Abstract
Three laryngeal properties associated with the production of masculine song—laryngeal muscle tension, fiber twitch type, and fiber recruitment—are markedly sexually dimorphic in adult Xenopus laevis frogs. To elucidate the pattern of sexual differentiation, tension and fiber recruitment in male and female larynges and fiber twitch type in male larynges were examined throughout postmetamorphic development. Masculinization of male laryngeal properties begins early in postmetamorphic development and continues until adulthood. In contrast, tension and fiber recruitment in females do not change after the end of metamorphosis. Laryngeal muscle tension and fiber type are gradually and progressively masculinized; the temporal pattern of masculinization is very similar for these properties. Fiber recruitment, on the other hand, appears to masculinize in a stepwise manner. Masculinization of all three properties is highly correlated with larynx weight in males. We have used this relation to divide postmetamorphic development into seven stages associated with key events in sexual differentiation. This staging scheme provides an important experimental tool for studying the hormonal regulation of sexual differentiation, the subject of the accompanying paper.
INTRODUCTION
Sexual differentiation is the developmental process whereby males and females acquire sex specific traits, including the capacity for courtship behaviors. Courtship song or mate calling in Xenopus laevis frogs is a male specific behavior requiring masculinized development of the vocal muscles and neuromuscular junction (Wetzel and Kelley, 1983; Hannigan and Kelley, 1986; Kelley and Tobias, 1989). Mate calls are alternating rapid (71 Hz) and slower (36 Hz) trills made up of clicks. During the fast trills, clicks become progressively louder (Wetzel and Kelley, 1983). In previous studies we have identified several properties of laryngeal muscle and the neuromuscular junction which contribute to the production of male song. Tension on laryngeal tendons produced by muscle contractions and the relative proportion of fast and slow twitch muscle fibers contribute to trill rate. Muscle fiber recruitment is correlated with amplitude modulation. In response to nerve stimulation at mate call rates, male laryngeal muscle contracts and relaxes completely; female laryngeal muscle cannot contract and relax at the rapid rate required for mate call production (Tobias and Kelley, 1987). The rapidly contracting male laryngeal muscle is composed entirely of fast twitch muscle fibers, while the more slowly contracting female laryngeal muscle is composed mostly of slow twitch fibers (Sassoon et al., 1987). Male laryngeal muscle fibers are progressively recruited throughout a rapid nerve stimulus train, while female laryngeal muscle fibers are not (Tobias and Kelley, 1987). Intracellular recordings from individual muscle fibers indicate that sex differences in fiber recruitment can result from sex differences in synaptic efficacy at the neuromuscular junction (Tobias and Kelley, 1988). Thus, the larynx is markedly dimorphic in adult males and females and these sex differences constrain vocal behavior. Sex differences in properties of the adult larynx result from sexual differentiation during development. The purpose of this study was to elucidate the pattern and timing of sexual differentiation of laryngeal tension, fiber type, and fiber recruitment.
In vertebrates, masculinization is driven by hormone secretion from the male gonad. In neuromuscular systems, masculinization is controlled by androgenic metabolites of the steroid hormone testosterone (reviewed in Kelley, 1988). The events which underly the ability of androgen to effect masculinization are poorly understood. We reason that these events can be better identified if the developmental stage at which they occur is known. Furthermore, it seems likely that androgen regulated cellular differentiation occurs while laryngeal properties are becoming sexually dimorphic. Thus, in this paper we identify the developmental stage corresponding to the onset of sexual differentiation for laryngeal muscle tension, fiber type, and fiber recruitment. In the accompanying paper, we determine when during development androgens are required to effect masculinization by manipulating endocrine levels at key stages in laryngeal development.
MATERIALS AND METHODS
Animals
X. laevis frogs, obtained from Nasco (Ft. Atkinson, WI), were maintained in the laboratory for up to 2 weeks. Frogs were housed in polycarbonate tanks in approximately 6 cm of standing tap water and were fed frog brittle or beef liver daily. Males and females at different postmetamorphic developmental stages were studied. The postmetamorphic period extends from the end of metamorphosis (tadpole stage 66 of Nieuwkoop and Faber, 1956) through sexual maturity.
Electrophysiologic
All laryngeal measurements were obtained from isolated larynges, a preparation in which electromyogram (EMG) and tension characteristics associated with mate calling have been defined (see Tobias and Kelley, 1987 for a full description of recording and stimulating procedures). Frogs were anesthetized by immersion in 0.1% MS 222 (ethyl m-amino benzoate methane sulfonic acid, Aldrich Chemical Co.). Larynges were removed and maintained in glucose supplemented saline throughout the experiment. The laryngeal nerve was stimulated via a suction electrode. Stimulus trains were designed to emulate the fast portion of a male mate call (250-ms duration, 71 Hz) and were separated by 10-sec intervals to avoid temporal summation or adaptation. Bipolar metal electrodes placed in the laryngeal muscle were used to record the EMG. The tendon which connects laryngeal muscles with the sound-producing cartilaginous disks was connected to a force transducer (Grass FT-103) to measure isotonic muscle tension.
EMG and tension recordings for each experiment were photographed directly from a storage oscilloscope and used to measure EMG potentiation and percentage (%) of transient tension. The amplitude of the EMG represents the relative number of muscle fibers responding to a stimulus; a progressive increase in EMG amplitude (EMG potentiation) during stimulation indicates fiber recruitment. EMG potentiation is measured by dividing the amplitude of the last compound action potential in a train by the amplitude of the first compound action potential. Because EMG amplitude is affected by the geometric relationship between muscle fibers and recording electrodes, measurements of EMG potentiation are variable. Muscle contractions produce tension on laryngeal tendons. The percentage of transient tension represents the relative proportion of transient to maintained tension during stimulus trains delivered to the nerve. The percentage of transient tension is obtained by dividing the amplitude of the transient portion of the tension record by the amplitude of total tension and then multiplying this value by 100.
Histochemistry
Fast and slow twitch muscle fibers were distinguished using histochemical methods that demonstrate acid stable ATPase activity (Guth and Samaha, 1970; Sassoon et al., 1987). Larynges were weighed, frozen in liquid nitrogen, and then transversely sectioned (18 μm) on a cryostat (Hacker). Sections were preincubated in acidic buffer (pH 4.6) and reacted with ATP before visualization with cobalt and ammonium sulfide.
Under these conditions, laryngeal slow twitch muscle fibers stain more intensely than fast twitch fibers (Fig. 4); slow twitch fibers are among the smallest in the larynx (Sassoon et al., 1987). Their small size and intense staining with the ATPase reaction make slow twitch fibers easy to identify and count. Photomicrographs of representative sections from the midrostro/caudal extent of each larynx were examined. From one section, a rectangular area 52,000 μm2 in size was chosen and the percentage of small, darkly staining fibers (percentage of slow twitch fibers) was determined.
Fig. 4.
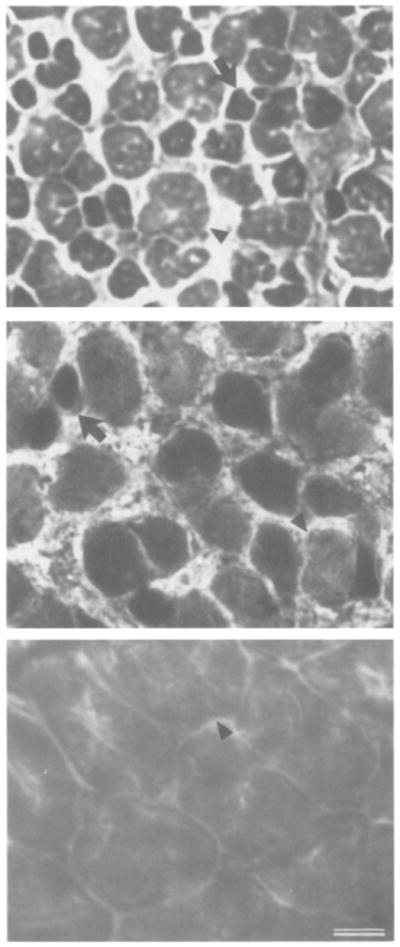
Photomicrographs of sections reacted for ATPase activity in male laryngeal muscle at three developmental stages. An example of a small dark muscle fiber (slow twitch) is indicated (arrow) at early developmental stages. A larger light muscle fiber (fast twitch) is also indicated (arrowhead) at each stage. At the end of metamorphosis (top, 9 mg larynx), male laryngeal muscle is composed of a heterogeneous population of fibers; 51% are slow twitch. During masculinization (middle, 102 mg larynx), male laryngeal muscle has 21% slow twitch fibers. In adulthood (bottom, 517 mg larynx), male laryngeal muscle is composed only of fast twitch fibers, no slow twitch fibers are present. Calibration bar: 10 μm.
Staging Postmetamorphic Development
We have used the relation between masculinization and larynx weight in males (see Results) to divide postmetamorphic development into seven stages (Table 1). These stages permit a concise description of masculinization in this system and are also useful in grouping animals of similar developmental states for experimental studies (see Tobias et al., 1991). The lower developmental limit, PM0, represents the end of metamorphosis and is recognized when the tail is no longer visible from the animal’s ventral side (tadpole stage 66, Nieuwkoop and Faber, 1956). The upper developmental limit, PM6, represents sexual maturity; male frogs are at least 2 years of age and weigh at least 25 g. For assignment to stages PM1–PM5, animals were selected by larynx weight.
TABLE 1.
Stages in the Postmetamorphic Masculinization of the Larynx
| Postmetamorphic stage | Larynx weight (mg) | Median body weight (g) | SIRa (g) | Age (months post- metamorphic) |
|---|---|---|---|---|
| 0 | 3–10 | 1.5 | .98–1.8 | 0b |
| 1 | 11–35 | 4.0 | 3.0–5.8 | 3 |
| 2 | 36–70 | 7.3 | 6.3–8.5 | 6 |
| 3 | 71–125 | 9.3 | 7.8–10.2 | 9 |
| 4 | 126–160 | 11.2 | 8.7–12.7 | 11 |
| 5 | 161–350 | 14.6 | 12.5–18.8 | >12 |
| 6 | >350 | 36.2 | 26.1–40.4 | >24 |
Semi-interquartile range.
The end of metamorphosis, tadpole stage 66 of Nieuwkoop and Faber (1956).
For endocrine manipulation studies, age and body weight are used to assign males to stages because animals must be sacrificed to obtain larynx weights. The body weights associated with stages PM0–PM6 were determined using the data illustrated in Fig. 1. Because the rate of masculinization is not constant over development, the upper and lower portions of body weight ranges for successive stages can overlap. To reduce overlap, we use body weight ranges representing 25% of values above and below the median body weight for each stage (the semi-interquartile range or SIR; see Table 1). This assignment scheme results in an average 19.4% total error rate. Most stage assignment errors are ±1 stage; 1.9% of errors are ±2 stages.
Fig. 1.
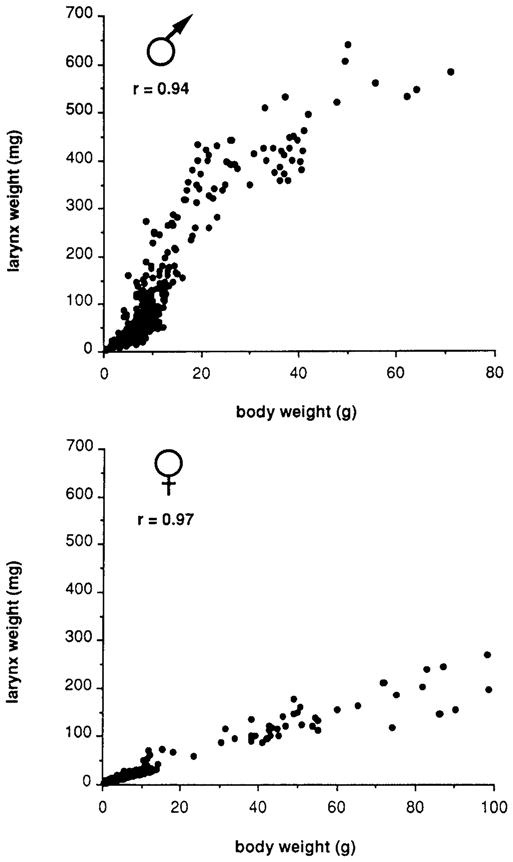
Laryngeal weight as a function of body weight for males and females throughout postmetamorphic development. Although larynx weight is highly correlated with body weight in both sexes, males increase larynx weight more rapidly than females. Note that both body weights and laryngeal weights are sexually dimorphic in adults.
We have also observed that the rate of growth can vary seasonally. Because both body and larynx weight are affected by breeding conditions (food, temperature, and density), weight may not be well correlated with age. The approximate ages most closely associated with a particular body weight range for the male frogs used in this study are indicated in Table 1.
RESULTS
Laryngeal Growth
The relation between larynx weight and body weight was determined for males and females throughout postmetamorphic development. For both sexes, the larynx enlarges as the body grows (Fig. 1). At the end of metamorphosis, both males and females weigh about 1 g and the larynx weighs less than 10 mg. From this stage on, the pattern of laryngeal growth is sexually dimorphic. In males, the larynx grows rapidly, weighing approximately 50 mg at a body weight of 7 g and about 100 mg at a body weight of 10 g. Laryngeal growth is slower in females than in males. A 50-mg laryngeal weight in females is not attained until 20 g body weight and a 100-mg laryngeal weight not until 30 g body weight. At sexual maturity, both body weight and larynx weight are sexually dimorphic and inversely related: laryngeal weight is greater in males (400 vs 150 mg) and body weight is greater in females (50 vs 25 g). After sexual maturity, body weight and larynx weight continue to increase slowly in both sexes.
Laryngeal Muscle Tension
At maturity, male laryngeal muscle produces entirely transient tension (Fig. 2g), while female laryngeal muscle produces primarily maintained tension (Fig. 2h). Laryngeal muscle tension was measured in male and female frogs throughout postmetamorphic development to determine how the adult phenotype is achieved. At the end of metamorphosis, laryngeal muscle produces only maintained tension in response to rapid nerve stimulation in both sexes (Figs. 2a, 2b). In females, there is little change in the tension records after the end of metamorphosis until maturity; tension is mostly maintained with small transients superimposed (Figs. 2d, 2f, 2h). In males, however, the relative proportion of transient to maintained tension increases markedly during postmetamorphic development (Figs. 2c, 2e, 2g). The percentage of transient tension is highly correlated with larynx weight (Fig. 3) in males (r = 0.85) and poorly correlated with larynx weight in females (r = 0.3). Thus, female tension characteristics are established early in postmetamorphic development and do not change, while male tension characteristics undergo a gradual increase in the percentage of transient tension to reach adult values.
Fig. 2.
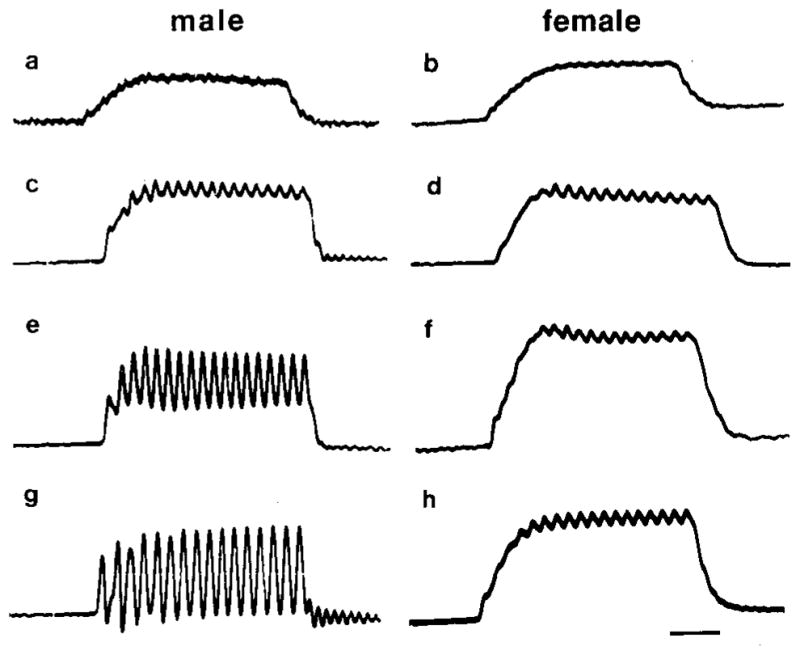
Tension records from developing male and female larynges. Adult males typically produce 100% transient tension, while females typically produce mostly maintained tension with small transients superimposed. Tension (t) is sexually monomorphic at the end of metamorphosis and during early postmetamorphic development. The percentage of transient tension then increases in males but remains unchanged in females. Calibration bar: 50 ms.
| male | female |
|---|---|
| a: end of metamorphosis, 0% t, 5 mg larynx | b: end of metamorphosis, 0% t, 5 mg larynx |
| c: early postmetamorphic, 27% t, 53 mg larynx | d: early postmetamorphic, 21% t, 59 mg larynx |
| e: late postmetamorphic, 67% t, 108 mg larynx | f: late postmetamorphic, 12% t, 113 mg larynx |
| g: mature, 100% t, 252 mg larynx | h: mature, 18% t, 240 mg larynx |
Fig. 3.
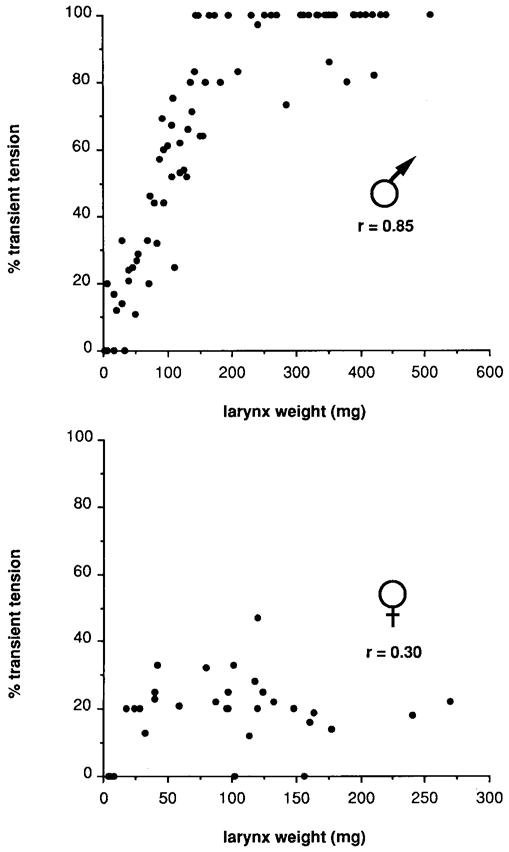
The percentage of transient tension as a function of larynx weight in males and females throughout postmetamorphic development. The percentage of transient tension is sexually monomorphic early in development and then increases rapidly in males but remains unchanged in females.
Laryngeal Muscle Fiber Type
The percentage of slow twitch fibers in laryngeal muscle is the same in males and females soon after metamorphosis (~50%); this value subsequently increases to ~70% in adult females and decreases to zero in adult males (Sassoon et al., 1987). Here we measure the percentage of slow twitch fibers in developing males to elucidate the pattern of slow fiber loss. The appearance of male muscle fibers using ATPase histochemistry at the end of metamorphosis, during subsequent slow fiber loss, and in adulthood is illustrated in Fig. 4. The percentage of slow twitch fibers is high early in development when the larynx is small and then undergoes a period of rapid decline until all slow twitch fibers are lost (Fig. 5). The percentage of slow twitch fibers is inversely correlated with larynx weight (r = −0.79).
Fig. 5.
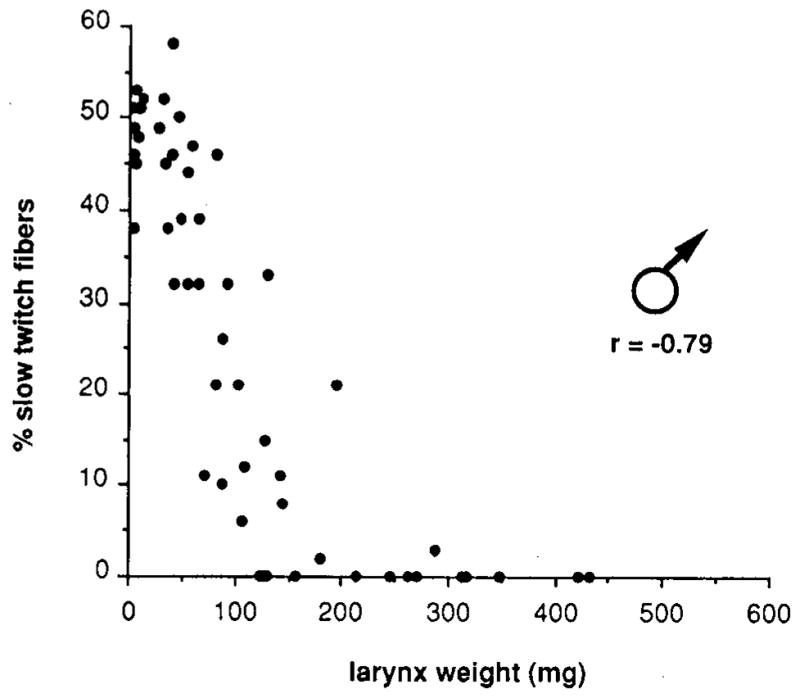
The percentage of slow twitch fibers as a function of larynx weight in males throughout postmetamorphic development. The percentage of slow twitch fibers begins to decline early in development and decreases rapidly. The loss of slow twitch fibers closely parallels the increase in the percentage of transient tension (Fig. 3).
Laryngeal Muscle Fiber Recruitment
At maturity, muscle fibers are recruited throughout the nerve stimulus train in males, but not in females (Figs. 6e, 6f). Fiber recruitment, measured by EMG potentiation, was determined for male and female laryngeal muscle throughout postmetamorphic development. At the end of metamorphosis, male and female larynges produce EMGs which decrease in amplitude during a stimulus train; EMG potentiation is <1.0 (Figs. 6a, 6b). Subsequently, both sexes produce EMGs which increase slightly during a stimulus train; EMG potentiation is >1.0 (Figs. 6c, 6d). In females, EMG potentiation does not progress any further during development. In males, the EMG record shows a marked increase in potentiation between developing and adult males (Figs. 6c, 6e). Values for EMG potentiation throughout postmetamorphic development (Fig. 7) indicate that the correlation between EMG potentiation and larynx weight is higher in males (r = 0.69) than in females (r = 0.35).
Fig. 6.
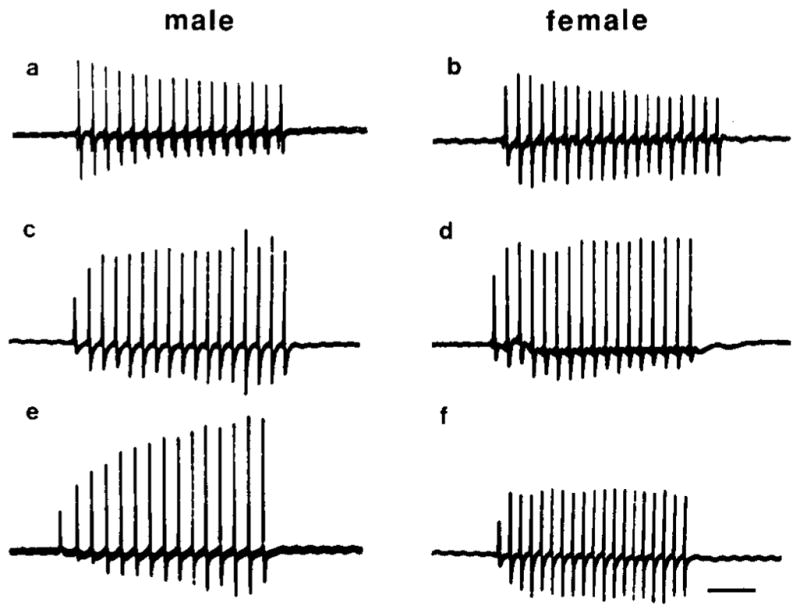
Electromyogram recordings from developing male and female larynges. In adulthood (bottom panels), male EMGs increase in amplitude throughout the stimulus train, while female EMGs have a nearly constant amplitude. At the end of metamorphosis (top panels), both sexes display a decrease in EMG amplitude in response to a stimulus train (EMG potentiation <1.0). Both sexes then increase EMG potentiation values somewhat (EMG potentiation ~1.0; middle panels). Males then further increase EMG potentiation during development while females do not. Calibration bar: 50 ms.
| male | female |
|---|---|
| a: end of metamorphosis, EMG = 0.6, 5 mg larynx | b: end of metamorphosis, EMG = 0.8, 6 mg larynx |
| c: postmetamorphic, EMG = 2.1,100 mg larynx | d: postmetamorphic, EMG = 1.5, 95 mg larynx |
| e: mature, EMG = 4.3, 309 mg larynx | f: mature, EMG = 1.7, 270 mg larynx |
Fig. 7.
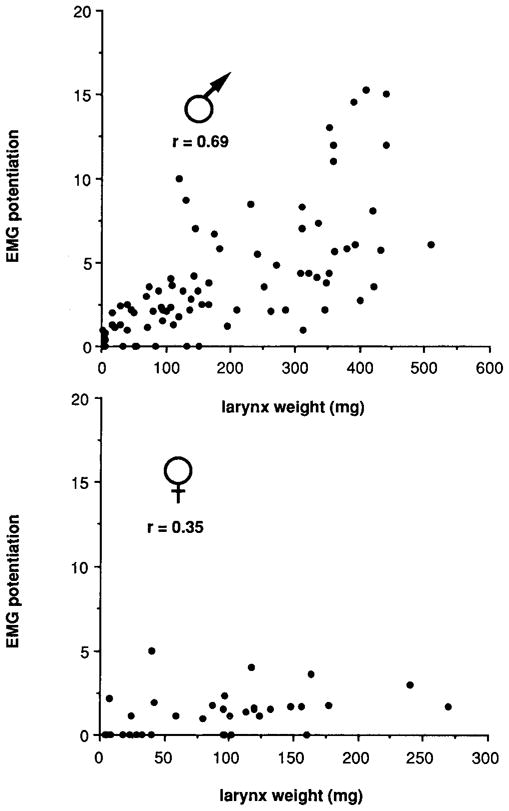
EMG potentiation as a function of larynx weight in males and females during postmetamorphic development. EMG potentiation is low in both sexes early in development. During postmetamorphic development, values increase in males and are not fully masculinized until adulthood. Female values remain low throughout postmetamorphic development.
Stages in Postmetamorphic Laryngeal Masculinization
The results of this study reveal that sexual differentiation of laryngeal tension, fiber type, and fiber recruitment is strongly correlated with larynx growth. Masculinization is a dynamic process which can be divided into phases. Each phase of masculinization is associated with a range of larynx weights. For the percentage of transient tension (Fig. 3), male values begin to reliably exceed female values when the larynx weighs ~70 mg. Adult male values are first produced when the larynx weighs ~130 mg and reliably produced when the larynx weighs over 160 mg. Masculinization of the percentage of transient tension thus occurs between larynx weights of 70 and 160 mg. For fiber type (Fig. 5), a similar pattern is observed. Male values begin to fall below those characteristic of the end of metamorphosis when the larynx weighs ~40 mg and are consistently lower than these values when the larynx weighs ~60 mg. Values characteristic of adult males are first obtained when the larynx weighs ~125 mg and reliably obtained when the larynx weighs over 150 mg. Masculinization of fiber type thus occurs between larynx weights of 40 and 150 mg. The pattern of masculinization for fiber recruitment (Fig. 7) is more difficult to discern due to greater variability for this measure. Values for EMG potentiation are consistently higher in males than in females when the male larynx weighs ~70 mg and continue to increase until the larynx weighs ~125 mg. Values for EMG potentiation are then maintained at intermediate values until a second phase of increase when the larynx weighs ~350 mg. Masculinization of fiber recruitment thus occurs between larynx weights of 70 and 350 mg. We have used the relation between masculinization and larynx weight to divide postmetamorphic development into seven stages (Table 1). The larynx weights, body weights (see Materials and Methods), and approximate age associated with each developmental stage are indicated. Each stage is associated with a characteristic phase of masculinization for laryngeal tension, fiber type, fiber recruitment, or muscle fiber number (Marin et al., 1990).
Average values for the percentage of slow twitch fibers, the percentage of transient tension, and EMG potentiation at each developmental stage are illustrated in Fig. 8. Average values for females are included for comparison. The percentage of slow twitch fibers begins to decline at PM2. There is a marked change in the percentage of slow twitch fibers, the percentage of transient tension, and EMG potentiation between PM2 and PM3. Thus we conclude that PM2 marks the end of the sexually monomorphic period. Fiber type and tension gradually masculinize between PMO and PM5, when masculinization is complete. In contrast, fiber recruitment masculinizes in two phases, one between PM3 and PM5 and a second at adulthood.
Fig. 8.
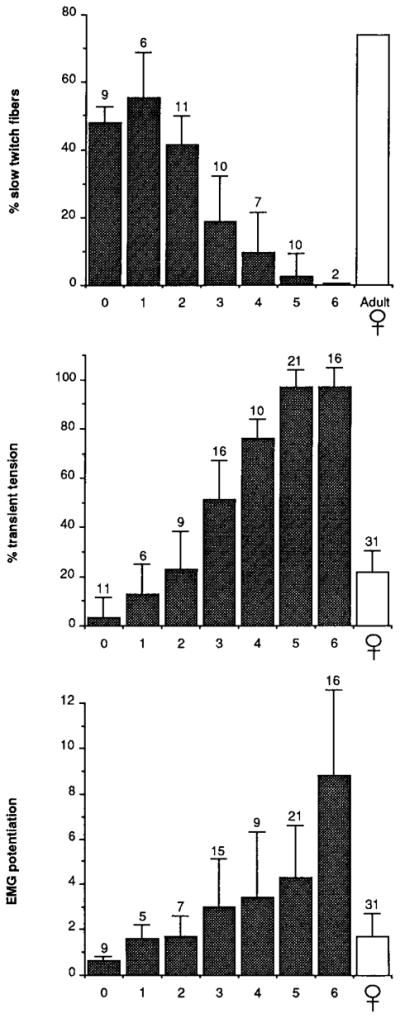
Average values for male percentage of slow twitch fibers, percentage of transient tension, and EMG potentiation at each stage in postmetamorphic development. Female values (mean of PM1 through PM6) are shown for comparison in percentage of transient tension and EMG potentiation graphs; adult female values (from Sassoon et al., 1987) are shown in percentage of slow twitch fibers graph. Bars represent mean ± SD. The number of animals included in each bar is indicated above the bar. All properties show a marked increase in masculinization between PM2 and PM3.
DISCUSSION
Patterns of Laryngeal Masculinization
Sex differences in adult laryngeal muscle tension, fiber type, and fiber recruitment result from sexually dimorphic developmental programs. All three laryngeal properties are sexually monomorphic at the end of metamorphosis and for a short time thereafter. Tension and fiber recruitment properties do not change throughout most of postmetamorphic development in females, but are progressively masculinized throughout most of postmetamorphic development in males. The percentage of slow twitch fibers increases in females but decreases in males during postmetamorphic development (Sassoon et al., 1987). Thus, sexual differentiation occurs during postmetamorphic development. Sex differences in adult laryngeal tension and fiber recruitment result solely from the process of masculinization, as male values increase above constant female values. Sex differences in adult fiber type result from both masculinization and feminization.
The temporal pattern of masculinization also differs for different laryngeal properties (Fig. 8). Masculinization of fiber recruitment appears to be a two-stage process; there are marked increases in EMG potentiation at stages PM3 and PM6, but no increase in EMG potentiation between these stages. In contrast, masculinization of tension and fiber type is gradual and progressive, increasing with each stage of development from PMO until masculinization is complete at PM5.
Stages for laryngeal masculinization (Table 1) are based on attainment of a characteristic phase of masculinization for laryngeal fiber number (Marin et al., 1990), tension, fiber type, and fiber recruitment (this study). At PM0, there is no sex difference in any of these properties. At PM1, sexual differentiation of laryngeal fiber number begins. At PM2, sexual differentiation of laryngeal fiber type, expressed as the decline in percentage of slow twitch fibers, begins. At PM3, sexual differentiation of laryngeal muscle tension and fiber recruitment begins and masculinization of laryngeal fiber number is complete. At PM4, laryngeal tension and fiber type are complete in some animals, and by PM5 these properties are fully masculinized in all animals. At PM6, there is a marked increase in fiber recruitment.
Laryngeal Fiber Recruitment
Sex differences in EMG potentiation are correlated with sex differences in synaptic efficacy at the laryngeal neuromuscular junction (Tobias and Kelley, 1988). Male laryngeal muscle fibers are innervated by weak synapses and female laryngeal muscle fibers are innervated by strong synapses. Since EMG potentiation is low in both sexes early in postmetamorphic development, it may be that male synapses are initially strong and become weak during development. Alternatively, both sexes may have strong synapses early in development which are maintained. Subsequently, newly added synapses may be weak in males and strong in females. Further studies on the synaptic efficacy of developing laryngeal muscle synapses are needed to determine how the sex difference in adults is achieved.
Although it may seem paradoxical that masculine synapses are weak, this property confers certain behavioral advantages to the male. Low transmitter release at the neuromuscular junction allows facilitation which permits graded muscle responses during nerve activity. If the number of contracting muscle fibers controls sound amplitude, then facilitation contributes to amplitude modulation, an important characteristic of the male call. In contrast, strong synapses would ensure a uniform muscle contraction in response to each motor neuron impulse, resulting in a constant sound amplitude. This is the situation in females, whose vocalizations are not amplitude modulated. Further, facilitating synapses in male larynx provide a single mechanism by which both click rate and click amplitude can be controlled by the temporal output from the brain.
Laryngeal Muscle Fiber Type and Tension
One of the most dramatic changes in the male larynx during postmetamorphic development is the complete loss of slow twitch fibers. While this loss might be attributable to massive muscle fiber death, we consider this possibility unlikely. Neither in this study nor in previous work (Sassoon and Kelley, 1986; Sassoon et al., 1987; Marin et al., 1990) have we observed any overt evidence of muscle fiber degeneration in muscle cell nuclei or cytoplasm of the postmetamorphic larynx. There is a marked increase in laryngeal muscle fiber number in postmetamorphic males (Sassoon and Kelley, 1986; Marin et al., 1990), and it is thus possible that loss of slow muscle fibers is due to addition of fast twitch fibers and transformation or loss of existing slow fibers. However, results of this study indicate that loss of slow twitch muscle fibers does not begin until muscle fiber addition is nearly complete (PM3; Marin et al., 1990). We infer then that muscle fiber addition prior to PM3 includes both fast and slow fibers. After PM3, existing muscle fibers must be transformed from slow twitch to fast twitch.
The rate limiting step in repetitive muscle contractions is muscle relaxation. Muscle relaxation is controlled by cleavage of the myosin/actin bond (formed during muscle contraction) by myosin ATPase. This process occurs at different rates for fast and slow twitch fibers and is controlled by different myosin isoforms (reviewed in Burke, 1978). Fiber histochemistry utilizes differences in ATPase activity at different pHs (an enzyme specific property) to identify particular myosin types. Our studies suggest that laryngeal muscle contains one type of myosin ATPase molecule (fast) in adult males and at least two types of myosin ATPase molecules (slow and fast) in females and developing males (see also Sassoon et al., 1987).
We believe that myosin isoform expression contributes to sex differences in click rate. The fastest trill of the male mate call is an order of magnitude faster than the click rate used in female ticking (Wetzel and Kelley, 1983; Hannigan and Kelley, 1986). A click is produced by one laryngeal muscle contraction/relaxation cycle; male laryngeal muscle contracts and relaxes more rapidly than female laryngeal muscle (Tobias and Kelley, 1987). Thus, female laryngeal muscle, which cannot contract and relax at mate call rates, also cannot mate call. Histochemical fiber typing reveals that male laryngeal muscle contains exclusively fast twitch fibers, while female laryngeal muscle contains primarily slow twitch fibers (Sassoon et al., 1987). Thus, there is good agreement among vocal behavior, laryngeal muscle tension properties, and muscle fiber twitch type. If tension and fiber composition are functionally related in adults, one would expect to see this relationship maintained during development. We show here that masculinization of fiber type shortly precedes masculinization of tension (Fig. 8).
In summary, changes in the synaptic efficacy of the male laryngeal neuromuscular junction during postmetamorphic development result in the weak facilitating synapses which permit amplitude modulation in the mate call. Masculinization of laryngeal muscle fiber type produces an all fast twitch muscle capable of the rapid tension transients prerequisite to male song. Since these transformations occur only in males, and since androgen levels in the sexes differ during postmetamorphic development (Lambdin and Kelley, 1986), we hypothesize that androgenic hormones are required for masculinization. This hypothesis is explored in the accompanying paper.
Acknowledgments
We thank Diana Catz, Leslie Fischer, Maria Moschella, and James Watson for helpful comments on the manuscript. We are indebted to John Robertson for his assistance with photomicrographs. This research was supported by NS 23684.
References
- Burke R. Motor units: Physiological/histochemical profiles, neuronal connectivity and functional specializations. Am Zool. 1978;18:127–134. [Google Scholar]
- Guth L, Samaha F. Procedure for histochemical demonstration of actomyosin ATPase. Exp Neurol. 1970;28:365–367. [PubMed] [Google Scholar]
- Hannigan P, Kelley D. Androgen-induced alterations in vocalizations of female Xenopus laevis: Modiflability and constraints. J Comp Physiol A. 1986;158:517–528. doi: 10.1007/BF00603797. [DOI] [PubMed] [Google Scholar]
- Kelley D. Sexually dimorphic behavior. Ann Rev Neurosci. 1988;11:225–251. doi: 10.1146/annurev.ne.11.030188.001301. [DOI] [PubMed] [Google Scholar]
- Kelley D, Tobias M. The genesis of courtship song: Cellular and molecular control of a sexually differentiated behavior. In: Carew TJ, Kelley DB, editors. Perspectives in Neural Systems and Behavior. A. R. Liss; New York: 1989. pp. 175–194. [Google Scholar]
- Lambdin L, Kelley D. Organization and activation of sexually dimorphic vocalizations: Androgen levels in developing and adult Xenopus laevis. Soc Neurosci Abstr. 1986;12:1213. [Google Scholar]
- Marin M, Tobias M, Kelley D. Hormone sensitive stages in the sexual differentiation of laryngeal muscle fiber number in Xenopus laevis. Development. 1990;110:703–711. doi: 10.1242/dev.110.3.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieuwkoop P, Faber J. Normal Table of Xenopus laevis (Daudin) North-Holland; Amsterdam: 1956. [Google Scholar]
- Sassoon D, Kelley D. The sexually dimorphic larynx of Xenopus laevis: Development and androgen regulation. Am J Anat. 1986;177:457–472. doi: 10.1002/aja.1001770404. [DOI] [PubMed] [Google Scholar]
- Sassoon D, Gray G, Kelley D. Androgen regulation of muscle fiber type in the sexually dimorphic larynx of Xenopus laevis. J Neurosci. 1987;7:3198–3206. doi: 10.1523/JNEUROSCI.07-10-03198.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobias M, Kelley D. Vocalizations by a sexually dimorphic isolated larynx: Peripheral constraints on behavioral expression. J Neurosci. 1987;7:3191–3197. doi: 10.1523/JNEUROSCI.07-10-03191.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobias M, Kelley D. Electrophysiology and dye-coupling are sexually dimorphic characteristics of individual laryngeal muscle fibers in Xenopus laevis. J Neurosci. 1988;8:2422–2429. doi: 10.1523/JNEUROSCI.08-07-02422.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobias M, Marin M, Kelley D. Temporal constraints on androgen directed laryngeal masculinization in Xenopus laevis. Dev Biol. 1991;147:260–270. doi: 10.1016/s0012-1606(05)80023-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetzel D, Kelley D. Androgen and gonadotropin control of the mate calls of male South African clawed frogs, Xenopus laevis. Horm Behav. 1983;17:388–404. doi: 10.1016/0018-506x(83)90048-x. [DOI] [PubMed] [Google Scholar]


