Abstract
In the present study, the authors investigated the role of the intrauterine environment in childhood adiposity by comparing the maternal-offspring body mass index (BMI) association with the paternal-offspring BMI association when the offspring were 3 years of age, using parental prepregnancy BMI (measured as weight in kilograms divided by height in meters squared). The parent-offspring trios (n = 29,216) were recruited during pregnancy from 2001 to 2008 into the Norwegian Mother and Child Cohort Study conducted by The Norwegian Institute of Public Health. Data from self-administered questionnaires were used in linear regression analyses. Crude analyses showed similar parental-offspring BMI associations; the mean difference in offspring BMI was 0.15 (95% confidence interval: 0.13, 0.16) per each 1-standard-deviation increase in maternal BMI and 0.15 (95% confidence interval: 0.13, 0.17) per each 1-standard-deviation increase in paternal BMI. After all adjustments, the mean difference in offspring BMI per each 1-standard-deviation increment of maternal BMI was 0.12, and the mean difference in offspring BMI per each 1-standard-deviation increment of paternal BMI was 0.13. There was no strong support for heterogeneity between the associations (P > 0.6). In conclusion, results from the present large population-based study showed similar parental-offspring BMI associations when the offspring were 3 years of age, which indicates that the maternal-offspring association may be explained by shared familial (environmental and genetic) risk factors rather than by the intrauterine environment.
Keywords: adiposity, body mass index; child, preschool, fathers, infant, mothers, overweight, pregnancy
Editor's note: An invited commentary on this article appears on page 93, and the authors' response appears on page 97.
Maintaining a reduced weight after weight loss is difficult (1–3). Thus, it is important to detect and address the causes of overweight before it becomes an issue. It has been suggested that some causes may operate during pregnancy and increase the fetus's risk of adiposity later in life (1, 2, 4–7). This is often referred to as developmental overnutrition. The mechanisms involved might include permanent changes in neuroendocrine functioning or energy metabolism (4, 5, 7). It is well known that higher maternal body mass index (BMI, measured as weight in kilograms divided by height in meters squared) is associated with an increased risk of adiposity in offspring (8). Maternal BMI is also associated with maternal diet and blood glucose levels during pregnancy (9), both of which influence the intrauterine environment and potentially lead to developmental overnutrition. The hypothesis that greater maternal within-pregnancy adiposity might influence offspring adiposity, if true, has important public health implications. This can result in ever-increasing body weight through generations independent of other genetic or environmental changes (2, 5).
Parents may influence the risk of adiposity in their offspring through genetics, the intrauterine environment, and behavioral and environmental factors (2, 10). In our analyses, we assumed that maternal prepregnancy BMI was a marker for all of these factors and that paternal BMI reflected the same factors except for intrauterine environment. By comparing the parental-offspring associations of BMI (using prepregnancy parental BMI), we may find a greater maternal-offspring association if the intrauterine environment influences childhood adiposity and there is no parent-of-origin effect on inheritance and expression of adiposity-related genetic variants (11, 12). Such comparisons have been carried out in a few studies, with somewhat inconsistent results (10, 13–16). In a recent study from Belarus, Patel et al. (11) found similar parental-offspring associations when parental and offspring BMIs were recorded at the same time. However, the levels of parental overweight were low, and so we wished to examine the parental-offspring associations in a large cohort from a high-income country with higher levels of overweight.
In the present population-based study, we compared the associations between parental prepregnancy BMI and offspring BMI at age 3 years. Further, we investigated the influence of important prenatal and postnatal factors that might confound or mediate any association and that have not previously been available for similar analyses.
MATERIAL AND METHODS
Our data are from the Norwegian Mother and Child Cohort Study (MoBa) (17, 18), which was conducted by the Norwegian Institute of Public Health. This is a population-based pregnancy cohort that recruited more than 108,000 children from all parts of Norway from 1999 to 2009. The aim of the cohort study was to elucidate the etiologies and pathogenesis of disorders that may originate in early life by following the children and their parents for years. Women and their partners were recruited via a postal invitation sent before the ultrasound examination offered to all pregnant women in Norway during gestational weeks 17–19. Informed consent was obtained from each participant before inclusion. The women were given 3 questionnaires during pregnancy (at weeks 17, 22, and 30) and at intervals after birth (when children were 6, 18, and 36 months of age). Some of the fathers received a questionnaire sometime during gestational weeks 17–19. The data were linked to the Medical Birth Registry of Norway (MBRN) (19, 20). MoBa has been approved by the Norwegian Regional Committee for Ethics in Medical Research and the Data Inspectorate.
Approximately 42% of the invited women agreed to participate. Ninety-four percent of the women who responded to the first questionnaire also responded to the last questionnaire during pregnancy (third questionnaire). Approximately 61% also responded to the questionnaire sent when offspring were 3 years of age (sixth questionnaire), and 78% of these children also had a participating father. Our analyses included data from MBRN and all 7 MoBa questionnaires.
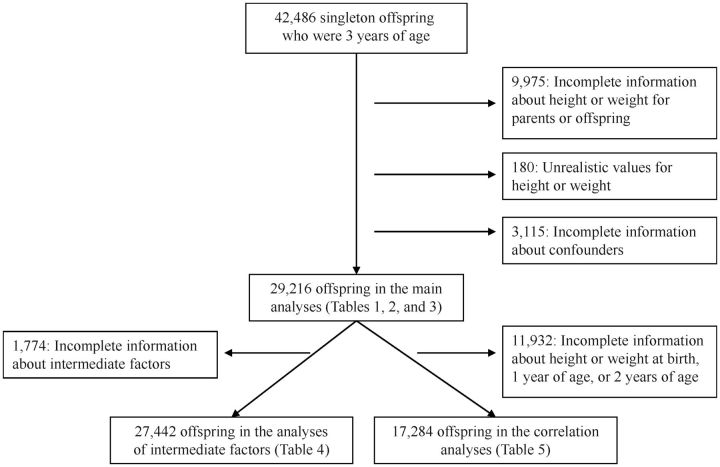
Our study population included singleton offspring who had reached the age of 3 years and for whom the mother had returned the first and sixth questionnaires with information about heights and weights (n = 42,486). We excluded participants for whom we had incomplete information about height or weight of the offspring, mother, or father (n = 9,975), as well as participants who listed unrealistic values for height or weight (n = 180). We also excluded persons for whom we did not have complete information about the relevant confounders (n = 3,115). Of the original 42,486 offspring, 29,216 (69%) were suitable for further analyses with their parents (Figure 1).
Figure 1.
Flow chart of the sample selection for the different analyses, the Norwegian Mother and Child Cohort Study, 1999–2009.
Variables
Main outcome and exposures
The outcome variable in the regression analyses was offspring BMI at 3 years of age, calculated from the mother's report of offspring height and weight when the child was 36 months of age (sixth questionnaire). The mean age of the offspring when the sixth questionnaire was sent out was 1,102 days (standard deviation (SD), 16.7 days). The main exposure variables were maternal and paternal BMI. Maternal BMI was calculated from mothers' retrospective reports of their prepregnancy height and weight reported around gestational week 17 (first questionnaire). Paternal BMI was calculated from fathers' self-report of height and weight for 20% of the trios (reported around the 17th week of gestation) or from maternal report (first questionnaire) if the father had not received or responded to the questionnaire (80% of the trios). The Pearson's correlations between paternal self-report and maternal report on behalf of the father were 0.975 (95% confidence interval (CI): 0.974, 0.977; n = 5,764) for weight, 0.973 (95% CI: 0.971, 0.974; n = 5,816) for height, and 0.961 (95% CI: 0.959, 0.963; n = 5,755) for BMI.
Confounders and intermediate factors
In our analyses, we adjusted for both prenatal and postnatal characteristics that are associated with parental and offspring BMI. Many of the postnatal characteristics are related to the shared familial environment. Most parents (95%) were living together when the offspring were 3 years of age. The following possible prenatal confounders were included in our analyses: parental educational level (years), maternal smoking during pregnancy (daily, occasionally, or never), paternal smoking (smoking during pregnancy and/or at offspring age of 18 months: daily, occasionally, or never), and maternal coffee consumption during pregnancy (cups/day). The possible postnatal confounders were: number of siblings (0, 1, 2, or ≥3), maternal smoking after pregnancy, paternal smoking, amount of breastfeeding for the first 4 months (only breast milk, breast milk and other fluids/solids, or no breast milk), place of day care at 3 years of age (home with mother, home with father, or in a day care institution), offspring's time spent watching television or video at age 3 years (hours/day), offspring's time spent outside at age 3 years (hours/day), and offspring's diet at age 3 years (slices of bread or crackers/day, frequency of candies/chips/soft drinks/deserts, etc, and frequency of vegetables).
In a subgroup for whom data were available (n = 27,442 trios; Figure 1), we adjusted for the potentially mediating factors in the maternal-offspring association of BMI that may be indicators of the intrauterine environment and developmental overnutrition: maternal weight change during pregnancy, diabetes status, and diet in the first 4–5 months of pregnancy (21), including intakes of calories (kilojoules/day), protein (g/day), fat (g/day), and carbohydrates (g/day). The dietary data were collected in the second questionnaire at approximately gestational week 22 (21). An altered regression coefficient for the maternal-offspring association of BMI could provide some support for maternal prepregnancy BMI being related to developmental overnutrition in utero through these intermediate factors.
Variables in sensitivity and correlation analyses
The other potential confounders and intermediate factors that we investigated in sensitivity analyses were parental age, parental physical activity level, cohabitant status, maternal alcohol consumption during pregnancy, offspring birth weight, gestational age, and gender of the offspring. To explore the potential of nonadditive simultaneous influences on offspring BMI in subsets of pairs of its determinants, we tested for interactions between parental BMI and the following determinants of childhood BMI: maternal weight change during pregnancy, maternal smoking during pregnancy, offspring birth weight, gestational age, gender of the offspring, and breastfeeding.
Offspring BMIs at several ages (birth, 1 year, 2 years, and 3 years) were included in crude correlation analyses of parental and offspring BMIs in a subgroup of offspring for which we had complete information about height and weight at all 4 ages (n = 17,284 trios; Figure 1). Weight and length at birth were reported in MBRN and by the mother 6 months after birth (fourth questionnaire), weight and height at 1 year of age were reported when offspring were 18 months of age (fifth questionnaire), and weight and height at ages 2 and 3 years were reported when offspring were 36 months of age (sixth questionnaire).
Statistical analyses
Regression analyses
By comparing the maternal-offspring BMI associations with the paternal-offspring BMI associations, it may be possible to find indications of intrauterine mechanisms underlying at least part of the maternal-offspring BMI association. This comparison was based on 2 assumptions. The first was that maternal prepregnancy BMI was a marker for the intrauterine environment that potentially influenced childhood adiposity, in addition to the mother's genetics, her lifestyle, and the obesogenic environment. The second was that the inheritance and expression of adiposity-related genetic variants in the offspring were equal from each parent.
The regression analyses were divided into 2 sets. The first investigated parental-offspring associations using absolute values of parental BMI, and the second used z-score values of parental BMI (SD). z score was used because 1 maternal BMI unit may reflect different biology or lifestyle-related behaviors and environment than 1 paternal BMI unit. The foundation for the comparison of the different parental BMI units may improve by transforming the BMI scales into z scores. Each set of analyses consisted of 6 regression models: 1) maternal BMI singularly, 2) paternal BMI singularly, 3) parental BMI simultaneously, 4) parental BMI simultaneously and prenatal factors, 5) parental BMI simultaneously and postnatal factors, and 6) all factors. For each regression model, we checked assumptions (linearity and constant variance) and looked for outliers with large influence.
Sensitivity and correlation analyses
Sensitivity analyses included 1) investigation of potential influence of other related factors and interactions as described earlier, 2) analyses of a smaller sample restricted to values of offspring height and weight that have been measured for Norwegian children who were 2–4 years old (22), 3) investigation of the effect of potential misreport of weights and heights based on published validation studies (23, 24), 4) analyses of a sample restricted to those parent-offspring trios for whom we had available self-reported data on the father's height and weight, 5) analyses investigating whether our results would change if 0%–10% of the included fathers were not the biological fathers (14, 25), and 6) analyses of potential time trends over the recruitment period.
We ran crude correlation analyses (Pearson's) between parental BMI (with maternal and paternal BMIs done separately) and offspring BMI at different ages, from birth to 3 years of age. This was carried out to compare the 2 parental-offspring correlations of BMI at different offspring ages and to investigate the potential change in correlations over time. The dependent correlation coefficients were compared by using the Stata command “corcor.” All analyses were conducted in SPSS, version 17.0 (SPSS, Inc., Chicago, Illinois), and Stata, version 9.2 (StataCorp LP, College Station, Texas).
RESULTS
Descriptive statistics
Table 1 shows the mean values and ranges of weights, heights, BMIs, and parental ages when the children were 3 years of age. Mean maternal and paternal ages were 33 years and 36 years, respectively, and mean parity was 0.7 children (range, 0–10 children). The mean offspring weight was 15.1 kg, the mean height was 97 cm, and the mean BMI was 16.1. The mean maternal BMI was 24.1 and the mean paternal BMI was 25.8. In Tables 2 and 3, several parental and offspring characteristics are shown in relation to mean parental BMI. Parental mean BMI was positively associated with offspring BMI, parental age, maternal parity, maternal smoking, offspring's intake of slices of bread/crackers per day, offspring's intake of chips/candies/deserts/soft drinks, and offspring's time spent watching television or video. Parental mean BMI was negatively associated with increasing amounts of breastfeeding and intake of vegetables in offspring. In addition, mean maternal BMI was associated with place of day care and offspring's time spent outdoors, and mean paternal BMI was associated with cohabitation status.
Table 1.
Participant Anthropometric Characteristics and Parental Age When Offspring Were 3 Years of Age Among 29,216 Parent-Offspring Trios in the Norwegian Mother and Child Cohort Study, 2001–2007
| Mean | Range | |
|---|---|---|
| Offspring | ||
| Weight, kg | 15.1 | 7.9, 31.0 |
| Height, m | 0.97 | 0.72, 1.20 |
| BMIa | 16.1 | 9.0, 33.4 |
| Mother | ||
| Weight, kg | 68.2 | 37.0, 160.0 |
| Height, m | 1.68 | 1.16, 1.98 |
| BMIa | 24.1 | 12.5, 59.5 |
| Age, years | 33.3 | 19, 50 |
| Father | ||
| Weight, kg | 85.2 | 46.0, 215.0 |
| Height, m | 1.82 | 1.48, 2.10 |
| BMIa | 25.8 | 13.1, 59.6 |
| Age, years | 35.9 | 20, 72 |
Abbreviation: BMI, body mass index.
a Weight (kg)/height (m)2.
Table 2.
Mean Parental Prepregnancy Body Mass Index According to Parental and Offspring Characteristics Among 29,216 Parent-Offspring Trios in the Norwegian Mother and Child Cohort Study, 2001–2007
| No. of Participants | % |
Maternal BMIa |
Paternal BMIa |
|||
|---|---|---|---|---|---|---|
| Mean (SD) | P Valueb | Mean (SD) | P Valueb | |||
| Offspring BMIa at 3 years of age | <0.001 | <0.001 | ||||
| <13.71 | 1,230 | 4.2 | 23.8 (4.3) | 25.5 (3.3) | ||
| 13.71–17.73 | 24,354 | 83.4 | 23.9 (4.1) | 25.7 (3.2) | ||
| 17.74–19.47 | 2,990 | 10.2 | 24.8 (4.5) | 26.4 (3.6) | ||
| ≥19.48 | 642 | 2.2 | 25.6 (4.9) | 26.8 (3.7) | ||
| Maternal age at birth, years | <0.001 | <0.001 | ||||
| 16–24 | 2,571 | 8.8 | 24.0 (4.4) | 25.6 (3.6) | ||
| 25–29 | 9,952 | 34.1 | 24.0 (4.2) | 25.8 (3.3) | ||
| 30–34 | 11,599 | 39.7 | 24.1 (4.1) | 25.8 (3.2) | ||
| 35–39 | 4,455 | 15.2 | 24.3 (4.1) | 25.9 (3.1) | ||
| 40–47 | 592 | 2.0 | 24.6 (4.3) | 26.1 (3.3) | ||
| Paternal age at birth, years | <0.001 | <0.001 | ||||
| 17–24 | 1,032 | 3.5 | 23.8 (4.4) | 25.1 (3.5) | ||
| 25–29 | 6,566 | 22.5 | 23.9 (4.1) | 25.5 (3.4) | ||
| 30–34 | 11,767 | 40.3 | 24.0 (4.1) | 25.8 (3.2) | ||
| 35–39 | 6,895 | 23.6 | 24.3 (4.3) | 26.0 (3.3) | ||
| 40–69 | 2,883 | 9.9 | 24.2 (4.2) | 26.1 (3.1) | ||
| Parity | <0.001 | <0.001 | ||||
| 0 children | 14,382 | 49.2 | 23.9 (4.1) | 25.7 (3.3) | ||
| 1 child | 10,139 | 34.7 | 24.2 (4.2) | 25.9 (3.3) | ||
| 2 children | 3,931 | 13.5 | 24.4 (4.2) | 25.9 (3.1) | ||
| ≥3 children | 764 | 2.6 | 24.9 (4.7) | 26.0 (3.3) | ||
| Maternal smoking during pregnancy | <0.001 | <0.001 | ||||
| None | 27,155 | 92.9 | 24.0 (4.1) | 25.8 (3.2) | ||
| Occasional | 610 | 2.1 | 24.4 (4.4) | 25.9 (3.3) | ||
| Daily | 1,451 | 5.0 | 24.8 (4.9) | 26.3 (3.9) | ||
| Paternal smoking | <0.001 | 0.971 | ||||
| None | 20,753 | 71.0 | 24.0 (4.1) | 25.8 (3.2) | ||
| Occasional | 3,336 | 11.4 | 23.8 (3.9) | 25.8 (3.2) | ||
| Daily | 5,127 | 17.5 | 24.7 (4.6) | 25.8 (3.5) | ||
| Maternal educational level, years | <0.001 | <0.001 | ||||
| ≤9 (secondary school) | 482 | 1.6 | 24.9 (5.3) | 26.3 (4.1) | ||
| 1–3 years of high school | 8,717 | 29.8 | 24.8 (4.7) | 26.2 (3.6) | ||
| 1–4 years of college/university | 12,958 | 44.4 | 24.0 (4.0) | 25.8 (3.1) | ||
| >4 years of college/university | 6,591 | 22.6 | 23.2 (3.5) | 25.3 (2.9) | ||
| Other | 468 | 1.6 | 23.9 (3.9) | 26.2 (3.3) | ||
| Paternal educational level, years | <0.001 | <0.001 | ||||
| ≤9 (secondary school) | 1,191 | 4.1 | 25.3 (5.2) | 26.5 (4.1) | ||
| 1–3 years of high school | 12,787 | 43.8 | 24.7 (4.5) | 26.2 (3.5) | ||
| 1–4 years of college/university | 7,998 | 27.4 | 23.7 (3.8) | 25.7 (3.1) | ||
| >4 years of college/university | 6,494 | 22.2 | 23.1 (3.4) | 25.1 (2.8) | ||
| Other | 746 | 2.6 | 24.0 (4.0) | 25.7 (3.1) | ||
| Parents living together | 0.225 | 0.020 | ||||
| Yes | 27,718 | 94.9 | 24.1 (4.2) | 25.8 (3.2) | ||
| No | 1,370 | 4.7 | 24.2 (4.7) | 25.6 (3.6) | ||
Abbreviations: BMI, body mass index; SD, standard deviation.
a Weight (kg)/height (m)2.
b F test for difference between the groups of maternal and paternal BMI, respectively.
Table 3.
Mean Parental Prepregnancy Body Mass Index According to Parental and Offspring Characteristics Among 29,216 Parent-Offspring Trios in the Norwegian Mother and Child Cohort Study, 2001–2007
| No. of Participants | % |
Maternal BMIa |
Paternal BMIa |
|||
|---|---|---|---|---|---|---|
| Mean (SD) | P Valueb | Mean (SD) | P Valueb | |||
| Breast milk intake until 5 months of age | <0.001 | <0.001 | ||||
| Only breastmilk | 19,831 | 67.9 | 23.8 (3.9) | 25.7 (3.2) | ||
| Breast milk and other fluids/solids | 8,826 | 30.2 | 24.7 (4.6) | 26.1 (3.5) | ||
| No breast milk | 559 | 1.9 | 25.4 (4.8) | 26.1 (3.3) | ||
| Offspring intake of bread/crackers at 3 years of age, slices/day | <0.001 | 0.001 | ||||
| 0–2 | 3,626 | 12.4 | 23.8 (4.0) | 25.7 (3.3) | ||
| 3–5 | 22,795 | 78.0 | 24.0 (4.1) | 25.8 (3.2) | ||
| ≥6 | 2,795 | 9.6 | 24.6 (4.6) | 26.0 (3.5) | ||
| Offspring intake of chips, candies, soft drinks, etc., at 3 years of age | <0.001 | <0.001 | ||||
| Seldom or <1 time/week | 2,990 | 10.2 | 23.8 (4.2) | 25.6 (3.1) | ||
| 1–3 times/week | 3,235 | 11.1 | 23.9 (4.0) | 25.6 (3.1) | ||
| 4–7 times/week | 20,401 | 69.8 | 24.0 (4.1) | 25.8 (3.3) | ||
| 2–3 times/day | 2,208 | 7.6 | 24.7 (4.6) | 26.1 (3.4) | ||
| ≥4 times/day | 382 | 1.3 | 25.6 (4.9) | 26.4 (3.4) | ||
| Offspring intake of vegetables at 3 years of age | <0.001 | <0.001 | ||||
| ≥5 times/week | 17,027 | 58.3 | 24.0 (4.1) | 25.7 (3.2) | ||
| 4 times/week | 3,921 | 13.4 | 24.2 (4.1) | 25.9 (3.3) | ||
| 2–3 times/week | 4,500 | 15.4 | 24.3 (4.3) | 26.0 (3.3) | ||
| 2–4 times/month | 2,174 | 7.4 | 24.3 (4.4) | 26.0 (3.4) | ||
| <2 times/month | 1,594 | 5.5 | 24.2 (4.3) | 25.9 (3.2) | ||
| Offspring's time doing outdoor activities at 3 years of age | 0.003 | 0.259 | ||||
| >3 hours/day | 9,970 | 34.1 | 24.2 (4.3) | 25.8 (3.2) | ||
| 1–3 hours/day | 18,284 | 62.6 | 24.0 (4.1) | 25.8 (3.3) | ||
| <1 hour/day | 962 | 3.3 | 24.1 (4.3) | 26.0 (3.3) | ||
| Offspring's time spent watching television/video at 3 years of age | <0.001 | <0.001 | ||||
| Seldom/never | 1,371 | 4.7 | 23.8 (4.0) | 25.4 (3.2) | ||
| <1 hour/day | 16,169 | 55.3 | 23.8 (4.0) | 25.6 (3.1) | ||
| 1–2 hours/day | 10,904 | 37.3 | 24.5 (4.4) | 26.1 (3.5) | ||
| ≥3 hours/day | 772 | 2.6 | 25.0 (4.9) | 26.5 (3.7) | ||
| Day care at 3 years of age | 0.013 | 0.204 | ||||
| With mother | 2,362 | 8.1 | 24.2 (4.4) | 25.9 (3.4) | ||
| With father | 98 | 0.3 | 24.9 (4.9) | 26.2 (3.4) | ||
| In kindergarten or other institutions | 26,756 | 91.6 | 24.1 (4.2) | 25.8 (3.3) | ||
Abbreviations: BMI, body mass index; SD, standard deviation.
a Weight (kg)/height (m)2.
b F test for difference between the groups of maternal and paternal BMI, respectively.
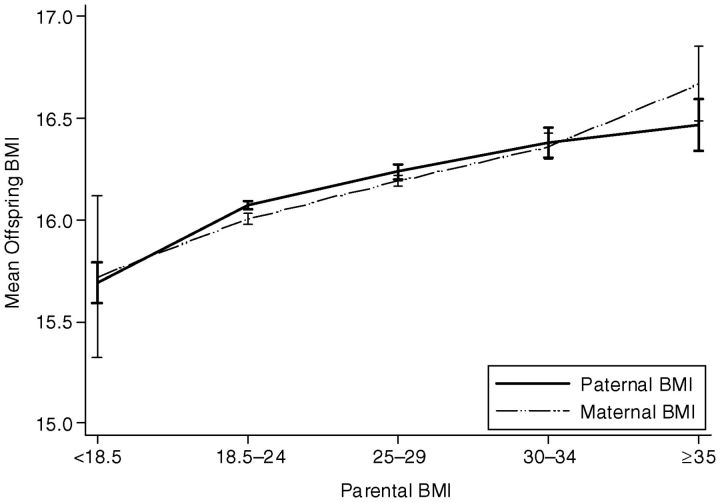
More than 83% of the offspring were normal weight, 4% were thin, 10% were overweight, and 2% were obese (Table 2) when compared with average BMI cut-offs for 3-year-old children (26, 27). Using the World Health Organization criteria (28), 22% of mothers were deemed overweight and 9% were obese; 45% of fathers were overweight and 10% were obese. The maternal-paternal BMI correlation was 0.222 (95% CI: 0.211, 0.233), the maternal-offspring BMI correlation was 0.095 (95% CI: 0.084, 0.106), and the paternal-offspring BMI correlation was 0.097 (95% CI: 0.086, 0.108). As shown in Figure 2, the mean offspring BMI was modestly positively associated with parental BMI. We found that the interaction between maternal and paternal BMI and had no impact on offspring BMI (P = 0.629).
Figure 2.
Mean offspring body mass index (BMI, measured as weight in kilograms divided by height in meters squared) at the age of 3 years according to parental prepregnancy BMI among 29,216 parent-offspring trios in the Norwegian Mother and Child Cohort Study, 2001–2007.
Regression analyses
In all regression analyses, both maternal and paternal BMI were positively associated with offspring BMI. The crude analyses using absolute BMI values showed that a 1-kg/m2 increase in maternal BMI was associated with a 0.04-kg/m2 increase in offspring BMI (95% CI: 0.031, 0.039; P < 0.001) (Table 4). A 1-kg/m2 increase in paternal BMI was associated with a 0.05-kg/m2 increase in offspring BMI (95% CI: 0.040, 0.051; P < 0.001). All regression models using absolute BMI values showed that paternal-offspring BMI associations were stronger than maternal-offspring BMI associations. The magnitudes of these differences were small (range, 0.01–0.009), but there was statistical support for real differences (P values = 0.001–0.022).
Table 4.
Regression Analyses of Offspring Body Mass Index at 3 Years of Age Among 29,216 Parent-Offspring Trios in the Norwegian Mother and Child Cohort Study, 2001–2007
| Offspring BMIa |
||||||
|---|---|---|---|---|---|---|
| Absolute Valuesb |
z Scorec |
|||||
| β | 95% CI | P Valued | β | 95% CI | P Valued | |
| Model 1e | 0.001 | 0.815 | ||||
| Maternal BMI | 0.035 | 0.031, 0.039 | 0.146 | 0.128, 0.163 | ||
| Paternal BMI | 0.045 | 0.040, 0.051 | 0.149 | 0.131, 0.166 | ||
| Model 2f | 0.020 | 0.799 | ||||
| Maternal BMI | 0.028 | 0.024, 0.033 | 0.119 | 0.101, 0.137 | ||
| Paternal BMI | 0.037 | 0.032, 0.043 | 0.122 | 0.104, 0.140 | ||
| Model 2 including prenatal factorsg | 0.022 | 0.900 | ||||
| Maternal BMI | 0.030 | 0.026, 0.034 | 0.125 | 0.107, 0.143 | ||
| Paternal BMI | 0.039 | 0.033, 0.044 | 0.127 | 0.109, 0.145 | ||
| Model 2 including postnatal factorsh | 0.014 | 0.670 | ||||
| Maternal BMI | 0.027 | 0.023, 0.032 | 0.115 | 0.097, 0.133 | ||
| Paternal BMI | 0.037 | 0.032, 0.043 | 0.121 | 0.103, 0.139 | ||
| Model 2 including both prenatal and postnatal factors | 0.018 | 0.805 | ||||
| Maternal BMI | 0.029 | 0.025, 0.033 | 0.122 | 0.104, 0.140 | ||
| Paternal BMI | 0.038 | 0.033, 0.044 | 0.125 | 0.107, 0.143 | ||
Abbreviations: BMI, body mass index; CI, confidence interval.
a Weight (kg)/height (m)2.
b Both parental and offspring BMI were analysed in absolute values.
c Parental BMIs were analyzed in z-score values (standard deviation), and offspring BMI were analyzed in absolute values.
d Wald test for difference between maternal-offspring association of BMI compared with paternal-offspring association.
e Model 1: univariate analyses of maternal and paternal BMI, respectively.
f Model 2: both parental BMIs included.
g Prenatal factors included parental educational level, parental prenatal smoking, and maternal coffee consumption.
h Postnatal factors included number of siblings, day care, breastfeeding, outdoor activities, watching television/video, diet, and parental postnatal smoking.
When analyses were completed using z scores (SD) for parental BMI, there was no strong statistical support for differences in their associations with offspring BMI (P values = 0.670–0.900) (Table 4). Because the differences in parental-offspring associations when analyzing absolute BMI values were small and the units of maternal and paternal BMI may not be directly comparable, we considered the analyses using z scores to be the most informative. As shown in model 2 (Table 4), a 1-standard-deviation increase in maternal BMI was associated with a 0.119-kg/m2 (95% CI: 0.101, 0.137) increase in offspring BMI, and a 1-standard-deviation increase in paternal BMI was associated with an increase of 0.122 kg/m2 (95% CI: 0.104, 0.140). Both parental-offspring associations of BMI decreased slightly after adjustments. The largest decrease occurred after adjusting for the other parent's BMI.
The additional regression analyses on a subpopulation included potentially mediating factors in the maternal-offspring association of BMI that may be related to developmental overnutrition in utero (maternal diabetes status, gestational weight change, and maternal diet). There were no differences between this population and the main population regarding the distribution of anthropometrics or confounding factors. The results are shown in Table 5. In these subanalyses, the maternal-offspring associations tended to be stronger, mainly because of the adjustment for gestational weight change. However, there was no strong statistical evidence for a real change in the parental-offspring associations of BMI.
Table 5.
Regression Analyses of Offspring Body Mass Index at 3 Years of Age Including Parental Body Mass Index Among 27,442 Parent-Offspring Trios in the Norwegian Mother and Child Cohort Study, 2001–2007
| Offspring BMIa |
||||||
|---|---|---|---|---|---|---|
| Absolute Values |
z Score Valuesb |
|||||
| β | 95% CI | P Valuec | β | 95% CI | P Valuec | |
| Model 2d with prenatale and postnatal factorsf | 0.748 | |||||
| Maternal BMI | 0.030 | 0.026, 0.035 | 0.127 | 0.109, 0.146 | ||
| Paternal BMI | 0.038 | 0.032, 0.043 | 0.079 | 0.123 | 0.104, 0.141 | |
| Model 2 with prenatale, postnatalf, and intrauterine nutritionalg factors | 0.065 | |||||
| Maternal BMI | 0.036 | 0.031, 0.040 | 0.149 | 0.130, 0.168 | ||
| Paternal BMI | 0.037 | 0.032, 0.043 | 0.695 | 0.122 | 0.103, 0.140 | |
Abbreviations: BMI, body mass index; CI, confidence interval.
a Weight (kg)/height (m)2.
b Parental BMI is analysed in z score values (SD), offspring BMI in absolute values.
c Wald test for difference between maternal-offspring association of BMI compared with paternal-offspring association.
d Model 2: both parental BMIs included.
e Prenatal factors included parental educational level, prenatal smoking, and maternal coffee consumption.
f Postnatal factors included number of siblings, day care, breastfeeding, outdoor activities, watching television/video, diet, and parental postnatal smoking.
g Intrauterine nutritional factors included maternal diabetic status during pregnancy, diet during pregnancy, and gestational weight change.
Sensitivity and correlation analyses
In general, the results were essentially the same as those in the main analyses presented in all of our sensitivity analyses. The one exception was that with adjustment for birth weight (model 2, which included prenatal and postnatal factors), the maternal-offspring BMI association became considerably weaker (β = 0.022; 95% CI: 0.017, 0.026) than the paternal-offspring association (β = 0.036; 95% CI: 0.031, 0.042).
Table 6 shows the correlations between parental BMI and offspring BMI at birth and when the children were 1, 2, and 3 years old. All of the correlations were weak (r = 0.04–0.11), and the maternal-offspring correlations were somewhat stronger than the paternal-offspring correlations at all times; however, there was only strong support for a difference at birth and at 1 year. The paternal-offspring correlation coefficients showed a tendency to strengthen by offspring age.
Table 6.
Crude Pearson's Correlations Between Parental Prepregnancy Body Mass Index and Offspring Body Mass Index Among 17,284 Parent-Offspring Trios in the Norwegian Mother and Child Cohort Study, 2001–2007
| Age of Offspring |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Birth |
1 Year |
2 Years |
3 Years |
|||||||||
| r | 95% CI | P Value | r | 95% CI | P Value | r | 95% CI | P Value | r | 95% CI | P Value | |
| Maternal BMIa | 0.10 | 0.09, 0.12 | 0.11 | 0.09, 0.12 | 0.08 | 0.07, 0.10 | 0.10 | 0.09, 0.12 | ||||
| Paternal BMIa | 0.04 | 0.02, 0.05 | 0.08 | 0.07, 0.10 | 0.07 | 0.06, 0.09 | 0.09 | 0.08, 0.11 | ||||
| Statistical differenceb | <0.001 | 0.010 | 0.234 | 0.327 | ||||||||
Abbreviations: BMI, body mass index; CI, confidence interval.
a Weight (kg)/height (m)2.
b Null hypothesis: No difference in correlation of the maternal-offspring association of BMI compared with the paternal-offspring association of BMI; testing dependent correlation coefficients by using Fisher's z transformation.
DISCUSSION
In the present study, we investigated the possibility that the association between maternal prepregnancy BMI and offspring BMI may in part be driven by intrauterine mechanisms related to maternal BMI by comparing the parental-offspring associations of BMI. A larger maternal-offspring association would provide some support for an effect of the intrauterine environment on childhood adiposity. We found similar parental-offspring associations of BMI when analyzing z-score values, which indicates that the parental-offspring BMI associations at 3 years of age are driven by shared familial genetic and environmental risk factors for variation in BMI.
The strengths of the present study are the large sample size, prospective design, and inclusion of several related obesogenic factors. The results of the earlier studies have been somewhat inconsistent (10, 13–16). Davey Smith et al. (14), Kivimäki et al. (10), and Knight et al. (16) found no strong support for differences between the maternal-offspring and paternal-offspring associations of BMI. However, Knight et al. (16) found a tendency for a greater paternal-offspring BMI association, as did we. Lawlor et al. found greater associations between maternal and offspring BMIs in an Australian cohort (15) and between maternal BMI and offspring fat mass in an English cohort (13), in which there previously had been found similar associations of BMI (14). The varying results may stem from smaller sample sizes and power to detect statistical differences between the associations, or they may be due to different confounding structures in the populations.
The strengths of the associations differed between studies. This may be related to offspring age at adiposity assessment, as the associations may get stronger with increasing offspring age (10, 14). It is well known that the maternal-offspring BMI association is stronger than the paternal-offspring BMI association at birth (8, 29), as was found in our correlation analyses. Our results suggest that the paternal-offspring association gets stronger as the offspring get older, whereas the maternal-offspring association seems to stay more stable. Thus, it is possible that differences between the parental-offspring associations may occur at later offspring ages. It should be noted that the reliability of BMI in assessing fat mass may change according to age (30) because of the changing height and body fat distribution. However, recent analyses using adiposity data in children who were 9–12 years of age suggested high correlations of BMI with measured fat mass (31).
Despite 2 of the previous studies finding that maternal-offspring BMI associations were larger than paternal-offspring associations, the authors noted that the difference was small and not likely to drive any obesity epidemic through generations (13, 15). Further, our results are consistent with a recent large study in which similar parental-offspring associations were found when parental and offspring BMIs were recorded at the same time (11). Our findings are also consistent with a large study of Swedish siblings in which the maternal early pregnancy BMI was different for each sibling; there was no strong support for a difference in the associations between maternal early pregnancy BMI and offspring BMI at 18 years of age (9).
Some genetic variants have been found to be robustly associated with BMI (32, 33), and we assume that the offspring inheritance and expression of variants from each parent is equal (i.e., no parent-of-origin effect). Previous studies support this theory, and they found no substantial gender-specific associations (mother-daughter and father-son associations) (11, 12). One previous study used maternal genetic variation in the FTO (fat mass and obesity associated) gene (adjusted for offspring FTO), a genetic variant associated with greater adiposity, as an instrumental variable for maternal BMI. That study concluded with no strong evidence of maternal adiposity influencing offspring adiposity via intrauterine mechanisms (13).
There are some potential limitations to our study. There is a skewed self-selection of women into MoBa regarding several characteristics (34), but this pregnancy cohort is large enough to represent a wide range of all the relevant characteristics (17). Further, our study indirectly investigates underlying biologic mechanisms. Most likely, such mechanisms are similar in all healthy women, an assumption that was investigated in MoBa previously (34). The declining response rate during the study period did not influence well-known estimates of association (34). It is known that people have a tendency to overreport their height and underreport their weight (24). However, our associations did not change in the sensitivity analysis investigating these phenomena. Some of the fathers in our study may not be the biological fathers, which may weaken the paternal-offspring association. Our nonpaternity analyses show a modest strengthening with an increasing proportion of nonpaternity from 0% to 10% and a weakening of the maternal-offspring association, but the parental-offspring associations remained similar to each other. High rates of split families in our population could violate the assumption about shared postnatal environment. However, 95% of the trios in our population live together.
Our findings of similar parental-offspring BMI associations suggest that both the mother and father influence their child's BMI in similar ways (10, 11, 14, 35). An alternative explanation could be that different influences from each of the parents counterbalance each other (36). For example, it could be that part of the maternal association is due to intrauterine effects but that there is a greater expression of adiposity-related genetic variants transmitted from fathers. However, this suggestion seems implausible because the likelihood of perfectly mimicked effects from mechanistically distinct factors is rather low (35). It may be difficult to disentangle the relative importance for offspring BMI of each parental BMI from the web of other obesogenic factors (37). However, adjustment for a range of obesogenic factors seems to be of little or no importance to the relative strengths of our associations.
In conclusion, we found similar associations between maternal-offspring BMI and paternal-offspring BMI, which indicates that the association of maternal BMI to offspring BMI at 3 years of age is likely to be explained by shared familial (environmental and genetic) risk factors rather than by the intrauterine environment related to maternal BMI. If this is true, prevention of childhood adiposity will benefit more from intervening on postnatal risk factors rather than the prenatal ones. However, the potential role of the intrauterine environment should be investigated further at several offspring ages, with different methodological approaches, and with other measures of the intrauterine environment.
ACKNOWLEDGMENTS
Author affiliations: Division of Epidemiology, Norwegian Institute of Public Health, Oslo, Norway (Caroline Fleten, Wenche Nystad, Hein Stigum, Øyvind Næss); Department of Public Health and Primary Health Care, University of Bergen, Bergen, Norway (Rolv Skjærven); Medical Birth Registry of Norway, Norwegian Institute of Public Health, Bergen, Norway (Rolv Skjærven); and Medical Research Council Centre for Causal Analyses in Translational Epidemiology, School of Social and Community Medicine, University of Bristol, Bristol, England (Debbie A. Lawlor, George Davey Smith).
This study is based on the Norwegian Mother and Child Cohort Study conducted by the Norwegian Institute of Public Health. The Norwegian Mother and Child Cohort Study is supported by the Norwegian Ministry of Health, the National Institutes of Health/National Institute of Environmental Health Sciences (grant NO-ES-75558), the National Institutes of Health/National Institute of Neurological Disorders and Stroke (grant 1 UO1 NS 047537-01), and the Norwegian Research Council/Functional Genomics (grant 151918/S10). D. A. L. and G. D. S. work in a center that receives funding from the United Kingdom Medical Research Council (grant G0600705) and the University of Bristol. C. F. receives financial support from the Norwegian Extra Foundation for Health and Rehabilitation and the Norwegian Health Association through EXTRA funds.
The authors thank Dr. Per Magnus for his important and useful comments on an earlier version of this manuscript.
The views expressed in this paper are those of the authors and not necessarily any funding body. The funding bodies did not influence data collection, analysis or interpretation of findings.
Conflict of interest: none declared.
REFERENCES
- 1.Lawlor DA, Chaturvedi N. Treatment and prevention of obesity—are there critical periods for intervention? Int J Epidemiol. 2006;35(1):3–9. doi: 10.1093/ije/dyi309. [DOI] [PubMed] [Google Scholar]
- 2.Oken E, Gillman MW. Fetal origins of obesity. Obes Res. 2003;11(4):496–506. doi: 10.1038/oby.2003.69. [DOI] [PubMed] [Google Scholar]
- 3.Ebbeling CB, Pawlak DB, Ludwig DS. Childhood obesity: public-health crisis, common sense cure. Lancet. 2002;360(9331):473–482. doi: 10.1016/S0140-6736(02)09678-2. [DOI] [PubMed] [Google Scholar]
- 4.Whitaker RC, Dietz WH. Role of the prenatal environment in the development of obesity. J Pediatr. 1998;132(5):768–776. doi: 10.1016/s0022-3476(98)70302-6. [DOI] [PubMed] [Google Scholar]
- 5.Levin BE. The obesity epidemic: metabolic imprinting on genetically susceptible neural circuits. Obes Res. 2000;8(4):342–347. doi: 10.1038/oby.2000.41. [DOI] [PubMed] [Google Scholar]
- 6.Huang JS, Lee TA, Lu MC. Prenatal programming of childhood overweight and obesity. Matern Child Health J. 2007;11(5):461–473. doi: 10.1007/s10995-006-0141-8. [DOI] [PubMed] [Google Scholar]
- 7.Freinkel N. Banting Lecture 1980: of pregnancy and progeny. Diabetes. 1980;29(12):1023–1035. doi: 10.2337/diab.29.12.1023. [DOI] [PubMed] [Google Scholar]
- 8.Xue F, Willett WC, Rosner BA, et al. Parental characteristics as predictors of birthweight. Hum Reprod. 2008;23(1):168–177. doi: 10.1093/humrep/dem316. [DOI] [PubMed] [Google Scholar]
- 9.Lawlor DA, Lichtenstein P, Långström N. Association of maternal diabetes mellitus in pregnancy with offspring adiposity into early adulthood: sibling study in a prospective cohort of 280,866 men from 248,293 families. Circulation. 2011;123(3):258–265. doi: 10.1161/CIRCULATIONAHA.110.980169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kivimäki M, Lawlor DA, Smith GD, et al. Substantial intergenerational increases in body mass index are not explained by the fetal overnutrition hypothesis: the Cardiovascular Risk in Young Finns Study. Am J Clin Nutr. 2007;86(5):1509–1514. doi: 10.1093/ajcn/86.5.1509. [DOI] [PubMed] [Google Scholar]
- 11.Patel R, Martin RM, Kramer MS, et al. Familial associations of adiposity: findings from a cross-sectional study of 12,181 parental-offspring trios from Belarus. PLoS One. 2011;6(1) doi: 10.1371/journal.pone.0014607. pe14607. ( doi:10.1371/journal.pone.0014607) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leary S, Davey Smith G, Ness A. No evidence of large differences in mother-daughter and father-son body mass index concordance in a large UK birth cohort. Int J Obes (Lond) 2010;34(7):1191–1192. doi: 10.1038/ijo.2010.60. [DOI] [PubMed] [Google Scholar]
- 13.Lawlor DA, Timpson NJ, Harbord RM, et al. Exploring the developmental overnutrition hypothesis using parental-offspring associations and FTO as an instrumental variable. PLoS Med. 2008;5(3) doi: 10.1371/journal.pmed.0050033. (doi:10.1371/journal.pmed.0050033) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davey Smith G, Steer C, Leary S, et al. Is there an intrauterine influence on obesity? Evidence from parent child associations in the Avon Longitudinal Study of Parents and Children (ALSPAC) Arch Dis Child. 2007;92(10):876–880. doi: 10.1136/adc.2006.104869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lawlor DA, Smith GD, O'Callaghan M, et al. Epidemiologic evidence for the fetal overnutrition hypothesis: findings from the mater-university study of pregnancy and its outcomes. Am J Epidemiol. 2007;165(4):418–424. doi: 10.1093/aje/kwk030. [DOI] [PubMed] [Google Scholar]
- 16.Knight B, Shields BM, Hill A, et al. The impact of maternal glycemia and obesity on early postnatal growth in a nondiabetic Caucasian population. Diabetes Care. 2007;30(4):777–783. doi: 10.2337/dc06-1849. [DOI] [PubMed] [Google Scholar]
- 17.Magnus P, Irgens LM, Haug K, et al. Cohort profile: the Norwegian Mother and Child Cohort Study (MoBa) Int J Epidemiol. 2006;35(5):1146–1150. doi: 10.1093/ije/dyl170. [DOI] [PubMed] [Google Scholar]
- 18.Norwegian Institute of Public Health. Norwegian Mother and Child Cohort Study–General Information. Bergen, Norway: Norwegian Institute of Public Health; 2011. (http://www.fhi.no/eway/default.aspx?pid=233&trg=MainArea_5661&MainArea_5661=5565:0:15,1216:1:0:0:::0:0. ). (Accessed August 17, 2011) [Google Scholar]
- 19.Irgens LM. The Medical Birth Registry of Norway. Epidemiological research and surveillance throughout 30 years. Acta Obstet Gynecol Scand. 2000;79(6):435–439. [PubMed] [Google Scholar]
- 20.Norwegian Institute of Public Health. The Medical Birth Registry of Norway–General Information. Bergen, Norway: Norwegian Institute of Public Health; 2011. (http://www.fhi.no/eway/default.aspx?pid=233&trg=MainArea_5661&MainArea_5661=5631:0:15,3278:1:0:0:::0:0. ). (Accessed August 17, 2011) [Google Scholar]
- 21.Meltzer HM, Brantsaeter AL, Ydersbond TA, et al. Methodological challenges when monitoring the diet of pregnant women in a large study: experiences from the Norwegian Mother and Child Cohort Study (MoBa) Matern Child Nutr. 2008;4(1):14–27. doi: 10.1111/j.1740-8709.2007.00104.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Júlíusson PB, Roelants M, Eide GE, et al. Growth references for Norwegian children [in Norwegian] Tidsskr Nor Laegeforen. 2009;129(4):281–286. doi: 10.4045/tidsskr.09.32473. [DOI] [PubMed] [Google Scholar]
- 23.Scholtens S, Brunekreef B, Visscher TL, et al. Reported versus measured body weight and height of 4-year-old children and the prevalence of overweight. Eur J Public Health. 2007;17(4):369–374. doi: 10.1093/eurpub/ckl253. [DOI] [PubMed] [Google Scholar]
- 24.Nyholm M, Gullberg B, Merlo J, et al. The validity of obesity based on self-reported weight and height: implications for population studies. Obesity (Silver Spring) 2007;15(1):197–208. doi: 10.1038/oby.2007.536. [DOI] [PubMed] [Google Scholar]
- 25.Clemons T. A look at the inheritance of height using regression toward the mean. Hum Biol. 2000;72(3):447–454. [PubMed] [Google Scholar]
- 26.Cole TJ, Bellizzi MC, Flegal KM, et al. Establishing a standard definition for child overweight and obesity worldwide: international survey. BMJ. 2000;320(7244):1240–1243. doi: 10.1136/bmj.320.7244.1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cole TJ, Flegal KM, Nicholls D, et al. Body mass index cut offs to define thinness in children and adolescents: international survey. BMJ. 2007;335(7612):194. doi: 10.1136/bmj.39238.399444.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.World Health Organization. WHO Technical Report Series 894. Obesity: Preventing and Managing the Global Epidemic: Report of a WHO Consultation. Geneva, Switzerland: World Health Organization; 2000. [PubMed] [Google Scholar]
- 29.Lie RT, Wilcox AJ, Skjaerven R. Maternal and paternal influences on length of pregnancy. Obstet Gynecol. 2006;107(4):880–885. doi: 10.1097/01.AOG.0000206797.52832.36. [DOI] [PubMed] [Google Scholar]
- 30.Rolland-Cachera MF, Cole TJ, Sempé M, et al. Body mass index variations: centiles from birth to 87 years. Eur J Clin Nutr. 1991;45(1):13–21. [PubMed] [Google Scholar]
- 31.Lawlor DA, Benfield L, Logue J, et al. Association between general and central adiposity in childhood, and change in these, with cardiovascular risk factors in adolescence: prospective cohort study. BMJ. 2010;341 doi: 10.1136/bmj.c6224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li S, Zhao JH, Luan J, et al. Cumulative effects and predictive value of common obesity-susceptibility variants identified by genome-wide association studies. Am J Clin Nutr. 2010;91(1):184–190. doi: 10.3945/ajcn.2009.28403. [DOI] [PubMed] [Google Scholar]
- 33.Loos RJ. Recent progress in the genetics of common obesity. Br J Clin Pharmacol. 2009;68(6):811–829. doi: 10.1111/j.1365-2125.2009.03523.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nilsen RM, Vollset SE, Gjessing HK, et al. Self-selection and bias in a large prospective pregnancy cohort in Norway. Paediatr Perinat Epidemiol. 2009;23(6):597–608. doi: 10.1111/j.1365-3016.2009.01062.x. [DOI] [PubMed] [Google Scholar]
- 35.Smith GD. Assessing intrauterine influences on offspring health outcomes: can epidemiological studies yield robust findings? Basic Clin Pharmacol Toxicol. 2008;102(2):245–256. doi: 10.1111/j.1742-7843.2007.00191.x. [DOI] [PubMed] [Google Scholar]
- 36.Cole TJ, Power C, Moore GE. Intergenerational obesity involves both the father and the mother. Am J Clin Nutr. 2008;87(5):1535–1536. doi: 10.1093/ajcn/87.5.1535. [DOI] [PubMed] [Google Scholar]
- 37.Monasta L, Batty GD, Cattaneo A, et al. Early-life determinants of overweight and obesity: a review of systematic reviews. Obes Rev. 2010;11(10):695–708. doi: 10.1111/j.1467-789X.2010.00735.x. [DOI] [PubMed] [Google Scholar]