Abstract
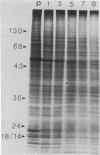
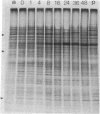
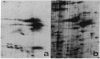
Cytodifferentiation in the transition cycle of the parasitic protozoan Leishmania mexicana amazonensis was studied in vitro. The flagellated motile promastigotes transform into the nonmotile amastigotes in 7 days at 35 degrees C intracellularly in the murine macrophage line J774G8. In medium 199 plus fetal bovine serum, the reverse transformation occurs extracellularly at 27 degrees C in 2 days. Slab gel electrophoresis of leishmanias labeled with [35S]methionine during transformation revealed changes in protein banding patterns. The intensity of two protein species with apparent molecular weights of approximately equal to 55,000 increased in the amastigote-to-promastigote differentiation and decreased during the reverse transformation. These two protein species comigrated approximately with alpha- and beta-tubulin of Chlamydomonas flagella in two-dimensional gel electrophoresis. The lower band was further identified as beta-tubulin by immunoprecipitation using rabbit antiserum specific to the beta-tubulin of Chlamydomonas axonemes. The biosynthetic change of tubulin was found to correlate with the morphological change of microtubules is leishmanial flagella and cytoskeleton during transformation.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akiyama H. J., McQuillen N. K. Interaction and transformation of Leishmania donovani within in vitro cultured cells. An electron microscopical study. Am J Trop Med Hyg. 1972 Nov;21(6):873–879. doi: 10.4269/ajtmh.1972.21.873. [DOI] [PubMed] [Google Scholar]
- Akiyama H. J., Taylor J. C. Effect of macrophage engulfment and temperature on the transformation process of Leishmania donovani. Am J Trop Med Hyg. 1970 Sep;19(5):747–754. doi: 10.4269/ajtmh.1970.19.747. [DOI] [PubMed] [Google Scholar]
- Bloodgood R. A. Resorption of organelles containing microtubules. Cytobios. 1974 Mar-Apr;9(35):142–161. [PubMed] [Google Scholar]
- Brun R., Berens R. L., Krassner S. M. Inhibition of Leishmania donovani transformation by hamster spleen homogenates and active human lymphocytes. Nature. 1976 Aug 19;262(5570):689–691. doi: 10.1038/262689a0. [DOI] [PubMed] [Google Scholar]
- Brun R., Krassner S. M. Quantitative ultrastructural investigations of mitochondrial development in Leishmania donovani during transformation. J Protozool. 1976 Nov;23(4):493–497. doi: 10.1111/j.1550-7408.1976.tb03824.x. [DOI] [PubMed] [Google Scholar]
- Chang K. P. Human cutaneous lieshmania in a mouse macrophage line: propagation and isolation of intracellular parasites. Science. 1980 Sep 12;209(4462):1240–1242. doi: 10.1126/science.7403880. [DOI] [PubMed] [Google Scholar]
- Chang K. P. Leishmania donovani: promastigote--macrophage surface interactions in vitro. Exp Parasitol. 1979 Oct;48(2):175–189. doi: 10.1016/0014-4894(79)90097-3. [DOI] [PubMed] [Google Scholar]
- Chang K. P. Leishmania infection of human skin fibroblasts in vitro: absence of phagolysosomal fusion after induced phagocytosis of promastigotes, and their intracellular transformation. Am J Trop Med Hyg. 1978 Nov;27(6):1084–1096. doi: 10.4269/ajtmh.1978.27.1084. [DOI] [PubMed] [Google Scholar]
- Dwyer D. M. Antibody-induced modulation of Leishmania donovani surface membrane antigens. J Immunol. 1976 Dec;117(6):2081–2091. [PubMed] [Google Scholar]
- Dwyer D. M. Isolation and partial characterization of surface membranes from Leishmania donovani promastigotes. J Protozool. 1980 May;27(2):176–182. doi: 10.1111/j.1550-7408.1980.tb04676.x. [DOI] [PubMed] [Google Scholar]
- Dwyer D. M., Langreth S. G., Dwyer N. K. Evidence for a polysaccharide surface coat in the developmental stages of Leishmania donovani: a fine structure-cytochemical study. Z Parasitenkd. 1974 Jun 21;43(4):227–249. doi: 10.1007/BF00328879. [DOI] [PubMed] [Google Scholar]
- Ebert F., Buse E., Mühlpfordt H. In vitro light and electron microscope studies on different virulent promastigotes of Leishmania donovani in hamster peritoneal macrophages. Z Parasitenkd. 1979 Jun 13;59(1):31–41. doi: 10.1007/BF00927844. [DOI] [PubMed] [Google Scholar]
- Erickson A. H., Blobel G. Early events in the biosynthesis of the lysosomal enzyme cathepsin D. J Biol Chem. 1979 Dec 10;254(23):11771–11774. [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Fulton C. Cell differentiation in Naegleria gruberi. Annu Rev Microbiol. 1977;31:597–629. doi: 10.1146/annurev.mi.31.100177.003121. [DOI] [PubMed] [Google Scholar]
- Guttman S. D., Gorovsky M. A. Cilia regeneration in starved tetrahymena: an inducible system for studying gene expression and organelle biogenesis. Cell. 1979 Jun;17(2):307–317. doi: 10.1016/0092-8674(79)90156-9. [DOI] [PubMed] [Google Scholar]
- Janovy J., Jr Respiratory changes accompanying Leishmania to leptomonad transformation in Leishmania donovani. Exp Parasitol. 1967 Feb;20(1):51–55. doi: 10.1016/0014-4894(67)90021-5. [DOI] [PubMed] [Google Scholar]
- Kowit J. D., Fulton C. Programmed synthesis of tubulin for the flagella that develop during cell differentiation in Naegleria gruberi. Proc Natl Acad Sci U S A. 1974 Jul;71(7):2877–2881. doi: 10.1073/pnas.71.7.2877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krassner S. M. Cytochromes, lactic dehydrogenase and transformation in Leishmania. J Protozool. 1966 May;13(2):286–290. [PubMed] [Google Scholar]
- Krassner S. M., Morrow C. D., Flory B. Inhibition of Leishmania donovani amastigote-to-promastigote transformation by infected hamster spleen lymphocyte lysates. J Protozool. 1980 Feb;27(1):87–92. doi: 10.1111/j.1550-7408.1980.tb04233.x. [DOI] [PubMed] [Google Scholar]
- Königk E., Putfarken B. Stage-specific differences of a perhaps signal-transferring system in Leishmania donovani. Tropenmed Parasitol. 1980 Dec;31(4):421–424. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lai E. Y., Walsh C., Wardell D., Fulton C. Programmed appearance of translatable flagellar tubulin mRNA during cell differentiation in Naegleria. Cell. 1979 Aug;17(4):867–878. doi: 10.1016/0092-8674(79)90327-1. [DOI] [PubMed] [Google Scholar]
- Lefebvre P. A., Nordstrom S. A., Moulder J. E., Rosenbaum J. L. Flagellar elongation and shortening in Chlamydomonas. IV. Effects of flagellar detachment, regeneration, and resorption on the induction of flagellar protein synthesis. J Cell Biol. 1978 Jul;78(1):8–27. doi: 10.1083/jcb.78.1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefebvre P. A., Silflow C. D., Wieben E. D., Rosenbaum J. L. Increased levels of mRNAs for tubulin and other flagellar proteins after amputation or shortening of Chlamydomonas flagella. Cell. 1980 Jun;20(2):469–477. doi: 10.1016/0092-8674(80)90633-9. [DOI] [PubMed] [Google Scholar]
- Lewis D. H., Peters W. The resistance of intracellular Leishmania parasites to digestion by lysosomal enzymes. Ann Trop Med Parasitol. 1977 Sep;71(3):295–312. doi: 10.1080/00034983.1977.11687192. [DOI] [PubMed] [Google Scholar]
- Maccecchini M. L., Rudin Y., Blobel G., Schatz G. Import of proteins into mitochondria: precursor forms of the extramitochondrially made F1-ATPase subunits in yeast. Proc Natl Acad Sci U S A. 1979 Jan;76(1):343–347. doi: 10.1073/pnas.76.1.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcaud L., Hayes D. RNA synthesis in starved deciliated Tetrahymena pyriformis. Eur J Biochem. 1979 Jul;98(1):267–273. doi: 10.1111/j.1432-1033.1979.tb13185.x. [DOI] [PubMed] [Google Scholar]
- Marsden P. D. Current concepts in parasitology. Leishmaniasis. N Engl J Med. 1979 Feb 15;300(7):350–352. doi: 10.1056/NEJM197902153000706. [DOI] [PubMed] [Google Scholar]
- Nogueira N., Chaplan S., Tydings J. D., Unkeless J., Cohn Z. Trypanosoma cruzi. Surface antigens of blood and culture forms. J Exp Med. 1981 Mar 1;153(3):629–639. doi: 10.1084/jem.153.3.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira N. M., de Souza W., Machado R. D., de Castro F. T. Isolation and properties of flagella of trypanosomatids. J Protozool. 1977 Nov;24(4):511–514. doi: 10.1111/j.1550-7408.1977.tb01002.x. [DOI] [PubMed] [Google Scholar]
- Piperno G., Huang B., Luck D. J. Two-dimensional analysis of flagellar proteins from wild-type and paralyzed mutants of Chlamydomonas reinhardtii. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1600–1604. doi: 10.1073/pnas.74.4.1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piperno G., Luck D. J. Microtubular proteins of Chlamydomonas reinhardtii. An immunochemical study based on the use of an antibody specific for the beta-tubulin subunit. J Biol Chem. 1977 Jan 10;252(1):383–391. [PubMed] [Google Scholar]
- Piras M. M., De Rodriguez O. O., Piras R. Trypanosoma cruzi: antigenic composition of axonemes and flagellar membranes of epimastigotes cultured in vitro. Exp Parasitol. 1981 Feb;51(1):59–73. doi: 10.1016/0014-4894(81)90042-4. [DOI] [PubMed] [Google Scholar]
- RUDZINSKA M. A., D ALESANDRO P. A., TRAGER W. THE FINE STRUCTURE OF LEISHMANIA DONOVANI AND THE ROLE OF THE KINETOPLAST IN THE LEISHMANI-LEPTOMONAD TRANSFORMATION. J Protozool. 1964 May;11:166–191. doi: 10.1111/j.1550-7408.1964.tb01739.x. [DOI] [PubMed] [Google Scholar]
- Simpson L. The leishmania-leptomonad transformation of Leishmania donovani: nutritional requirements, respiration changes and antigenic changes. J Protozool. 1968 Feb;15(1):201–207. doi: 10.1111/j.1550-7408.1968.tb02112.x. [DOI] [PubMed] [Google Scholar]
- Strangways-Dixon J., Lainson R. The epidemiology of dermal leishmansiasis in British Honduras. 3. The transmission of Leishmania mexicana to man by Phlebotomus pessoanus, with observations on the development of the parasite in different species of Phlebotomus. Trans R Soc Trop Med Hyg. 1966;60(2):192–207. doi: 10.1016/0035-9203(66)90027-7. [DOI] [PubMed] [Google Scholar]
- Studier F. W. Analysis of bacteriophage T7 early RNAs and proteins on slab gels. J Mol Biol. 1973 Sep 15;79(2):237–248. doi: 10.1016/0022-2836(73)90003-x. [DOI] [PubMed] [Google Scholar]
- Walter R. D., Buse E., Ebert F. Effect of cyclic AMP on transformation and proliferation of leishmania cells. Tropenmed Parasitol. 1978 Dec;29(4):439–442. [PubMed] [Google Scholar]