Abstract
The authors conducted a 2-year follow-up of 40 cardiovascular disease patients (mean age = 65.6 years (standard deviation, 5.8)) who underwent repeated measurements of cardiovascular response before and during the 2008 Beijing Olympics (Beijing, China), when air pollution was strictly controlled. Ambient levels of particulate matter with an aerodynamic diameter less than 2.5 µm (PM2.5), black carbon, nitrogen dioxide, sulfur dioxide, ozone, and carbon monoxide were measured continuously, with validation of concurrent real-time measurements of personal exposure to PM2.5 and carbon monoxide. Linear mixed-effects models were used with adjustment for individual risk factors, time-varying factors, and meteorologic effects. Significant heart rate variability reduction and blood pressure elevation were observed in association with exposure to air pollution. Specifically, interquartile-range increases of 51.8 µg/m3, 2.02 µg/m3, and 13.7 ppb in prior 4-hour exposure to PM2.5, black carbon, and nitrogen dioxide were associated with significant reductions in the standard deviation of the normal-to-normal intervals of 4.2% (95% confidence interval (CI): 1.9, 6.4), 4.2% (95% CI: 1.8, 6.6), and 3.9% (95% CI: 2.2, 5.7), respectively. Greater heart rate variability declines were observed among subjects with C-reactive protein values above the 90th percentile, subjects with a body mass index greater than 25, and females. The authors conclude that autonomic and vascular dysfunction may be one of the mechanisms through which air pollution exposure can increase cardiovascular disease risk, especially among persons with systemic inflammation and overweight.
Keywords: air pollution, carbon, heart rate, inflammation, obesity, particulate matter, soot
Epidemiologic evidence suggests that acute and chronic cardiovascular disease (CVD) mortality and morbidity are related to exposure to ambient pollutants (especially particulate matter (PM)) that may trigger cardiovascular events within hours or days of exposure (1). Increased incidences of specific acute CVDs, including myocardial infarction, ischemic stroke, heart failure, cardiac arrhythmia, atrial fibrillation, and peripheral arterial and venous diseases, have been found to be associated with exposure to increased ambient PM (2–8). Among the hypothesized mechanisms for ambient pollution-associated cardiovascular effects, PM and its heterogeneous constituent exposures are thought to induce systemic inflammation, oxidative stress, increased blood coagulability, and autonomic and vascular imbalance (9–11). Studies have suggested that pollution from traffic-related sources, estimated by PM, black carbon, and gaseous pollutants, might be responsible for the cardiovascular endpoints (12–16).
Extensive studies have examined associations between decreased heart rate variability (HRV) and elevated blood pressure and exposure to fine particulates (particulate matter with an aerodynamic diameter less than 2.5 µm (PM2.5)) (17–20). Recent studies have focused on examining potential roles of CVD risk factors, such as inflammation, overweight, and metabolic syndrome, in modifying particulate-associated autonomic and vascular dysfunction (21–28). These studies have suggested that the air pollution-related cardiovascular responses may be enhanced in subjects with the noted risk factors for adverse cardiac outcomes. Thus, better understanding of the pathophysiologic mechanisms of ambient PM-mediated cardiovascular risk has important public health implications for the larger population with and without health condition impairments.
In 2008, unprecedented air pollution control actions focusing on control of traffic pollution throughout the Beijing Summer Olympic (August 8–24) and Paralympic (September 6–17) games temporarily resulted in dramatic improvements in Beijing's air quality (29–31). Since some sources of air pollution were controlled more intensively than others, the PM chemical composition and concentrations of PM and gaseous copollutants may have differed dramatically before and during the Olympics, thus providing a unique opportunity to address several prominently hypothesized pathophysiologic mechanisms of air pollution cardiovascular health effects that have not been clearly answered by previous studies. In this 2-year longitudinal follow-up of CVD patients before and during the 2008 Beijing Olympics, we hypothesized that increased exposure to air pollution would trigger cardiovascular dysfunction in vulnerable subjects with high baseline exposure to air pollution. We further assessed whether systemic inflammation, diabetes, overweight, and/or gender might modify cardiovascular responses in association with exposure to air pollution.
MATERIALS AND METHODS
Study subjects and design
Our study population consisted of 40 nonsmoking CVD patients (mean age = 65.6 years (standard deviation, 5.8) recruited through the on-campus clinic of Peking University Health Science Center (PKUHSC), Beijing, China (32). All subjects were retired employees of PKUHSC and lived on campus. Of these subjects, 40 patients participated in 24-hour ambulatory electrocardiographic (ECG) monitoring repeatedly, during summer 2007 and summer 2008. A subset of 23 patients participated in 24-hour ambulatory blood pressure monitoring conducted in summer 2008. The institutional review board of PKUHSC approved the study protocol. Informed consent was obtained from each subject prior to study participation.
Outcome measurements
Demographic information on each subject's age, gender, body mass index (weight (kg)/height (m)2), smoking status, and medical history was obtained through a baseline questionnaire interview administered during the participant recruitment process. During each visit, subjects were given a diary to record any symptoms such as shortness of breath during physical activity, at the time of the visit and 24 hours before. Fasting venous blood samples were obtained from each participant at the end of each visit. Blood samples were analyzed by the clinical laboratory of PKUHSC Third Affiliated Hospital for high-sensitivity C-reactive protein, von Willebrand factor, fibrinogen, and viscosity.
Each subject was fitted with an ambulatory ECG monitor that recorded heart rate data between 8:00 am and 10:00 am the next day, when it was removed. The hookup of a V7 bipolar lead placement of the ECG monitor and skin preparation was conducted by trained personnel at each subject's home, following a standard protocol. ECG data were recorded digitally on removable flash cards using a standard 3-channel Holter Monitor (model MGY H7; DM Software Inc., Stateline, Nevada). The ECG digital recordings were reviewed and processed by trained cardiologists. Consecutive 5-minute measurements of heart rate and various measures of HRV were calculated for each monitoring session of each subject using personal computer-based software (Holter System, version 12.Net for Windows; DM Software Inc.). Each 5-minute segment was processed to measure the normal-to-normal intervals of the heartbeat, which were used to calculate the time-domain HRV index: the standard deviation of all normal-to-normal intervals (SDNN) and the root mean square of successive differences between adjacent normal cycles (rMSSD), as well as the frequency-domain HRV metrics of low frequency (0.04–0.15 Hz) and high frequency (0.15–0.4 Hz). On average, approximately 240–280 successful segments of 5-minute HRV measurements were obtained for data analysis from each subject during each visit.
Concurrent with ECG monitoring, ambulatory blood pressure measurements were taken every 30 minutes throughout the 24-hour monitoring period (MGY-ABP1; DM Software Inc.). The blood pressure recordings were reviewed and processed to obtain 30-minute systolic blood pressure and diastolic blood pressure readings, using personal computer-based software (Ambulatory Blood Pressure Monitor Analysis Software 1.0 for Windows; DM Software Inc.).
Air pollution measurements
Concurrent with ambulatory ECG monitoring, ambient pollution concentrations and meteorologic conditions were monitored continuously at a long-term air monitoring station located on the main campus of Peking University, which is approximately 3 km west of the PKUHSC campus. Both the Peking University air monitoring station and the PKUHSC campus study site are within 500 m of the fourth traffic ring road, which is one of the major traffic roads surrounding the urban area of Beijing.
The Peking University air monitoring station is located on the roof of a 4-story teaching building on campus. Routinely monitored pollutants include PM2.5, black carbon, sulfur dioxide, nitrogen dioxide, carbon monoxide, and ozone. Minute-to-minute average PM2.5 mass concentrations were determined with Tapered Element Oscillating Microbalance monitors (Thermo Fisher Scientific Inc., Franklin, Massachusetts). Five-minute black carbon concentrations were measured using the Multi-Angle Absorption Photometer (model 5012; Thermo Fisher Scientific Inc.). Minute-to-minute average sulfur dioxide, nitrogen dioxide, carbon monoxide, and ozone concentrations were monitored by means of EC9800 series ambient gas analyzers (EcoTech Pty. Ltd., Knoxfield, Victoria, Australia), where the instruments were maintained by a contract instrument maintenance company and autocalibrated between midnight and 1:00 am on a daily basis. Temperature (°C) and relative humidity (%) were measured using a Met One unit (Met One Instruments Inc., Grants Pass, Oregon) at the same location. All minute-to-minute environmental observations were processed into 5-minute and 30-minute averages corresponding to concurrent 5-minute HRV and 30-minute blood pressure measurements for further analysis.
Concurrent with fixed-location air monitoring, we conducted personal exposure assessment for each subject, using portable samplers measuring real-time PM2.5 concentrations with a DustTrak instrument (model 8520; TSI Inc., Shoreview, Minnesota) and real-time concentrations of carbon monoxide, carbon dioxide, temperature, and relative humidity with a QTrak instrument (model 7565; TSI Inc.). Integrated personal samples of PM2.5 mass were concurrently collected using an impactor (PEM200; MSP Corporation, Shoreview, Minnesota) for daily calibration of the average DustTrak response and for PM2.5 mass composition analyses. High correlations between ambient and personal measurements of PM2.5, using DustTrak monitors, were observed (r ≈ 0.95; data not shown).
Statistical analysis
The initial descriptive statistical analysis was conducted for all 5-minute HRV and 30-minute blood pressure variables and environmental measurements. Data for all HRV variables were logarithmically transformed because of right-skewed distributions. Collinearity of pollutants was examined using Spearman correlation analyses. Linear mixed-model analysis was used to estimate the association between ambient pollution and repeatedly measured outcomes.
We first built basic models without including pollutants. Subjects' age, gender, and body mass index were included in the basic models. Temperature and relative humidity were modeled with linear, squared, and cubic terms controlling for meteorologic effects on autonomic and vascular function. Day of the week and visit were included as categorical variables controlling for environmental changes over time. Because our elderly subjects may have engaged in more quiet and sedentary activities during the morning and night and may have become more active during the late morning to early afternoon, we chose to include centered time and time-squared terms controlling for the quadratic activity pattern of our subjects. The first-order autoregressive model was chosen to account for temporal autocorrelation of outcome variables on the basis of minimizing Akaike's Information Criterion.
Single-pollutant models were then developed by including pollutant variables to examine the association between various autonomic and vascular outcomes and exposure to air pollution during prior hours. We used residual diagnostics to investigate deviations from standard linear mixed-model assumptions, the presence of influential observations, and subject clusters. We further explored whether the association could be partially explained by certain blood markers by including high-sensitivity C-reactive protein, fibrinogen, and von Willebrand factor in the models. Results were insensitive to inclusion of any of the blood markers, so they were not considered further.
To evaluate potential interaction by systemic inflammation, overweight, diabetes, and gender, we conducted stratified analysis for SDNN to obtain the effect estimates for each air pollutant and compared effect differences between strata for statistical significance (P < 0.05). The stratification cutpoint for systemic inflammation status was determined by the C-reactive protein value at the 90th percentile, 1.76 mg/L. Stratification by overweight status was conducted for subjects with a body mass index less than 25 (normal weight; n = 24) and subjects with a body mass index greater than or equal to 25 (n = 16; only 1 of these subjects had a body mass index greater than or equal to 30). The interactions were examined with a Z test.
To assess whether the associations observed in single-pollutant models were attributable to other pollutants from the same source or different sources, we then examined the effects of 2-pollutant mixtures with the joint exposure contrasts. Further, we derived exposure-response curves of HRV reduction versus lagged air pollution exposures to examine the linear association assumption of mixed models.
All estimates are presented as percent changes (with 95% confidence intervals) associated with interquartile-range (IQR) increases in pollutant concentrations. All analyses were conducted using SAS statistical software, version 9.1 (SAS Institute Inc., Cary, North Carolina).
RESULTS
Eligible study subjects included 40 CVD patients, who had repeated measurements taken on multiple outcomes of interest (Table 1). Subjects were on average 65.6 years of age (standard deviation, 5.8); 60% were female, 40% were overweight, and 22.5% had diagnosed diabetes. The correlations among log-transformed HRV indices were modest (coefficients ranged from 0.54 to 0.82; data not shown).
Table 1.
Demographic Characteristics and Cardiac Outcomes for 40 Cardiovascular Disease Subjects Followed Before and During the Summer Olympics in Beijing, China, 2007–2008
| Characteristic | No. | % | Mean (SD) |
|---|---|---|---|
| Age, years | 65.6 (5.8) | ||
| Body mass indexa | 24 (4.0) | ||
| Cardiac outcome measureb | |||
| High-sensitivity C-reactive protein, mg/L | 1.05 (1.83) | ||
| Fibrinogen, g/L | 3.10 (1.07) | ||
| von Willebrand factor, μmol/L | 154.0 (45.0) | ||
| Heart rate, beats/minute | 64.2 (16.8) | ||
| SDNN, msec | 44.5 (27.0) | ||
| r-MSSD, msec | 26.5 (20.1) | ||
| Low frequency, ms2 | 338.3 (602.1) | ||
| High frequency, ms2 | 162.2 (296.9) | ||
| Gender | |||
| Male | 16 | 40 | |
| Female | 24 | 60 | |
| Primary cardiovascular diagnosis | |||
| Coronary artery disease | 6 | 15 | |
| Angina pectoris | 18 | 45 | |
| Arrhythmia | 16 | 40 | |
| High-sensitivity C-reactive protein, mg/L | |||
| >1.76 | 6 | 15 | |
| ≤1.76 | 34 | 85 | |
| Diabetic status | |||
| Diabetes | 9 | 22.5 | |
| No diabetes | 31 | 77.5 | |
| Overweight status (body mass index ≥25) | |||
| ≥25 (overweight) | 16 (40) | ||
| <25 (normal weight) | 24 (60) |
Abbreviations: r-MSSD, square root of the mean squared difference between adjacent normal-to-normal intervals; SD, standard deviation; SDNN, standard deviation of the normal-to-normal intervals.
a Weight (kg)/height (m)2.
b Mean values for outcome measures were calculated on the basis of the total number of measurements, ignoring the intrasubject correlation from repeated measures.
Ambient air quality changes before and during the Olympics
We observed significant reductions in mean air pollutant concentrations during the 2008 Olympic period (visit 4), when air pollution control measures were in place (Table 2), as compared with the pre-Olympic period (visit 3): PM2.5 levels decreased by 28%, black carbon levels decreased by 27.6%, and reductions of 12%–47% were observed for levels of gaseous pollutants. Compared with visit 2, conducted in 2007, the pollution level reductions from visit 2 to visit 4 were also significant but mostly of smaller magnitude. Among the pollutants, correlations were highest between PM2.5 and the traffic markers black carbon and carbon monoxide (coefficients were 0.57 and 0.60, respectively) and between black carbon, nitrogen dioxide, and carbon monoxide (coefficients ranged from 0.53 to 0.73), whereas sulfur dioxide and ozone were not correlated with other pollutants or were negatively correlated with other pollutants (Table 3).
Table 2.
Summary Statistics (Mean Values) for Hourly Ambient Air Pollution Concentrations and Meteorologic Parameters Measured Before and During the Summer Olympics in Beijing, China, 2007–2008
| Summer 2007 |
Summer 2008 |
Change in Pollution Level, % |
||||
|---|---|---|---|---|---|---|
| Visit 1 (July 1–9) | Visit 2 (August 15–25) | Visit 3 (July 4–11) | Visit 4 (August 23–29) | Visit 4 vs. Visit 3 | Visit 4 vs. Visit 2 | |
| PM2.5, µg/m3 | 112.5 (61.3)a | 78.3 (50.6) | 89.2 (53.9) | 64.2 (39.9) | −28.0 | −18.0 |
| Black carbon, µg/m3 | 4.0 (2.0) | 2.9 (1.5) | 2.1 (1.1) | −27.6 | −47.5 | |
| Sulfur dioxide, ppb | 2.4 (2.8) | 8.2 (4.7) | 11.2 (9.1) | 5.9 (4.0) | −47.3 | −28.0 |
| Nitrogen dioxide, ppb | 33.8 (14.3) | 26.3 (11.2) | 29.2 (9.0) | 22.9 (11.6) | −21.6 | −12.9 |
| Ozoneb, ppb | 51.8 (27.7) | 57.8 (23.4) | 60.5 (39.1) | 53.4 (17.9) | −11.7 | −7.6 |
| Carbon monoxide, ppm | 2.6 (1.6) | 1.3 (0.6) | 1.1 (0.4) | 0.9 (0.3) | −18.2 | −30.1 |
| Temperature, °C | 29.7 (3.8) | 30.2 (2.9) | 30.3 (4.4) | 27.7 (5.3) | ||
| Relative humidity, % | 60.7 (15.1) | 54.2 (15.6) | 63.0 (19.6) | 65.4 (22.5) | ||
Abbreviation: PM2.5, particulate matter with an aerodynamic diameter less than 2.5 µm.
a Numbers in parentheses, standard deviation.
b 10-hour maximum values for hourly ozone were used.
Table 3.
Spearman Correlation Coefficients for Correlations Between Daily Air Pollutant Concentrations and Meteorologic Parameters Measured Before and During the Summer Olympics in Beijing, China, 2007–2008
| PM2.5 | Black Carbon | Nitrogen Dioxide | Sulfur Dioxide | Carbon Monoxide | Ozone | Temperature | Relative Humidity | |
|---|---|---|---|---|---|---|---|---|
| PM2.5 | 1.00 | 0.57 | 0.34 | −0.01 | 0.60 | −0.06 | −0.02 | 0.32 |
| Black carbon | 1.00 | 0.61 | 0.09 | 0.73 | −0.46 | −0.24 | 0.36 | |
| Nitrogen dioxide | 1.00 | −0.03 | 0.53 | −0.43 | −0.20 | 0.28 | ||
| Sulfur dioxide | 1.00 | −0.04 | −0.07 | −0.03 | −0.06 | |||
| Carbon monoxide | 1.00 | −0.19 | −0.03 | 0.27 | ||||
| Ozone | 1.00 | 0.65 | −0.55 | |||||
| Temperature | 1.00 | −0.86 | ||||||
| Relative humidity | 1.00 |
Abbreviation: PM2.5, particulate matter with an aerodynamic diameter less than 2.5 µm.
Association between air pollution and autonomic and vascular dysfunction
Significant inverse associations between air pollution and the HRV index were observed in cumulative exposure assessment (Table 4). In association with an IQR increase of 48.4 µg/m3 in preceding 12-hour average exposure to PM2.5, we observed the greatest reductions for SDNN (4.7%, 95% confidence interval (CI): 1.7, 7.6) and low frequency (14.2%, 95% CI: 6.8, 21.0). For PM2.5 exposure, the reductions in the frequency-domain variables low frequency and high frequency were 2- to 3-fold greater than those in time-domain variables across all of the time periods examined. Per an IQR increase of 2.02 µg/m3 in preceding 4-hour exposure to black carbon, the decreases in SDNN (4.2%, 95% CI: 1.8, 6.6) and low frequency (10.5%, 95% CI: 4.4, 16.3) were of similar magnitude and in the same direction as PM2.5 exposure associated with SDNN and low frequency reductions. We observed some negative effects of nitrogen dioxide and carbon monoxide on HRV reductions associated within hours of exposure, but not for sulfur dioxide and ozone overall.
Table 4.
Adjusted Percent Change in Heart Rate Variability Indices Per Interquartile-Range Increase in Prior 1-Hour, 4-Hour, and 12-Hour Moving Average Exposure to Ambient Pollutants in Single-Pollutant, Mixed-Effects Modelsa, Beijing, China, 2007–2008
| Heart Rate Variability Index | PM2.5 |
Black Carbon |
Nitrogen Dioxide |
Sulfur Dioxide |
Carbon Monoxide |
Ozone |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Effect | 95% CI | Effect | 95% CI | Effect | 95% CI | Effect | 95% CI | Effect | 95% CI | Effect | 95% CI | |
| SDNN | ||||||||||||
| 1-hour | −3.5 | −5.6, −1.4 | −3.3 | −5.7, −0.8 | −1.9 | −3.4, −0.3 | 0.5 | −0.6, 1.6 | −0.3 | −2.5, 2.0 | −1.1 | −3.1, 0.8 |
| 4-hour | −4.2 | −6.4, −1.9 | −4.2 | −6.6, −1.8 | −3.9 | −5.7, −2.2 | 0.6 | −0.4, 1.5 | −2.7 | −5.3, 0.4 | −0.5 | −2.5, 1.5 |
| 12-hour | −4.7 | −7.6, −1.7 | −4.2 | −7.5, −0.8 | −3.6 | −5.5, −1.6 | −0.8 | −2.5, 0.9 | −2.2 | −6.2, 2.1 | 0.8 | −1.8, 3.5 |
| r-MSSD | ||||||||||||
| 1-hour | −4.2 | −7.5, −0.8 | −4.2 | −8.4, 0.1 | 1.4 | −1.1, 3.9 | 1.4 | 0.0, 2.8 | −2.5 | −5.6, 0.5 | −2.6 | −5.6, 0.5 |
| 4-hour | −5.5 | −9.4, −1.5 | −4.1 | −8.4, 0.4 | −2.2 | −5.7, 1.5 | 1.0 | −0.5, 2.5 | −4.0 | −7.5, −0.4 | −4.0 | −7.5, −0.4 |
| 12-hour | −10.7 | −15.5, −5.7 | −5.5 | −11.8, 1.3 | −2.2 | −6.1, 2.0 | 2.6 | −1.1, 6.5 | −3.0 | −7.6, 1.9 | −3.0 | −7.6, 1.9 |
| Low frequency | ||||||||||||
| 1-hour | −8.8 | −14.0, −3.4 | −9.1 | −15.0, −2.7 | −5.4 | −9.3, −1.4 | 1.1 | −1.7, 4.0 | 0.2 | −4.8, 5.5 | 0.2 | −4.8, 5.5 |
| 4-hour | −9.9 | −15.4, −4.1 | −10.5 | −16.3, −4.4 | −8.9 | −13.2, −4.3 | 0.7 | −1.8, 3.2 | −2.4 | −7.4, 2.9 | −2.4 | −7.4, 2.9 |
| 12-hour | −14.2 | −21.0, −6.8 | −9.3 | −17.6, −0.2 | −7.9 | −12.8, −2.8 | −4.8 | −9.1, −0.3 | −6.6 | −12.8, 0.0 | −6.6 | −12.8, 0.0 |
| High frequency | ||||||||||||
| 1-hour | −9.3 | −15.2, −2.9 | −12.1 | −19.1, −4.6 | −3.5 | −8.2, 1.4 | 2.5 | −0.5, 5.7 | −2.3 | −8.1, 3.9 | −2.3 | −8.1, 3.9 |
| 4-hour | −8.4 | −15.2, −1.0 | −9.8 | −17.0, −2.1 | −5.1 | −11.0, 1.3 | 3.4 | 0.3, 6.5 | −6.0 | −12.1, 0.5 | −6.0 | −12.1, 0.5 |
| 12-hour | −16.3 | −24.3, −7.5 | −4.0 | −15.1, 8.6 | −3.7 | −10.4, 3.5 | 4.1 | −2.2, 10.9 | −8.7 | −16.4, -0.2 | −8.7 | −16.4, −0.2 |
Abbreviations: CI, confidence interval; PM2.5, particulate matter with an aerodynamic diameter less than 2.5 µm; r-MSSD, square root of the mean squared difference between adjacent normal-to-normal intervals; SDNN, standard deviation of the normal-to-normal intervals.
a Results were adjusted for age, body mass index, gender, time of day, day of the week, visit, temperature, and relative humidity.
Both systolic blood pressure and diastolic blood pressure were found to be significantly increased within minutes to hours of exposure to PM2.5 (Table 5). We observed 2.2- to 8.5-mm Hg increases in blood pressure with an IQR increase of 56.9 µg/m3 in prior 30-minute exposure to PM2.5. There was a significant increase of 3.1 mm Hg (95% CI: 0.4, 5.7) in diastolic blood pressure associated with prior 12-hour exposure to black carbon. We also observed significant blood pressure increases of up to 6.7 mm Hg with prior 12-hour exposure to carbon monoxide.
Table 5.
Adjusted Change in Blood Pressure Indices per Interquartile-Range Increase in Prior 30-Minute, 2-Hour, 12-Hour, and 24-Hour Moving Average Exposure to Ambient Pollutants in Single-Pollutant, Mixed-Effects Modelsa, Beijing, China, 2008
| PM2.5 |
Black Carbon |
Nitrogen Dioxide |
Sulfur Dioxide` |
Carbon Monoxide |
Ozone |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Effect | 95% CI | Effect | 95% CI | Effect | 95% CI | Effect | 95% CI | Effect | 95% CI | Effect | 95% CI | |
| Systolic blood pressure | ||||||||||||
| 30-minute | 5.7 | 2.9, 8.5 | 0.4 | −1.8, 2.5 | 1.8 | −0.5, 4.0 | −0.9 | −2.0, 0.2 | −0.2 | −2.0, 1.7 | −0.3 | −1.3, 0.8 |
| 2-hour | 3.9 | 0.0, 7.7 | −1.1 | −3.1, 1.1 | 0.0 | −2.6, 2.6 | −0.4 | −1.7, 0.8 | 0.5 | −2.1, 3.2 | −1.6 | −3.4, 0.2 |
| 12-hour | 5.3 | −1.3, 11.8 | 2.2 | −1.0, 5.5 | 0.7 | −3.2, 4.6 | −0.4 | −2.5, 1.7 | 3.3 | 0.0, 6.7 | −0.2 | −2.5, 2.1 |
| 24-hour | −1.2 | −3.6, 1.2 | −0.5 | −5.4, 4.4 | −0.8 | −6.6, 5.0 | −0.6 | −2.2, 0.9 | -0.4 | −2.9, 2.1 | 1.5 | −0.2, 3.2 |
| Diastolic blood pressure | ||||||||||||
| 30-minute | 4.6 | 2.2, 7.0 | −0.2 | −2.0, 1.6 | 1.1 | -0.9, 3.0 | −0.3 | −1.2, 0.6 | 0.3 | −1.2, 1.8 | −0.3 | −1.2, 0.6 |
| 2-hour | 1.9 | −1.3, 5.0 | −1.4 | −3.2, 0.3 | -0.1 | −2.3, 2.1 | 0.0 | −1.1, 1.1 | 0.4 | −1.8, 2.5 | −1.1 | −2.7, 0.4 |
| 12-hour | 5.2 | −0.1, 10.5 | 3.1 | 0.4, 5.7 | 1.2 | −2.1, 4.5 | 0.3 | −1.3, 2.0 | 3.5 | 0.9, 6.1 | −0.5 | −2.5, 1.4 |
| 24-hour | −0.7 | −2.8, 1.3 | 1.8 | −2.2, 5.8 | 1.5 | −3.4, 6.4 | 0.1 | −1.2, 1.3 | 0.6 | −1.4, 2.6 | 1.0 | −0.5, 2.4 |
Abbreviations: CI, confidence interval; PM2.5, particulate matter with an aerodynamic diameter less than 2.5 µm.
a Results were adjusted for age, body mass index, gender, time of day, day of the week, visit, temperature, and relative humidity.
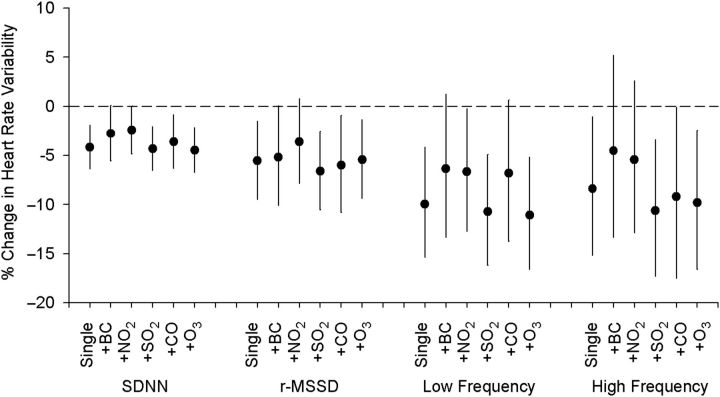
To assess whether the observed effects of PM2.5 on HRV decline were attributable to other pollutants, we compared the effects estimated in single-pollutant models of PM2.5 exposure alone with those estimated in 2-pollutant models including black carbon, nitrogen dioxide, sulfur dioxide, carbon monoxide, and ozone, respectively, as the second variable (Figure 1). After adjustment for the other pollutants, the overall PM2.5 effects remained significant, with some apparent confounding effects from black carbon, nitrogen dioxide, and carbon monoxide on the PM2.5-HRV association.
Figure 1.
Adjusted percent change in heart rate variability indices per interquartile-range increase in prior 4-hour moving average exposure to PM2.5 (particulate matter with an aerodynamic diameter less than 2.5 µm) in single- and 2-pollutant, mixed-effects models, Beijing, China, 2007–2008. Models were adjusted for age, body mass index, gender, time of day, day of the week, visit, temperature, and relative humidity. SDNN, standard deviation of the normal-to-normal intervals; r-MSSD, square root of the mean squared difference between adjacent normal-to-normal intervals; BC, black carbon; NO2, nitrogen dioxide; SO2, sulfur dioxide; CO, carbon monoxide; O3, ozone. Bars, 95% confidence interval.
Mixed-model analyses with restricted spline functions confirmed the linear relation between HRV reduction and increased exposures to PM2.5, black carbon, nitrogen dioxide, and carbon monoxide (data not shown). We observed similar exposure-response patterns for SDNN, rMSSD, low frequency, and high frequency, with prior 1-, 4-, and 12-hour averaged exposure to air pollution.
Interactions of systemic inflammation, overweight, and gender
We examined possible interaction of systemic inflammation, overweight, diabetes, and gender for the stratified subgroups (Table 6). Within the subgroups, greater HRV reductions were observed with exposure to PM2.5, black carbon, and nitrogen dioxide among obese subjects and females. In association with IQR increases of 51.8 µg/m3 and 2.02 µg/m3 in preceding 4-hour average exposures to PM2.5 and black carbon, the decreases in SDNN were significant (9.3% (95% CI: 5.4, 13.0) and 8.8% (95% CI: 4.9, 12.6), respectively) for overweight subjects (body mass index ≥25) but not significant in subjects with normal body weight. For females, significant decreases in SDNN (4.8% (95% CI: 2.4, 7.2) and 9.3% (95% CI: 6.8, 11.7)) were observed as compared with nonsignificant declines in males.
Table 6.
Interactions of Systemic Inflammation, Overweight, Diabetes, and Gender With Adjusted Percent Change in SDNN per Interquartile-Range Increase in Prior 4-Hour Moving Average Exposure to Air Pollution in Single-Pollutant, Mixed-Effects Modelsa, Beijing, China, 2007–2008
| Interaction Variable | PM2.5 |
Black Carbon |
Nitrogen Dioxide |
Sulfur Dioxide |
Carbon Monoxide |
Ozone |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Effect | 95% CI | Effect | 95% CI | Effect | 95% CI | Effect | 95% CI | Effect | 95% CI | Effect | 95% CI | |
| High-sensitivity C-reactive protein, mg/L | ||||||||||||
| >1.76 | −3.2 | −7.2, 1.1 | −2.5 | −6.8, 2.0 | −15.5 | −19.3, −11.5 | 19.3 | 9.6, 29.8 | −8.2 | −15.3, −0.4 | 7.3 | 2.2, 12.7 |
| ≤1.76 | −1.6 | −4.0, 1.0 | −0.3 | −2.9, 2.4 | −1.4 | −3.3, 1.0 | 0.1 | −0.8, 1.0 | −1.7 | −4.9, 1.5 | −2.0 | −4.0, 0.1 |
| P valueb | 0.51 | 0.44 | <0.001 | <0.001 | 0.13 | <0.001 | ||||||
| Overweight status (body mass indexc ≥25) | ||||||||||||
| ≥25 (overweight) | −9.3 | −13.0, −5.4 | −8.8 | −12.6, −4.9 | −4.9 | −7.7, −2.0 | 0.8 | −0.4, 2.0 | −2.3 | −7.2, 2.8 | 0.7 | −2.6, 4.2 |
| <25 (normal weight) | 1.4 | −1.0, 3.9 | 0.9 | −1.7, 3.7 | −3.7 | −5.7, −1.5 | −2.1 | −3.9, −0.2 | 3.6 | −0.1, 7.5 | −2.2 | −4.3, 0.0 |
| P value | <0.001 | <0.001 | 0.49 | 0.01 | 0.07 | 0.16 | ||||||
| Diabetic status | ||||||||||||
| Diabetes | 1.2 | −2.1, 4.6 | 1.5 | −2.0, 5.2 | 3.0 | 0.3, 5.7 | 3.9 | 2.3, 5.5 | 2.5 | −2.3, 7.4 | −7.2 | −9.8, −4.5 |
| No diabetes | −5.1 | −7.6, −2.5 | −5.1 | −7.7, −2.3 | −6.0 | −7.9, −3.9 | −0.1 | −1.2, 0.9 | −1.2 | −4.8, 2.6 | 1.2 | −1.1, 3.6 |
| P value | 0.003 | 0.003 | <0.001 | <0.001 | 0.24 | <0.001 | ||||||
| Gender | ||||||||||||
| Female | −4.8 | −7.2, −2.4 | −9.3 | −11.7, −6.8 | −8.2 | −10.1, −6.3 | −0.8 | −1.8, 0.3 | −10.4 | −13.6, −7.2 | 1.6 | −0.7, 4.0 |
| Male | −2.7 | −6.7, 1.52 | 1.6 | −2.7, 6.0 | 0.6 | −2.4, 3.7 | 2.6 | 1.0, 4.4 | 7.5 | 1.9, 13.4 | −10.3 | −18.0, −1.9 |
| P value | 0.38 | <0.001 | <0.001 | <0.001 | <0.001 | 0.01 | ||||||
Abbreviations: CI, confidence interval; PM2.5, particulate matter with an aerodynamic diameter less than 2.5 µm; SDNN, standard deviation of the normal-to-normal intervals.
a Results were adjusted for age, body mass index, gender, time of day, day of the week, visit, temperature, and relative humidity.
b P for difference between subgroups, by Z test.
c Weight (kg)/height (m)2.
In the 6 subjects with C-reactive protein values in the upper 10th percentile range, we also observed significant reductions in HRV with increased exposure to nitrogen dioxide and carbon monoxide and greater reduction in exposure to PM2.5 and black carbon. However, the diabetic subjects were found to be less susceptible to air pollution exposure.
DISCUSSION
CVD is now the leading cause of death among Chinese adults aged 40 years or older, accounting for approximately 40% of total mortality in China (33). Although the increased cardiovascular mortality risks associated with air pollution observed in the Chinese population might be similar in magnitude, per the amount of pollution, to the risks found in other parts of the world (34, 35), few studies have assessed cardiovascular morbidity or subclinical variables in relation to ambient pollution exposure among vulnerable urban residents in China.
In this 2-year longitudinal follow-up of a group of CVD patients, we observed significant declines in levels of most pollutants (except ozone) during the 2008 Summer Olympic period, when air pollution reductions efforts were in place. Over the exposure range measured during the study period, we observed significant HRV reduction in CVD patients associated with short-term exposure to air pollution, and the largest effects were found for the particulate measures PM2.5 and black carbon. The associations were found to be stronger for females, overweight persons, and those with increased systemic inflammation. We also observed some significant increases in blood pressure with acute exposure to PM2.5, black carbon, and carbon monoxide. Our results are largely consistent with existing literature on the short-term effects of PM on autonomic and vascular function (1, 17, 26, 36). Our study also adds to the cumulative evidence that exposure to traffic-related pollutants such as black carbon, nitrogen dioxide, and carbon monoxide may alter cardiovascular function (23, 37, 38).
Constituents of ambient particulates from traffic emissions were found to be more toxic than those from other sources (14, 39). Recent analyses have provided evidence that exposure to the ambient particulates PM2.5 and black carbon can mediate autonomic dysfunction (19, 38). In the current study, the observed time course, direction, and magnitude of HRV and blood pressure changes in association with exposure to PM2.5, black carbon, and other pollutants support the biologic plausibility of a causal relation between particulate air pollution and autonomic and vascular dysfunction: Following inhalation of ambient particulates, the toxic constituents in ambient particulates may have direct and immediate effects on blood and cardiovascular systems through pulmonary receptors (40). Toxic substances present in PM2.5 (i.e., black carbon, primary and secondary aerosols, metals) and gaseous pollutants (e.g., carbon monoxide, nitrogen dioxide) can further cross airway epithelium, reaching the vasculature, and induce the production of proinflammatory cytokines and reactive oxygen species (41, 42). The effects of those agents might subsequently lead to hypertensive responses and changes in autonomic cardiac control (40, 43).
In this study, we observed greater declines in HRV in the subgroups of overweight patients and those with increased systemic inflammation, in association with exposure to ambient particulate pollution. Given that a number of clinical and epidemiologic studies have found that overweight and systemic inflammation are important in modifying air pollution PM-associated CVD risk (21, 36, 44– 46), our findings highlight the importance of examining risk factors that could potentially modify cardiovascular responses to air pollution exposure.
Previous research suggested that gender could modify the associations between air pollution and CVD risk; however, the findings are heterogeneous (47–49). In the Chinese population, women have been found to be more susceptible to the effects of air pollution exposure (50). Although we observed larger HRV declines in association with exposures to black carbon and gaseous pollutants among female CVD patients, the mechanisms for gender-specific interaction are not yet clear and deserve further investigation.
Recent studies have also suggested that the metabolic abnormalities and neuropathophysiologic activities underlying the diabetic state may confer PM-associated autonomic and cardiovascular dysfunction. Some studies found that associations between PM exposure and autonomic dysfunction are stronger among persons with type 2 diabetes (18, 36, 45), whereas others suggested that subjects with individual components of the metabolic syndrome, such as abdominal overweight, type 2 diabetes, hypertension, and dyslipidemia, might be at higher risk of PM-associated cardiovascular dysfunction (26, 27). However, the diagnosed diabetic subjects appeared less susceptible to air pollution exposure in our analyses. Lack of individual data on the metabolic syndrome might have limited our capability for further examining the biologic plausibility of the metabolic syndrome in modifying PM2.5-associated cardiovascular dysfunction. Nevertheless, future research is needed to determine how underlying metabolic mechanisms may impart differential autonomic responses between metabolic syndrome groups.
Compared with most of the published studies examining acute effects of ambient particulate pollution on short-term cardiovascular biomarker variations (in each 5-minute ECG and 30-minute blood pressure segment), our study design had the following strengths. First, it took advantage of a once-in-a-lifetime natural experiment in which the Chinese government mandated temporary closures or relocations of industry and reductions in motor vehicle use before and during the 2008 Beijing Summer Olympics. Exposure measurements taken before and during the Olympics clearly showed that there were major reductions in air pollutant concentrations. Second, there was sufficient time to establish a suitable group of CVD subjects who could be followed during this major change in ambient exposures. The collection of data from four 24-hour ECG sessions and two 24-hour blood pressure sessions over an extended 2-year follow-up period allowed us to assess the acute effects of measured air pollution on autonomic and vascular function under contrasting exposure conditions. Lastly, the large variations in levels of the ambient pollutants during the study period provided unique contrasts in ambient pollution exposures with which to examine the exposure-response relations over a wide range of pollution levels. The linear relations observed between HRV reduction and exposure to PM2.5 and traffic pollutants extend our knowledge of the CVD risks of ambient pollution exposure at higher levels and a wider range than would normally occur in the developed world.
Despite advantages of this natural experimental study, several limitations should also be noted in interpreting our findings. First, use of nearby fixed-location monitoring data and lack of information on personal exposure to ambient pollution may have resulted in potential exposure misclassification errors and may have biased the effect estimates toward the null. Second, we did not observe a consistently negative association pattern across pollutants, suggesting the possibility of differential measurement errors between pollutants. If the monitoring instrument error were independent of the true ambient pollution level, such differential measurement error would be expected to attenuate the effect estimates and may have biased some results. Lastly, we did not consider exposure from indoor sources, given that our subjects tended to spend a large portion of their time indoors. However, other studies have suggested that daily population average concentrations of pollutants derived from indoor sources are approximately independent of ambient pollution levels (51). When this is true, failure to measure indoor sources will not introduce further bias in the estimated effects of ambient pollutants (52).
In summary, our study documents acute effects of exposure to air pollution on autonomic and vascular dysfunction among CVD subjects, over an extended follow-up period. Our results suggest effects of interactions between overweight, systemic inflammation, and gender on air pollution-attributable cardiovascular dysfunction among populations at risk of CVD.
ACKNOWLEDGMENTS
Author affiliations: SKJ Laboratory of Environmental Simulation and Pollution Control, College of Environmental Sciences and Engineering, Peking University, Beijing, China (Wei Huang, Tong Zhu, Min Hu, Yuanhang Zhang, Xiaoyan Tang); Centre for Environment and Health, Peking University, Beijing, China (Wei Huang, Tong Zhu, Min Hu, Yuanhang Zhang, Xiaoyan Tang); Department of Occupational and Environmental Health, School of Public Health, Peking University, Beijing, China (Xiaochuan Pan); Department of Biostatistics, School of Public Health, University of Medicine and Dentistry of New Jersey, Piscataway, New Jersey (Shou-en Lu, Yong Lin); and Beijing Municipal Institute of Labor Protection, Beijing, China (Tong Wang).
This study was supported by the Chinese National Science Foundation (grant 20637020), the China State Ministry of Environmental Protection (grant 201009032), and the Chinese Ministry of Science and Technology (grant 2008AA062503). Dr. Lu was partially supported by grant P30ES005022-21 from the US National Institute of Environmental Health Sciences.
The authors thank Ai Wang, Ya Chen, Dr. Yuping Jia, Dr. Liwen Zhang, Yuming Guo, and Hong Cheng for assisting with data collection. The authors also thank Dr. Thomas J. Smith of the Harvard School of Public Health for his comments.
Conflict of interest: none declared.
REFERENCES
- 1.Brook RD, III, Rajagopalan S, Pope CA, III, et al. Particulate matter air pollution and cardiovascular disease: an update to the scientific statement from the American Heart Association. Circulation. 2010;121(21):2331–2378. doi: 10.1161/CIR.0b013e3181dbece1. [DOI] [PubMed] [Google Scholar]
- 2.Peters A, Liu E, Verrier RL, et al. Air pollution and incidence of cardiac arrhythmia. Epidemiology. 2000;11(1):11–17. doi: 10.1097/00001648-200001000-00005. [DOI] [PubMed] [Google Scholar]
- 3.Pope CA, III, Thun MJ, Namboodiri MM, et al. Particulate air pollution as a predictor of mortality in a prospective study of U.S. adults. Am J Respir Crit Care Med. 1995;151(3):669–674. doi: 10.1164/ajrccm/151.3_Pt_1.669. [DOI] [PubMed] [Google Scholar]
- 4.Zanobetti A, Schwartz J. Particulate air pollution, progression, and survival after myocardial infarction. Environ Health Perspect. 2007;115(5):769–775. doi: 10.1289/ehp.9201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Krewski D, Jerrett M, Burnett RT, et al. Extended follow-up and spatial analysis of the American Cancer Society study linking particulate air pollution and mortality. Res Rep Health Eff Inst. 2009;(140):5–114. [PubMed] [Google Scholar]
- 6.Rich DQ, Schwartz J, Mittleman MA, et al. Association of short-term ambient air pollution concentrations and ventricular arrhythmias. Am J Epidemiol. 2005;161(12):1123–1132. doi: 10.1093/aje/kwi143. [DOI] [PubMed] [Google Scholar]
- 7.Dominici F, Peng RD, Bell ML, et al. Fine particulate air pollution and hospital admission for cardiovascular and respiratory diseases. JAMA. 2006;295(10):1127–1134. doi: 10.1001/jama.295.10.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baccarelli A, Martinelli I, Zanobetti A, et al. Exposure to particulate air pollution and risk of deep vein thrombosis. Arch Intern Med. 2008;168(9):920–927. doi: 10.1001/archinte.168.9.920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chuang KJ, Chan CC, Su TC, et al. The effect of urban air pollution on inflammation, oxidative stress, coagulation, and autonomic dysfunction in young adults. Am J Respir Crit Care Med. 2007;176(4):370–376. doi: 10.1164/rccm.200611-1627OC. [DOI] [PubMed] [Google Scholar]
- 10.Pope CA, III, Dockery DW. Health effects of fine particulate air pollution: lines that connect. J Air Waste Manag Assoc. 2006;56(6):709–742. doi: 10.1080/10473289.2006.10464485. [DOI] [PubMed] [Google Scholar]
- 11.Brook RD, Urch B, Dvonch JT, et al. Insights into the mechanisms and mediators of the effects of air pollution exposure on blood pressure and vascular function in healthy humans. Hypertension. 2009;54(3):659–667. doi: 10.1161/HYPERTENSIONAHA.109.130237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smith KR, Jerrett M, Anderson HR, et al. Public health benefits of strategies to reduce greenhouse-gas emissions: health implications of short-lived greenhouse pollutants. Lancet. 2009;374(9707):2091–2103. doi: 10.1016/S0140-6736(09)61716-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Franklin M, Koutrakis P, Schwartz P. The role of particle composition on the association between PM2.5 and mortality. Epidemiology. 2008;19(5):680–689. doi: 10.1097/ede.0b013e3181812bb7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grahame TJ. Does improved exposure information for PM2.5 constituents explain differing results among epidemiological studies? Inhal Toxicol. 2009;21(5):381–393. doi: 10.1080/08958370802380495. [DOI] [PubMed] [Google Scholar]
- 15.Maynard D, Coull BA, Gryparis A, et al. Mortality risk associated with short-term exposure to traffic particles and sulfates. Environ Health Perspect. 2007;115(5):751–755. doi: 10.1289/ehp.9537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Adar SD, Gold DR, Coull BA, et al. Focused exposures to airborne traffic particles and heart rate variability in the elderly. Epidemiology. 2007;18(1):95–103. doi: 10.1097/01.ede.0000249409.81050.46. [DOI] [PubMed] [Google Scholar]
- 17.Brook RD, Rajagopalan S. Particulate matter air pollution blood pressure. J Am Soc Hypertens. 2009;3(5):332–350. doi: 10.1016/j.jash.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 18.Park SK, O'Neill MS, Vokonas PS, et al. Effects of air pollution on heart rate variability: the VA Normative Aging Study. Environ Health Perspect. 2005;113(3):304–309. doi: 10.1289/ehp.7447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schwartz J, Litonjua A, Suh H, et al. Traffic related pollution and heart rate variability in a panel of elderly subjects. Thorax. 2005;60(6):455–461. doi: 10.1136/thx.2004.024836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gold DR, Litonjua A, Schwartz J, et al. Ambient pollution and heart rate variability. Circulation. 2000;101(11):1267–1273. doi: 10.1161/01.cir.101.11.1267. [DOI] [PubMed] [Google Scholar]
- 21.Luttmann-Gibson H, Suh HH, Coull BA, et al. Systemic inflammation, heart rate variability and air pollution in a cohort of senior adults. Occup Environ Med. 2010;67(9):625–630. doi: 10.1136/oem.2009.050625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dubowsky SD, Suh H, Schwartz J, et al. Diabetes, obesity, and hypertension may enhance associations between air pollution and markers of systemic inflammation. Environ Health Perspect. 2006;114(7):992–998. doi: 10.1289/ehp.8469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Delfino RJ, Tjoa T, Gillen DL, et al. Traffic-related air pollution and blood pressure in elderly subjects with coronary artery disease. Epidemiology. 2010;21(3):396–404. doi: 10.1097/EDE.0b013e3181d5e19b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.de Hartog JJ, Lanki T, Timonen KL, et al. Associations between PM2.5 and heart rate variability are modified by particle composition and beta-blocker use in patients with coronary heart disease. Environ Health Perspect. 2009;117(1):105–111. doi: 10.1289/ehp.11062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mordukhovich I, Wilker E, Suh H, et al. Black carbon exposure, oxidative stress genes, and blood pressure in a repeated-measures study. Environ Health Perspect. 2009;117(11):1767–1772. doi: 10.1289/ehp.0900591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Park SK, Auchincloss AH, O'Neill MS, et al. Particulate air pollution, metabolic syndrome, and heart rate variability: the Multi-Ethnic Study of Atherosclerosis (MESA) Environ Health Perspect. 2010;118(10):1406–1411. doi: 10.1289/ehp.0901778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Whitsel EA, Quibrera PM, Christ SL, et al. Heart rate variability, ambient particulate matter air pollution, and glucose homeostasis: the environmental epidemiology of arrhythmogenesis in the Women's Health Initiative. Am J Epidemiol. 2009;169(6):693–703. doi: 10.1093/aje/kwn400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schwartz J, Park SK, O'Neill MS, et al. Glutathione-S-transferase M1, obesity, statins, and autonomic effects of particles: gene-by-drug-by-environment interaction. Am J Respir Crit Care Med. 2005;172(12):1529–1533. doi: 10.1164/rccm.200412-1698OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang M, Zhu T, Zheng J, et al. Use of mobile laboratory to evaluate changes in on-road air pollutants during the Beijing 2008 Summer Olympics. Atmos Chem Phys. 2009;9(21):8247–8263. [Google Scholar]
- 30.Kipen H, Rich D, Huang W, et al. Measurement of inflammation and oxidative stress following drastic changes in air pollution during the Beijing Olympics: a panel study approach. Ann N Y Acad Sci. 2010;1203:160–167. doi: 10.1111/j.1749-6632.2010.05638.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu S, Deng F, Niu J, et al. Association of heart rate variability in taxi drivers with marked changes in particulate air pollution in Beijing in 2008. Environ Health Perspect. 2010;118(1):87–91. doi: 10.1289/ehp.0900818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jia YP, Guo YM, Wang ZY, et al. The correlations between air quality and heart rate variability in aged susceptible people during the Beijing Olympic Games 2008 [in Chinese] Zhonghua Yu Fang Yi Xue Za Zhi. 2009;43(8):669–673. [PubMed] [Google Scholar]
- 33.He J, Gu D, Wu X, et al. Major causes of death among men and women in China. N Engl J Med. 2005;353(11):1124–1134. doi: 10.1056/NEJMsa050467. [DOI] [PubMed] [Google Scholar]
- 34.Kan HD, Chen BH, Chen CH, et al. Establishment of exposure-response functions of air particulate matter and adverse health outcomes in China and worldwide. Biomed Environ Sci. 2005;18(3):159–163. [PubMed] [Google Scholar]
- 35.Aunan K, Pan XC. Exposure-response functions for health effects of ambient air pollution applicable for China—a meta-analysis. Sci Total Environ. 2004;329(1–3):3–16. doi: 10.1016/j.scitotenv.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 36.Schneider A, Neas LM, Graff DW, et al. Association of cardiac and vascular changes with ambient PM2.5 in diabetic individuals. Part Fibre Toxicol. 2010;7(14) doi: 10.1186/1743-8977-7-14. doi:10.1186/1743-8977-7-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fan ZT, Meng Q, Weisel C, et al. Acute exposure to elevated PM2.5 generated by traffic and cardiopulmonary health effects in healthy older adults. J Expo Sci Environ Epidemiol. 2009;19(5):525–533. doi: 10.1038/jes.2008.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zanobetti A, Gold DR, Stone PH, et al. Reduction in heart rate variability with traffic and air pollution in patients with coronary artery disease. Environ Health Perspect. 2010;118(3):324–330. doi: 10.1289/ehp.0901003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brook RD. Cardiovascular effects of air pollution. Clin Sci (Lond) 2008;115(6):175–187. doi: 10.1042/CS20070444. [DOI] [PubMed] [Google Scholar]
- 40.Simkhovich BZ, Kleinman MT, Kloner RA. Air pollution and cardiovascular injury epidemiology, toxicology, and mechanisms. J Am Coll Cardiol. 2008;52(9):719–726. doi: 10.1016/j.jacc.2008.05.029. [DOI] [PubMed] [Google Scholar]
- 41.O'Toole TE, Zheng YT, Hellmann J, et al. Acrolein activates matrix metalloproteinases by increasing reactive oxygen species in macrophages. Toxicol Appl Pharmacol. 2009;236(2):194–201. doi: 10.1016/j.taap.2009.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ghio AJ, Carter JD, Dailey LA, et al. Respiratory epithelial cells demonstrate lactoferrin receptors that increase after metal exposure. Am J Physiol. 1999;276(6):L933–L940. doi: 10.1152/ajplung.1999.276.6.L933. [DOI] [PubMed] [Google Scholar]
- 43.Sun Q, Yue P, Deiuliis JA, et al. Ambient air pollution exaggerates adipose inflammation and insulin resistance in a mouse model of diet-induced obesity. Circulation. 2009;119(4):538–546. doi: 10.1161/CIRCULATIONAHA.108.799015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fang SC, Cavallari JM, Eisen EA, et al. Vascular function, inflammation, and variations in cardiac autonomic responses to particulate matter among welders. Am J Epidemiol. 2009;169(7):848–856. doi: 10.1093/aje/kwn405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Baja ES, Schwartz JD, Wellenius GA, et al. Traffic-related air pollution and QT interval: modification by diabetes, obesity, and oxidative stress gene polymorphisms in the Normative Aging Study. Environ Health Perspect. 2010;118(6):840–846. doi: 10.1289/ehp.0901396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen JC, Cavallari JM, Stone PH, et al. Obesity is a modifier of autonomic cardiac responses to fine metal particulates. Environ Health Perspect. 2007;115(7):1002–1006. doi: 10.1289/ehp.9609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gold DR. Vulnerability to cardiovascular effects of air pollution in people with diabetes. Curr Diab Rep. 2008;8(5):333–335. doi: 10.1007/s11892-008-0058-2. [DOI] [PubMed] [Google Scholar]
- 48.Ito K, Thurston GD, Silverman RA. Characterization of PM2.5, gaseous pollutants, and meteorological interactions in the context of time-series health effects models. J Expo Sci Environ Epidemiol. 2007;17(suppl 2):S45–S60. doi: 10.1038/sj.jes.7500627. [DOI] [PubMed] [Google Scholar]
- 49.Künzli N, Jerrett M, Mack WJ, et al. Ambient air pollution and atherosclerosis in Los Angeles. Environ Health Perspect. 2005;113(2):201–206. doi: 10.1289/ehp.7523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kan H, London SJ, Chen G, et al. Season, sex, age, and education as modifiers of the effects of outdoor air pollution on daily mortality in Shanghai, China: The Public Health and Air Pollution in Asia (PAPA) Study. Environ Health Perspect. 2008;116(9):1183–1188. doi: 10.1289/ehp.10851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wilson WE, Suh HH. Fine particles and coarse particles: concentration relationships relevant to epidemiologic studies. J Air Waste Manag Assoc. 1997;47(12):1238–1249. doi: 10.1080/10473289.1997.10464074. [DOI] [PubMed] [Google Scholar]
- 52.Zeger SL, Thomas D, Dominici F, et al. Exposure measurement error in time-series studies of air pollution: concepts and consequences. Environ Health Perspect. 2000;108(5):419–426. doi: 10.1289/ehp.00108419. [DOI] [PMC free article] [PubMed] [Google Scholar]