over the course of the study, data have been collected on demographic characteristics (age, sex, ethnicity, and race), self-reported physical health and functioning, and depression. Physical measurements were taken for a small subsample of survey respondents in 2004. In 2006 this effort was expanded; physical measurements were taken for one-half of the full survey sample in 2006, and the other half in 2008. Among the physical measures were height (in inches), weight (in pounds), and a PEF lung function test. The PEF test consisted of 3 PEF measurements taken 30 seconds apart using a Mini-Wright peak flow meter (Clement Clarke International Ltd., Harlow, United Kingdom). The maximum value from the 3 measurements was used as the respondent's PEF.
The survey is described in detail on the Health and Retirement Study website (21), and a summary of measures used in our study is available as a Web Appendix (http://aje.oxfordjournals.org/). Biennially, respondents have provided information on their status with regard to chronic conditions: high blood pressure, diabetes, cancer, heart disease, chronic obstructive pulmonary disease (COPD) (not including asthma), stroke, and arthritis. Depression among respondents has been assessed biennially using a validated instrument containing 8 items, where a higher score indicates a higher level of depressive symptoms (22). Two functional measures have also been included: difficulty with mobility, strength, and fine motor skills (MSFMS) and Activities of Daily Living (ADLs) (23). Briefly, MSFMS comprise 12 physical activities such as jogging, walking several blocks, stooping, picking up a dime, and extending one's arms; ADLs comprise 6 daily functions such as walking, eating, and getting into/out of bed. Each measure is constructed as a sum of item responses; MSFMS scores range from 0 to 12 and ADL scores from 0 to 6, with higher scores indicating greater functional limitations. As part of the biennial survey, respondents have also been questioned on their utilization of health-care services in the prior 2 years: the number of visits to a physician, whether they have been hospitalized during the last 2 years, and, if so, how many times. Lastly, respondents less than 65 years of age have been asked to provide an estimate of their chances of living to age 75 years on a scale of 0–100, 0 being equivalent to no expectation that they would live that long and 100 to certainty that they would.
Predicted PEF model and statistical analyses
We developed a predicted PEF regression equation using a “normal” lung function population of survey respondents. In keeping with previous definitions (24, 25), the regression development population consisted of respondents without a history of respiratory impairment—no history of smoking, no indication of COPD or use of asthma or respiratory medication, and no reports of frequent shortness of breath or coughing/wheezing. Separate regression models were constructed for men and women. PEF was regressed on respondent age (in 1-year increments), height (in inches), and race/ethnicity (being Hispanic or African-American) using a linear multiple regression model. Goodness of fit was assessed using the overall R2 value. For each respondent, percentage of predicted PEF was calculated as actual PEF divided by the regression-predicted PEF.
Two methods were used to determine “low” PEF values. Our main analyses used a traditional fixed cutoff point of 80% of predicted PEF, with scores less than 80% of predicted PEF indicating low PEF values. In secondary analyses, the lower limit of normal (LLN), a relational method that bases the cutoff point on the lower 5th percentile of normal subjects, was calculated using regression parameter estimates less −1.645 × the standard error of the parameter estimate (26). This is a statistically rigorous definition that accounts for the greater variability in lung function that may be seen among the very elderly (26). All analyses were also stratified by COPD status (persons with and without COPD) to verify that the associations between reduced PEF and the outcomes of interest were also valid in persons without known lung disease.
Associations between lower-than-normal PEF values and physical functioning difficulties and subjective prediction of survival were investigated in multiple logistic regression models for persons with physical measures obtained in 2006 and 2008. Data distributions for physical functioning difficulties were examined to determine cutoff points for dichotomizing difficulty with MSFMS and ADLs. As a binary outcome, difficulty with MSFMS and difficulty with ADLs were each regressed on low PEF status, adjusting for age (65–74, 75–84, or ≥85 years as compared with age 50–64 years), male sex, race/ethnicity (Hispanic, African-American, or other as compared with non-Hispanic white), and self-reported morbidity. A subsample of respondents, those aged 60–65 years, was used to investigate the association between low PEF values and subjective probability of living to at least age 75 years by regressing low probability of survival (an estimate of <50% probability) on low PEF status, adjusting for male sex, race/ethnicity, and self-reported morbidity. A narrow range of ages was used to reduce confounding due to judgment adjustments that might occur with increasing age (27).
Association between low PEF status in 2006 and use of health-care resources in the subsequent 2 years was investigated using the subset of participants for whom physical measures were obtained in the 2006 survey and who also provided survey responses in 2008 about health-care utilization in the previous 2 years. In separate multivariate regression models, having any hospitalization and having high outpatient utilization of health-care services (third quartile of physician visits or higher) were regressed on low PEF status, with adjustment for age, male sex, race/ethnicity, and self-reported morbidity.
Finally, the sampling design of the Health and Retirement Study included probabilities that vary across older cohorts and involved oversampling of African-American and Hispanic respondents. Recognizing these aspects of the Health and Retirement Study, we conducted sensitivity analyses incorporating survey strata, clusters, and observation weights for all statistical analyses. SAS statistical software, version 9.2 (SAS Institute Inc., Cary, North Carolina), was used to perform analyses. All P values are 2-sided.
RESULTS
For 2006 and 2008, we identified 13,129 survey respondents with information on PEF, age, and height. Of these persons, 7,498 (57.1%) had no history of respiratory impairment and were used to develop PEF predictive equations (the normal respiratory function group). There was no difference in mean age (69 years) between the two groups (P = 0.12). The normal respiratory function group was 43.1% male, as compared with 40.5% male in the impaired lung function group (P = 0.002). The mean actual PEF for women in the regression development population was 309.6 L/minute (95% confidence interval (CI): 306.9, 312.22); for men it was 464.4 L/minute (95% CI: 459.86, 468.86). Table 1 shows the predicted PEF regression equation parameter estimates for men and women. The percentage of variance explained, as measured by the R2 statistic, was similar to that of other studies in which PEF regression models have been constructed (24, 28). Applying our predictive equations to the entire study population and determining low PEF using our 2 methods, we found that 27.0% (n = 3,551) of the survey respondents had PEF values that were less than 80% of predicted values, and 9.4% (n = 1,236) had PEF values that were below the LLN cutpoint.
Table 1.
Regression Equations for Percentage of Predicted Peak Expiratory Flowa Among Respondents With Normal Respiratory Function, by Sex, Health and Retirement Study, 2006 and 2008*
| Women (n = 4,260) |
Men (n = 3,238) |
|||
|---|---|---|---|---|
| Coefficient (β) | SE | Coefficient (β) | SE | |
| Intercept | 350.7 | 33.75 | 320.9 | 58.70 |
| Age (per year) | −4.3 | 0.12 | −5.6 | 0.22 |
| Height (per inch)b | 4.2 | 0.48 | 7.9 | 0.77 |
| Race/ethnicity | ||||
| Hispanic | −26.0 | 4.23 | −39.7 | 7.79 |
| African-American | −27.8 | 3.68 | −65.9 | 7.11 |
| R2 | 0.29 | 0.25 | ||
Abbreviation: SE, standard error.
* P < 0.001 for all coefficient estimates.
a Regression model for peak expiratory flow: intercept + bage × age + bheight × height + bHispanic × Hispanic + bAfrican-American × African-American.
b 1 inch = 2.54 cm.
One would expect PEF values to be lower among persons with respiratory disease, and in this population the mean percentage of predicted PEF was 76.4% among respondents with COPD, as compared with 84.6% among respondents with a history of stroke, percentages in the lower 90s for heart disease and diabetes, and approximately 94% otherwise (Table 2). The demographic and self-reported clinical characteristics of participants with normal PEF and low PEF characterized using the fixed percentage cutoff of <80% are shown in Table 3. Physical function limitations and health-care utilization are shown in Table 4. Differences in utilization between the two groups were similar when LLN was used to determine reduced PEF status (results not shown).
Table 2.
Percentage of Predicted Peak Expiratory Flow According to Self-Reported Chronic Conditions, Health and Retirement Study, 2006 and 2008
| No. of Respondents | % of Predicted PEF |
||
|---|---|---|---|
| Mean | 95% CI | ||
| Entire study population | 13,129 | 94.6 | 94.1, 95.1 |
| Reported morbidity | |||
| Cancer | 2,035 | 94.2 | 92.9, 95.4 |
| COPD | 1,367 | 76.4 | 74.9, 77.9 |
| Diabetes | 2,787 | 91.9 | 90.9, 92.9 |
| Heart disease | 3,440 | 90.9 | 90.0, 91.9 |
| High blood pressure | 7,777 | 93.7 | 93.1, 94.3 |
| Stroke | 884 | 84.6 | 82.7, 86.5 |
Abbreviations: CI, confidence interval; COPD, chronic obstructive pulmonary disease; PEF, peak expiratory flow.
Table 3.
Percentage of Predicted Peak Expiratory Flow According to Demographic Characteristics and Morbidity, Health and Retirement Study, 2006 and 2008
| % of Predicted PEF |
||||||
|---|---|---|---|---|---|---|
| Total |
<80 |
≥80 |
||||
| No. | Mean (SD) | Row % | Mean (SD) | Row % | Mean (SD) | |
| No. of respondents | 13,129 | 27.0 | 73.0 | |||
| Age group, years | ||||||
| 50–64** | 4,477 | 22.2 | 77.8 | |||
| 65–74** | 4,872 | 27.6 | 72.4 | |||
| 75–84** | 2,863 | 31.4 | 68.6 | |||
| ≥85** | 917 | 34.6 | 65.4 | |||
| Age, years** | 69.1 (9.7) | 70.3 (9.8) | 68.6 (9.6) | |||
| Sex | ||||||
| Female | 7,612 | 26.6 | 73.4 | |||
| Male | 5,517 | 27.7 | 72.3 | |||
| Race/ethnicity | ||||||
| Hispanic | 1,095 | 28.1 | 71.9 | |||
| Non-Hispanic white** | 10,054 | 26.2 | 73.8 | |||
| African-American** | 1,781 | 31.4 | 68.6 | |||
| Other | 199 | 25.6 | 74.4 | |||
| Body mass indexa** | 29.4 (6.3) | 28.8 (6.7) | 29.6 (6.1) | |||
| Reported morbidity | ||||||
| High blood pressure** | 7,777 | 29.0 | 71.0 | |||
| Diabetes** | 2,787 | 31.0 | 69.0 | |||
| Cancer* | 2,035 | 29.5 | 70.5 | |||
| COPD** | 1,367 | 55.8 | 44.2 | |||
| Heart disease** | 3,440 | 33.1 | 66.9 | |||
| Stroke** | 884 | 43.0 | 57.0 | |||
| Arthritis** | 8,219 | 28.2 | 71.8 | |||
| Depression (score ≥3 on CES-D8)** | 2,689 | 37.6 | 62.4 | |||
| % of predicted PEF** | 94.6 (27.0) | 60.6 (14.3) | 107.3 (18.3) | |||
| Respiratory symptoms | ||||||
| Persistent cough/wheeze** | 2,322 | 45.9 | 54.1 | |||
| Shortness of breath** | 2,553 | 45.3 | 54.7 | |||
| Persistent cough and shortness of breath** | 1,154 | 57.6 | 42.4 | |||
| Use of medication for lung disease, asthma, or other respiratory disorder** | 2,082 | 42.9 | 57.1 | |||
Abbreviations: CES-D8, Center for Epidemiologic Studies Depression Scale 8; COPD, chronic obstructive pulmonary disease; PEF, peak expiratory flow; SD, standard deviation.
* P < 0.01; **P < 0.001 (chi-square test for frequencies and Student's t test for means).
a Weight (pounds)/height (inches)2 × 703.
Table 4.
Percentage of Predicted Peak Expiratory Flow According to Functional Limitations and Health-Care Utilization, Health and Retirement Study, 2006 and 2008
| % of Predicted PEF |
||||||
|---|---|---|---|---|---|---|
| Total (n = 13,129)a |
<80 (n = 3,551) |
≥80 (n = 9,578) |
||||
| No. | Mean (SD) | Column % | Mean (SD) | Column % | Mean (SD) | |
| Functional limitations | ||||||
| Mobility, strength, and fine motor skills (maximum = 12 items) | ||||||
| Difficulty with >3 items* | 5,911 | 59.4 | 39.7 | |||
| Difficulty with >4 items* | 4,688 | 50.4 | 30.2 | |||
| Difficulty with >5 items* | 3,690 | 41.3 | 23.2 | |||
| No. of difficulties* | 3.8 (3.1) | 4.9 (3.3) | 3.5 (2.9) | |||
| Activities of Daily Living (maximum = 6 items) | ||||||
| Any difficulty* | 2,103 | 23.5 | 13.2 | |||
| No. of difficulties* | 0.3 (0.9) | 0.5 (1.2) | 0.2 (0.7) | |||
| Outpatient health-care utilization | ||||||
| Any physician visit in past 2 years | 11,835 | 87.3 | 91.2 | |||
| No. of physician visits in past 2 years* | 10.2 (17.8) | 12.1 (24.4) | 9.5 (14.6) | |||
| ≥Third quartile of no. of physician visits in past 2 years* | 3,164 | 27.3 | 22.9 | |||
| Inpatient health-care utilization | ||||||
| Any overnight stay in hospital in past 2 years* | 3,507 | 34.5 | 23.8 | |||
| No. of hospitalizations in past 2 years* | 0.4 (1.0) | 0.6 (1.3) | 0.4 (0.9) | |||
Abbreviations: PEF, peak expiratory flow; SD, standard deviation.
* P < 0.001 (chi-square test for frequencies and Student's t test for means).
a Number of respondents.
Respondents with reduced PEF in this cohort were older (P < 0.001), but there was no difference in the percentages of men and women (P = 0.17). The prevalence of reduced PEF was relatively consistent within race/ethnicity categories. Respondents with reduced PEF were significantly more likely to have chronic illnesses, especially COPD, heart disease, and stroke; to have a high depression score; and to have a slightly lower BMI. As expected, persons with a reduced PEF had an increased incidence of respiratory symptoms and increased use of respiratory medication (Table 3).
Physical functioning was more limited among participants with reduced PEF (Table 4). Higher MSFMS and ADL scores were both associated with reduced PEF (P < 0.001). A much larger percentage of persons with a PEF less than 80% of the predicted value than of persons with normal PEF values had difficulty with more than 3 MSFMS items (P < 0.001). There was less variation concerning ADLs: Only 13.2% of participants with normal PEF values reported difficulty with at least one ADL, and 23.5% of those with a PEF less than 80% of that predicted reported some difficulty. In both 2006 and 2008, respondents with reduced PEF were more likely to have a greater number of outpatient physician visits and to have been hospitalized within the last 2 years (Table 4).
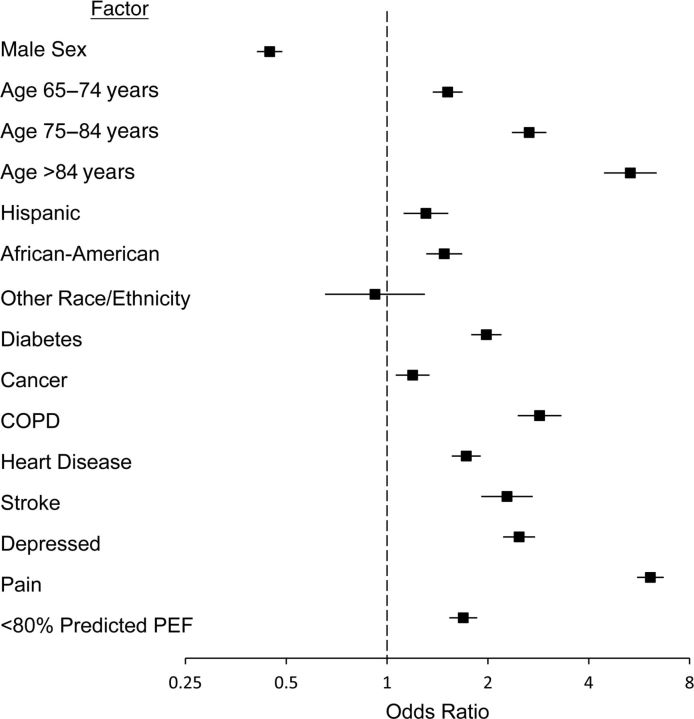
In a multiple logistic regression model adjusting for age, sex, race/ethnicity, and morbidity, respondents with a reduced PEF had 40% higher odds of having difficulty with any of the ADLs (odds ratio (OR) = 1.42, 95% CI: 1.27, 1.60). Greater difficulty with MSFMS was dichotomized in separate models as having difficulty with >3 items, difficulty with >4 items, and difficulty with >5 items. In each of the multiple logistic regression models, a peak flow rate less than 80% of that predicted was found to be an independent predictor of greater difficulty with MSFMS, with odds ratios being successively higher for each increasing level of discrimination. For difficulty with >3 items, the odds ratio was 1.69 (95% CI: 1.53, 1.86), and it compared favorably with other health status morbidity measures (Figure 1). If >5 items was used as the cutpoint, the odds ratio increased to 1.82 (95% CI: 1.64, 2.02).
Figure 1.
Adjusted odds ratio for reduced lung function (<80% of predicted peak expiratory flow (PEF)) according to chronic diseases and demographic factors among respondents who had more than 3 functional limitations in mobility, strength, and fine motor skills (n = 13,129), Health and Retirement Study, 2006 and 2008. Bars, 95% confidence interval. (COPD, chronic obstructive pulmonary disease).
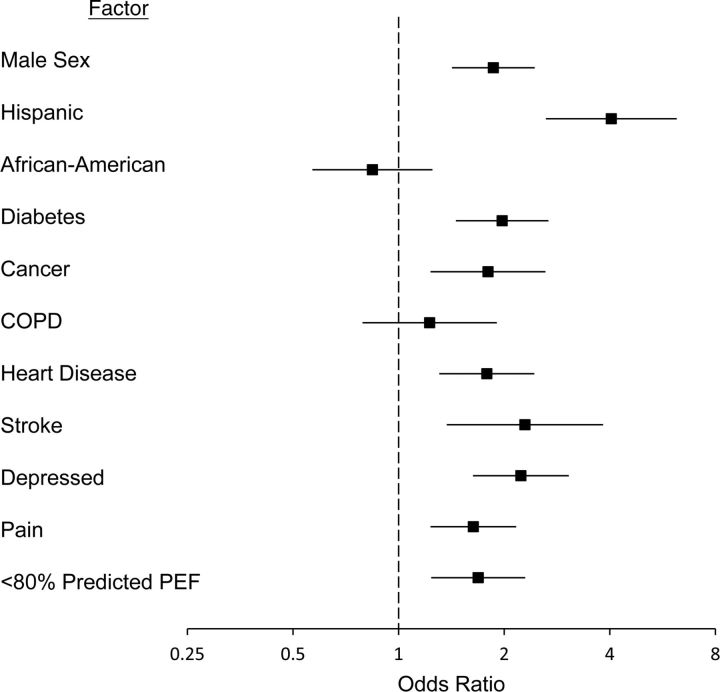
In 2006 and 2008, of the 1,568 respondents aged 60–65 years who were queried on the subjective probability of living to age 75 years, 20.9% reported a probability less than 50%. Among respondents with PEF less than 80% of that predicted, 32.4% reported an estimate of <50%, as compared with 17.8% among respondents with normal PEF (P < 0.001). In a multiple logistic regression model for an estimate of <50%, a peak flow rate less than 80% of that predicted was an independent predictor of a low survival estimate (OR = 1.69, 95% CI: 1.24, 2.30) (Figure 2).
Figure 2.
Adjusted odds ratio for reduced lung function (<80% of predicted peak (PEF)) according to chronic diseases and demographic factors among respondents who predicted that they had a <50% probability of living at least 10 more years (n = 1,568), Health and Retirement Study, 2006 and 2008. Bars, 95% confidence interval. (COPD, chronic obstructive pulmonary disease).
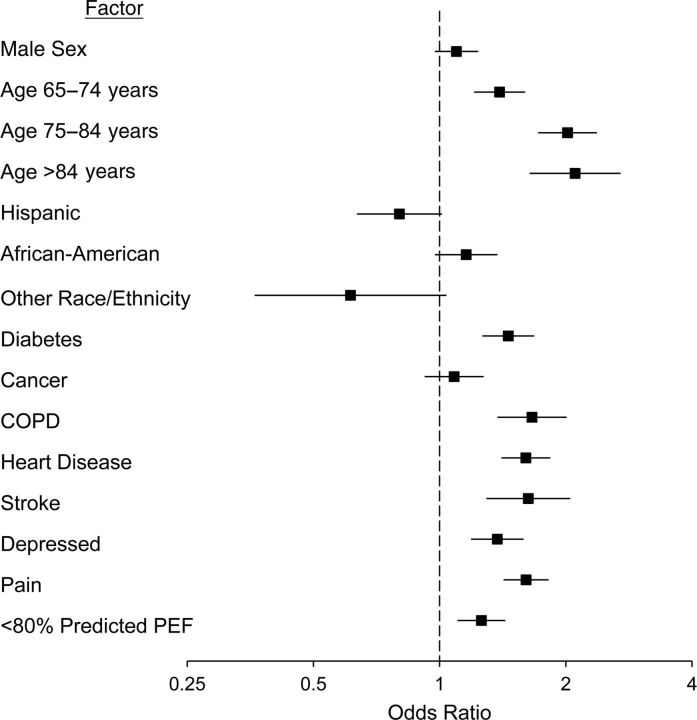
Predictive models for health-care utilization were based on the 2006 survey respondents who provided information in 2008 on physician visits (n = 5,986) and hospitalizations (n = 6,389) between the 2006 and 2008 survey points. These participants were a subset of the 13,129 respondents used for the previous analyses. Among those with PEF less than 80% of that predicted, 34.5% reported any hospitalization, as compared with 23.8% with normal PEF (P < 0.001); for increased outpatient physician visits, percentages were 27.3% and 22.9%, respectively (P < 0.001). Low PEF proved to be an independent predictor of hospitalization (OR = 1.26, 95% CI: 1.10, 1.43) after adjustment for age, sex, race/ethnicity, and morbidity (Figure 3), but not for increased physician visits (OR = 1.05, 95% CI: 0.91, 1.21).
Figure 3.
Adjusted odds ratio for reduced lung function (<80% of predicted peak expiratory flow (PEF)) according to chronic diseases and demographic factors among respondents who were hospitalized at any time in the subsequent 2 years (n = 6,398), Health and Retirement Study, 2006. Bars, 95% confidence interval. (COPD, chronic obstructive pulmonary disease).
Regression models in which reduced PEF was determined using LLN results showed similarly greater odds ratios for reduced PEF. Since the LLN is a more restrictive assessment, persons identified as having reduced PEF had even lower levels of PEF, and odds ratios using LLN were always higher than those calculated using <80% predicted PEF. For example, the odds ratio for difficulty with >3 items using the LLN was 2.00 (95% CI: 1.73, 2.30) as compared with 1.69 (95% CI: 1.53, 1.86) using <80% predicted PEF. Persons with COPD comprised approximately 10% of the survey respondents. In stratified models where reduced PEF was determined either by <80% predicted PEF or by LLN, persons with COPD had higher odds ratios for each of the reviewed outcomes, but because of the low percentage of COPD respondents, the odds ratios for respondents without COPD were not appreciably changed. For example, overall, persons with reduced PEF (using <80% of predicted PEF) had greater odds of poor survival (OR = 1.69, 95% CI: 1.24, 2.30), and among respondents without COPD, the odds were still higher (OR = 1.65, 95% CI: 1.14, 2.40). Results for regression models using the survey strata, cluster, and weights produced results very similar to those of the models without the probability sampling information (data not shown).
DISCUSSION
Our analysis demonstrated that PEF is correlated with other standard measures of health status and, in adjusted regression models, is an independent factor associated with limitations in MSFMS and ADLs. Reduced PEF was evident in a variety of chronic illnesses, and its validity as a health status measure was confirmed not only among persons with a prior diagnosis of COPD but also among persons without chronic lung disease. In our study sample, reduced PEF predicted increased risk of hospitalization within the next 2 years and was associated with a lower self-estimated probability of living to age 75 years among persons aged 60–65 years.
There are several advantages to the use of PEF as a health status measure for elderly persons, as compared with other physiologic tests. Peak flow meters are inexpensive, readily available, and simple to use, and it is relatively easy to train staff and patients to perform the peak flow maneuver. Patients do not have to be able to walk or stand, or even be able to hold the meter, in order to engage in reproducible PEF efforts. Among Health and Retirement Study participants able to perform the PEF test who were aged 65 years or older, 10.5% were unable to engage in a timed walk, and among those with COPD, 15% could not engage in a timed walk.
Although the PEF measure lacks the diagnostic utility of the FVC and FEV1 measures obtained by formal spirometry, it does not require the patient to exhale with maximum effort for at least 6 seconds—a difficult task for many elderly or debilitated patients. The PEF test also uses technically simpler and less expensive equipment, making it possible for PEF data to be captured efficiently from a broader range of older adult participants. In community surveys of older adults, the proportion of persons unable to perform spirometry has ranged from 12% to 17% (13, 29). Fewer studies using PEF in older adults have been conducted, but the proportion of those unable to produce an acceptable PEF measurement has ranged from 0.5% to 10% (6, 28, 30). Direct comparisons of PEF with FEV1 as a health status measure are rare, but in a prospective study of patients with COPD, both parameters were independent predictors of survival, and PEF appeared to capture limitations in physical functioning missed by FEV1 (31).
We do not propose the PEF measure as a substitute for formal spirometry where diagnostic assessment of lung function is needed; but as a simple objective physiologic measure of health status in elderly and even middle-aged populations, PEF is more than suitable. Findings from our analyses support previous studies suggesting that PEF is a useful measure of health status in older adults. In a cross-sectional survey of 3,812 persons aged 65 years or older, low PEF was associated with a history of stroke, angina, and tachycardia, as well as measures of functional ability and physical activity, cognition, and health self-assessment (32). In a follow-up analysis, PEF was a predictor of 5-year mortality even after adjustment for these cofactors plus smoking history, age, and demographic factors (17). In another cross-sectional survey of 1,354 persons aged 70–79 years, PEF was correlated with several physical measures, tests of cognitive performance, and urinary norepinephrine levels (20). In a prospective study of 753 persons with a mean age of 78 years, PEF was a predictor of disability and death after adjustment for multiple confounders, including age, smoking, and chronic lung disease (6). Our study added to previous work by studying PEF within a population that included persons who were still working (age >50 years), and to our knowledge, it is the first study to have demonstrated that low PEF is associated with an increased risk of hospitalization in the subsequent 2 years.
There are a few known and potential limitations of this study. Information on chronic illnesses and health-care utilization was obtained by respondent self-report, which can be inaccurate. Although the survey was designed to capture a representative sample of an older (age >50 years) US population, sampling biases are still likely and may have affected the results. We chose the cutoff value of 80% of predicted PEF in our main analyses largely on the basis of the traditional definition of “normal” for FEV1 and FVC. However, we conducted secondary analyses using the statistically defined LLN, which generally corresponds to the lowest 5th percentile, and results did not substantially change.
We used a surrogate measure for mortality, individual assessment of mortality risk, but several studies have found that individuals' subjective assessments covary with risk factors and have a high predictive ability with regard to actual mortality (33, 34). In our study, persons with reduced PEF values were slightly older than persons with normal PEF values; however, our analysis of the relation between reduced PEF and subjective mortality assessment included only participants aged 60–65 years, reducing bias due to age.
In summary, we conclude that PEF is a useful measure of physical functioning and health status in older adults with or without COPD and that it can be an independent predictor of increased health-care utilization. As this prospective cohort study evolves, we will be able to further examine relations between PEF and other clinical outcomes and how PEF complements other health status measures to provide a more thorough assessment of individuals and populations.
ACKNOWLEDGMENTS
Author affiliation: LCF Research, Albuquerque, New Mexico (Melissa H. Roberts, Douglas W. Mapel).
The Health and Retirement Study was sponsored by the National Institute on Aging (grant U01AG009740). No financial support was received for the current analysis.
Conflict of interest: none declared.
REFERENCES
- 1.Newman AB, Simonsick EM, Naydeck BL, et al. Association of long-distance corridor walk performance with mortality, cardiovascular disease, mobility limitation, and disability. JAMA. 2006;295(17):2018–2026. doi: 10.1001/jama.295.17.2018. [DOI] [PubMed] [Google Scholar]
- 2.Studenski S, Perera S, Patel K, et al. Gait speed and survival in older adults. JAMA. 2011;305(1):50–58. doi: 10.1001/jama.2010.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cooper R, Kuh D, Hardy R, et al. Objectively measured physical capability levels and mortality: systematic review and meta-analysis. BMJ. 2010;341:c4467. doi: 10.1136/bmj.c4467. doi:10.1136/bmj.c4467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miller MR, Pedersen OF, Lange P, et al. Improved survival prediction from lung function data in a large population sample. Respir Med. 2009;103(3):442–448. doi: 10.1016/j.rmed.2008.09.016. [DOI] [PubMed] [Google Scholar]
- 5.Eisner MD, Iribarren C, Yelin EH, et al. Pulmonary function and the risk of functional limitation in chronic obstructive pulmonary disease. Am J Epidemiol. 2008;167(9):1090–1101. doi: 10.1093/aje/kwn025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fragoso CA, Gahbauer EA, Van Ness PH, et al. Peak expiratory flow as a predictor of subsequent disability and death in community-living older persons. J Am Geriatr Soc. 2008;56(6):1014–1020. doi: 10.1111/j.1532-5415.2008.01687.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Young RP, Hopkins R, Eaton TE. Forced expiratory volume in one second: not just a lung function test but a marker of premature death from all causes. Eur Respir J. 2007;30(4):616–622. doi: 10.1183/09031936.00021707. [DOI] [PubMed] [Google Scholar]
- 8.Sin DD, Wu L, Man SF. The relationship between reduced lung function and cardiovascular mortality: a population-based study and a systematic review of the literature. Chest. 2005;127(6):1952–1959. doi: 10.1378/chest.127.6.1952. [DOI] [PubMed] [Google Scholar]
- 9.Coultas DB, Mapel D, Gagnon R, et al. The health impact of undiagnosed airflow obstruction in a national sample of United States adults. Am J Respir Crit Care Med. 2001;164(3):372–377. doi: 10.1164/ajrccm.164.3.2004029. [DOI] [PubMed] [Google Scholar]
- 10.Schünemann HJ, Dorn J, Grant BJ, et al. Pulmonary function is a long-term predictor of mortality in the general population: 29-year follow-up of the Buffalo Health Study. Chest. 2000;118(3):656–664. doi: 10.1378/chest.118.3.656. [DOI] [PubMed] [Google Scholar]
- 11.Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J. 2005;26(2):319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 12.Carvalhaes-Neto N, Lorino H, Gallinari C, et al. Cognitive function and assessment of lung function in the elderly. Am J Respir Crit Care Med. 1995;152(5):1611–1615. doi: 10.1164/ajrccm.152.5.7582303. [DOI] [PubMed] [Google Scholar]
- 13.Bellia V, Pistelli R, Catalano F, et al. Quality control of spirometry in the elderly. The SA.R.A. study. Am J Respir Crit Care Med. 2000;161(4):1094–1100. doi: 10.1164/ajrccm.161.4.9810093. [DOI] [PubMed] [Google Scholar]
- 14.Pezzoli L, Giardini G, Consonni S, et al. Quality of spirometric performance in older people. Age Ageing. 2003;32(1):43–46. doi: 10.1093/ageing/32.1.43. [DOI] [PubMed] [Google Scholar]
- 15.Allen S, Yeung P, Janczewski M, et al. Predicting inadequate spirometry technique and the use of FEV1/FEV3 as an alternative to FEV1/FVC for patients with mild cognitive impairment. Clin Respir J. 2008;2(4):208–213. doi: 10.1111/j.1752-699X.2008.00063.x. [DOI] [PubMed] [Google Scholar]
- 16.Bellia V, Pistelli F, Giannini D, et al. Questionnaires, spirometry and PEF monitoring in epidemiological studies on elderly respiratory patients. Eur Respir J. 2003;21(40 suppl):21S–27S. doi: 10.1183/09031936.03.00402303. [DOI] [PubMed] [Google Scholar]
- 17.Cook NR, Evans DA, Scherr PA, et al. Peak expiratory flow rate and 5-year mortality in an elderly population. Am J Epidemiol. 1991;133(8):784–794. doi: 10.1093/oxfordjournals.aje.a115957. [DOI] [PubMed] [Google Scholar]
- 18.Tilvis R, Valvanne J, Sairanen S, et al. Peak expiratory flow is a prognostic indicator in elderly people. BMJ. 1997;314(7080):605–606. [PMC free article] [PubMed] [Google Scholar]
- 19.Klein BE, Moss SE, Klein R, et al. Peak expiratory flow rate: relationship to risk variables and mortality: the Wisconsin Epidemiologic Study of Diabetic Retinopathy. Diabetes Care. 2001;24(11):1967–1971. doi: 10.2337/diacare.24.11.1967. [DOI] [PubMed] [Google Scholar]
- 20.Cook NR, Albert MS, Berkman LF, et al. Interrelationships of peak expiratory flow rate with physical and cognitive function in the elderly: MacArthur Foundation studies of aging. J Gerontol A Biol Sci Med Sci. 1995;50(6):M317–M323. doi: 10.1093/gerona/50a.6.m317. [DOI] [PubMed] [Google Scholar]
- 21.National Institutes of Health. Growing Older in America: The Health and Retirement Study. (NIH publication no. 07-5757) Ann Arbor, MI: HRS/AHEAD Documentation Report Survey Research Center, Institute for Social Research, University of Michigan; 2007. (http://hrsonline.isr.umich.edu/index.php?p=dbook. ). (Accessed February 21, 2011) [Google Scholar]
- 22.Steffick DE. Documentation of Affective Functioning Measures in the Health and Retirement Study. Ann Arbor, MI: HRS/AHEAD Documentation Report Survey Research Center, Institute for Social Research, University of Michigan; 2000. [Google Scholar]
- 23.Fonda S, Herzog AR. Documentation of Physical Functioning Measured in the Health and Retirement Study and the Asset and Health Dynamics Among the Oldest Old Study. Ann Arbor, MI: HRS/AHEAD Documentation Report Survey Research Center, Institute for Social Research, University of Michigan; 2004. [Google Scholar]
- 24.Nunn AJ, Gregg I. New regression equations for predicting peak expiratory flow in adults. BMJ. 1989;298(6680):1068–1070. doi: 10.1136/bmj.298.6680.1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Quanjer PH, Lebowitz MD, Gregg I, et al. Peak expiratory flow: conclusions and recommendations of a Working Party of the European Respiratory Society. Eur Respir J. 1997;10(24 suppl):2S–8S. [PubMed] [Google Scholar]
- 26.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med. 1999;159(1):179–187. doi: 10.1164/ajrccm.159.1.9712108. [DOI] [PubMed] [Google Scholar]
- 27.Tversky A, Kahneman D. Judgment under uncertainty: heuristics and biases. Science. 1974;185(4157):1124–1131. doi: 10.1126/science.185.4157.1124. [DOI] [PubMed] [Google Scholar]
- 28.Vaz Fragoso CA, Gahbauer EA, Van Ness PH, et al. Reporting peak expiratory flow in older persons. J Gerontol A Biol Sci Med Sci. 2007;62(10):1147–1151. doi: 10.1093/gerona/62.10.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hegewald MJ, Lefor MJ, Jensen RL, et al. Peak expiratory flow is not a quality indicator for spirometry: peak expiratory flow variability and FEV1 are poorly correlated in an elderly population. Chest. 2007;131(5):1494–1499. doi: 10.1378/chest.06-2707. [DOI] [PubMed] [Google Scholar]
- 30.Klein R, Klein BE, Knudtson MD. Frailty and age-related macular degeneration: the Beaver Dam Eye Study. Am J Ophthalmol. 2005;140(1):129–131. doi: 10.1016/j.ajo.2004.12.049. [DOI] [PubMed] [Google Scholar]
- 31.Hansen EF, Vestbo J, Phanareth K, et al. Peak flow as predictor of overall mortality in asthma and chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001;163(3):690–693. doi: 10.1164/ajrccm.163.3.2006120. [DOI] [PubMed] [Google Scholar]
- 32.Cook NR, Evans DA, Scherr PA, et al. Peak expiratory flow rate in an elderly population. Am J Epidemiol. 1989;130(1):66–78. doi: 10.1093/oxfordjournals.aje.a115324. [DOI] [PubMed] [Google Scholar]
- 33.Hurd MD, McGarry K. The predictive validity of subjective probabilities of survival. Econ J. 2002;112(482):966–985. [Google Scholar]
- 34.Salm M. Subjective mortality expectations and consumption and saving behaviours among the elderly. Can J Econ. 2010;43(3):1040–1057. [Google Scholar]