Abstract
Mate calling by South African clawed frogs, Xenopus laevis, is under the control of androgens. Autoradiographic studies demonstrate androgen-concentrating neurons in a motor nucleus that controls mate calling and a midbrain nucleus that is stimulated by sound. Hormone concentration by laryngeal motor neurons suggests that steroids regulate the final common path for vocal behavior. Modulation of auditory sensitivity by hormones could explain seasonal variations in behavioral responsiveness to conspecific vocalizations.
Sex hormones are powerful modulators of reproductive behaviors. Gonadectomy eliminates and steroid hormones restore sexual responses in most vertebrates (1). Hormone accumulation by target cells in specific brain nuclei is believed to regulate the activity of neural circuits that mediate sexual behaviors. Most steroid-concentrating cells are found in the hypothalamus and infundibulum (1). Recent autoradiographic evidence has shown that motor neurons in nuclei of cranial nerves and in the spinal cord concentrate androgen (2–4) and that some nuclei in the central nervous system (CNS) that receive sensory information are labeled by estrogen (5). I have been investigating mate calling, an androgen-dependent male sexual behavior in the South African clawed frog, Xenopus laevis, and now report that a CNS nucleus that receives auditory information and one that controls vocal behavior contain androgen-concentrating cells.
Male mate calling is stimulated by injection with human chorionic gonadotropin, abolished by castration, and reinstated by treatment with the androgens testosterone or dihydrotestosterone (DHT), but not with estradiol (6). Both DHT and testosterone are present in X. laevis blood; treatment with chorionic gonadotropin increases concentrations of both androgens to five times those seen in untreated males (7). Since testosterone can be metabolized to estradiol by CNS target cells (8), I investigated androgen-specific hormone accumulation in the frog brain with the non-aromatizable hormone DHT. The gonads of five adult male X. laevis were removed. One week later, 200 μCi of octalabeled, tritiated DHT (9) was injected into the dorsal lymph sac; the frogs were allowed to survive for 2 hours, after which the brains (3) and spinal cords were removed, frozen, and processed for steroid autoradiography. Slides were exposed for 4, 6, or 8 weeks, developed, lightly stained with cresyl violet acetate, and microscopically examined for the presence of labeled cells (10). The locations of such cells were plotted on enlarged microprojector drawings of the entire section with the aid of an x-y plotter coupled to linear potentiometers on the microscope stage.
After DHT injection, labeled cells were found in the anterior pituitary, the posterior thalamus, the laminar nucleus of the torus semicircularis, the dorsal tegmental area of the medulla, the principal nucleus of cranial nerve V, the motor nucleus of cranial nerves IX and X (N IX-X), the medullary tegmentum, and in large and small neurons in the ventral portion of the anterior spinal cord [for neuroanatomical nomenclature see (3)] (Fig. 1). Autoradiograms of cells in N IX-X are shown in Fig. 2, A and B. No DHT-labeled cells were ever seen in the anterior preoptic area or the ventral infundibular nucleus, the two regions with the greatest number of heavily labeled neurons after injection of tritiated estradiol or testosterone (3); this absence supports the hypothesis that testosterone-labeled cells in these nuclei are attributable to aromatization to estradiol (11, 12).
Fig. 1.
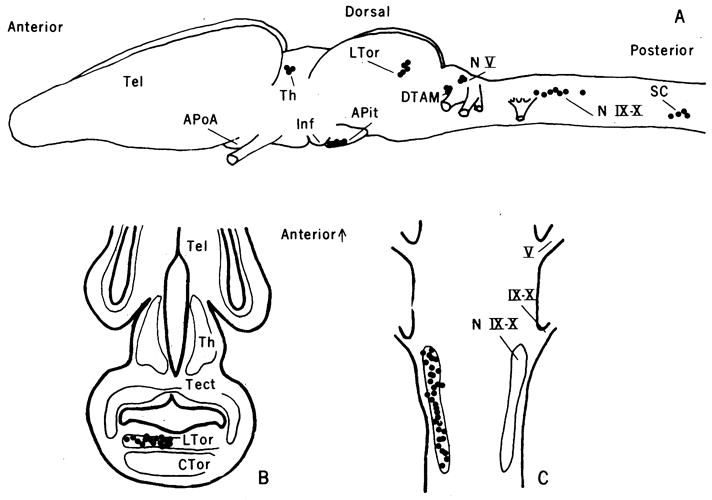
(A) A lateral view of the brain and anterior spinal chord of X. laevis. Locations of DHT-concentrating cells (●) are indicated schematically. (B) A representative horizontal section through the torus semicircularis. Locations of labeled cells (●) are shown on the left side; major brain nuclei are identified on the right. The figure represents all labeled cells from one 10-μm autoradiogram projected onto a standard reference section. (C) A representative horizontal section through the nucleus of cranial nerves IX and X. Abbreviations: APit, anterior pituitary; ApoA, anterior preoptic area; CTor, caudal nucleus of the torus semicircularis; DTAM, dorsal tegmental area of the medulla; Inf, infundibulum; LTor, laminar nucleus of the torus semicircularis; NV, sensory nucleus of the fifth cranial nerve; N IX-X, motor nucleus of the ninth and tenth cranial nerves; SC, ventral horn of the anterior spinal cord; Tect, optic tectum; Tel, telencephalon; Th, thalamus; V, fifth cranial nerve; and IX-X, ninth and tenth cranial nerves.
Fig. 2.
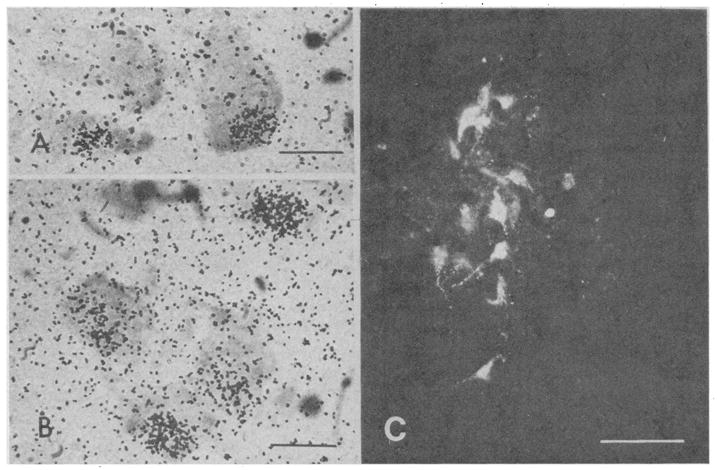
(A and B) Photomicrographs of autoradiograms illustrating DHT-concentrating cells in N IX-X of X. laevis after 6 weeks exposure. Cells are lightly stained with cresyl violet acetate. Calibration bar, 20 μm. (C) Dark-field photomicrograph of HRP-labeled cells in N IX-X after insertion of enzyme into the ipsilateral bipennate muscles of the larnyx. Intracellular HRP was visualized by reaction with tetramethylbenzidine and hydrogen peroxide. Calibration bar; 50 μm.
The anatomical location of DHT-labeled cells adjacent to cranial nerves IX and X - suggested that these neurons might be responsible for innervation of the larynx, the vocal organ of Xenopus, and thus might play a direct role in modulating mate calling. The cells of origin of laryngeal efferents were determined according to the method of retrograde transport of horseradish peroxidase (HRP) (13). Male frogs were anesthetized, and the larynx was exposed. Crystalline HRP was inserted into the arytenoid dilator or bipennate muscle, which controls the production of the click, the basic unit of X. laevis calls (14). Frogs were allowed to survive for 1 or 2 days, anesthetized, and transcardially perfused with fixative; the brains were removed and sectioned at 50 μm. intracellular HRP was visualized by reaction with tetramethylbenzidine or cobalt-intensified diaminobenzidine and hydrogen peroxide (15). All sections were examined microscopically for HRP-labeled cells (Fig. 2C).
After small amounts of HRP were inserted into the muscles of one side of the larynx, HRP-labeled cells were found only in N IX-X of the ipsilateral medulla. This nucleus consists of a rostral division, adjacent to cranial nerves IX and X, and a caudal extension. Cell sizes range from 15 to 33 μm. Both divisions of N IX-X contained labeled cells after HRP injection into the larynx, and both contained 3H-labeled DHT cells in autoradiograms. Large injections of HRP into the bipennate muscles labeled approximately 97 percent of the neurons in the ipsilateral N IX-X. Systemic administration of hormone labeled approximately 60 percent of N IX-X cells (76). The figures suggest that some of the neurons innervating the larynx also concentrate DHT. Thus, behavioral modulation of mate calling by exogenous DHT or testosterone may be mediated by direct effects on the final efferent output of the vocal pathway, the laryngeal motor neurons. As these cells were also labeled after injection of tritiated testosterone but not of tritiated estradiol, cellular accumulation of hormone appears to be selective for androgen.
The laminar nucleus of the torus semicircularis seemed a promising candidate for a role in the neural processing of auditory information (12). Neurophysiological studies by Potter (17) demonstrated the presence of auditorily responsive units in that nucleus in Rana catesbeiana. Xenopus laevis both makes and receives sound while underwater. Under these conditions, the 2-deoxy-D-glucose (2DG) autoradiographic technique (18) appeared a convenient initial approach for determining the auditory sensitivity of the nucleus. Male frogs were injected with radioactive 2DG and exposed to a 2-hour tape of conspecific vocalizations or to ambient noise. The frogs were killed and their brains removed and sectioned on a cryostat according to the method of Sokoloff for 14C-labeled 2DG or according to a precoated slide method (19) for 3H-tabeled 2DG. After autoradiography, cryostat sections were Nissl-stained and brain areas corresponding to regions of high 2DG uptake identified. For intact frogs exposed to mate calls, the nucleus appears as a distinct dark bar, just caudal to the tectal ventricle (Fig. 3A). Unilateral removal of the tympanum and middle ear bones prior to exposure to taped calls resulted in a 50 percent decrease in grain density in the contralateral nucleus compared with the ipsilateral side (Fig. 3B). When frogs were bilaterally deafened and exposed to ambient noise, the nucleus could not be distinguished in autoradiograms, although the adjacent caudal nucleus of the torus semicircularis is clearly visible (Fig. 3C). Thus, the laminar nucleus of the torus semicircularis is a mesencephalic auditory nucleus that contains cells that concentrate DHT and estradiol (3, 20).
Fig. 3.
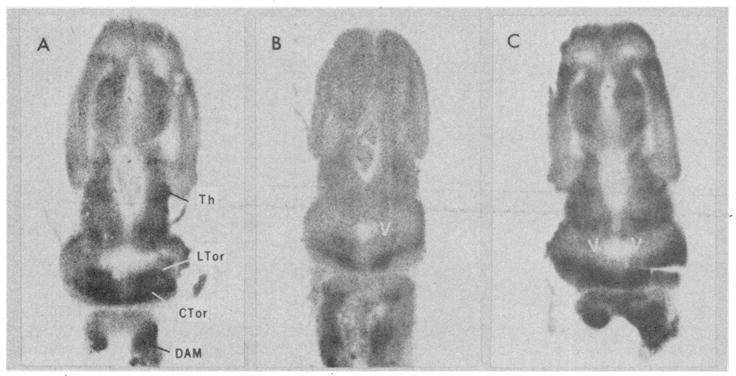
Autoradiograms of horizontal sections through the torus semicircularis of frogs exposed to 14C-labeled 2DG. (A) Intact male frog stimulated with a 2-hour tape of conspecific vocalizations. Regions of heavy accumulation of radioactivity include the dorsal acoustic medulla (DAM), the caudal nucleus of the torus semicircularis (CTor), the laminar nucleus of the torus semicircularis (LTor), and anterior and posterior thalamus (Th). (B) Male frog deafened on one side and stimulated with a 2-hour tape of conspecific vocalizations. Arrow, reduction of label in the contralateral LTor. (C) Bilaterally deafened, male frog exposed only to ambient noise. While CTor and Th are clearly visible, LTor cannot be distinguished (arrows).
To my knowledge this is the first report of steroid-concentrating cells in a functionally identified vertebrate auditory nucleus. Other stations of the auditory neural pathway in frogs may contain steroid-sensitive cells as well. With regard to the efferent pathway for vocal control in frogs, calling behavior is modulated by the anterior preoptic area, the ventral infundibulum, and the dorsal tegmental area of the medulla (21). As all these nuclei contain steroid-concentrating cells in X. laevis (3), we should consider the possibility that all stations of the neural pathway controlling calling are sensitive to hormones (4). Multiple sites of hormone action on neural pathways for reproductive behavior may ensure a high frequency of sexual behaviors during the breeding season and may synchronize the receptivity of females with the attraction behavior of males. Such evolutionary specializations are probably not confined to anurans but may be present in other vertebrates that breed seasonally, including birds and mammals.
Acknowledgments
I thank B. Goun and C. Szmauz for expert technical assistance; J. Paton for preparing some of the 2DG autoradiograms; H. Feder for providing the radioimmunoassay data; B. Campbell, D. Griffin, C. Gross, and B. Hoebel for lending equipment. Supported by grant HD12126 from the National Institutes of Health.
References and Notes
- 1.Kelley D, Pfaff D. In: Biological Determinants of Sexual Behavior. Hutchison J, editor. Wiley-Interscience; New York: 1978. pp. 225–254. [Google Scholar]; Morrell J, Kelley D, Pfaff D. In: Proceedings of the Second Brain-Endocrine Interaction Symposium. Knigge K, Scott D, Kobayashi M, Ishii S, editors. Karger; Basel: 1975. pp. 230–256. [Google Scholar]
- 2.Examples of testosterone-concentrating motor neurons include the nucleus of cranial nerves IX and X in frogs (3), hypoglossal neurons in zebra finches (4), and motor neurons in the ventral horn in rats [ Sar M, Stumpf WE. Science. 1977;197:77. doi: 10.1126/science.867053.].
- 3.Kelley D, Morrell J, Pfaff D. J Comp Neurol. 1975;164:47. doi: 10.1002/cne.901640105. [DOI] [PubMed] [Google Scholar]; Morrell J, Kelley D, Pfaff D. :63. ibid. [Google Scholar]
- 4.As has also been described for CNS control of song in birds [ Arnold A, Nottebohm F, Pfaff D. 1976;165:487. doi: 10.1002/cne.901650406. ibid.
- 5.For example, dorsal horn cells in the spinal cord concentrate estradiol [ Keefer DA, Stumpf WE. Proc Soc Exp Biol Med. 1973;143:414. doi: 10.3181/00379727-143-37333.
- 6.Kelley D, Pfaff D. Horm Behav. 1976;7:159. doi: 10.1016/0018-506x(76)90045-3. [DOI] [PubMed] [Google Scholar]; Kelley D. unpublished observations [Google Scholar]
- 7.Blood concentrations are given in nanograms per milliliter (N = 2) for each hormone condition. Intact maleX. laevis: testosterone, 3.6/2.8; DHT, 1.4/3.5. Intact males injected with human chorionic gonadotropin: testosterone, 19.5/22.3; DHT 24.6/20.9. Castrated males injected with gonadotropin: testosterone, 0.7/0.9; DHT 4.9/1.0.
- 8.Ryan K. J Biol Chem. 1959;234:268. [PubMed] [Google Scholar]; Acta Endocrinol. 1960;35:697. [Google Scholar]
- 9.New England Nuclear 200 Ci/mmole. Each frog (mean weight, 44 g) was injected with 300 ng of DHT. This dose results in blood concentrations equivalent to those of intact males injected with gonadotropin (7).
- 10.The criterion for a labeled cell was that the number of silver grains over the stained cell body equal or exceed five times the number of grains over adjacent, cell-sized areas of neuropil (background).
- 11.Kelley D. Am Zool. 1978;18:477. [Google Scholar]
- 12.Kelley D, Lieberburg I, McEwen B, Pfaff D. Brain Res. 1978;140:287. doi: 10.1016/0006-8993(78)90461-4. [DOI] [PubMed] [Google Scholar]
- 13.LaVail J, LaVail M. J Comp Neurol. 1974;157:303. doi: 10.1002/cne.901570304. [DOI] [PubMed] [Google Scholar]
- 14.Ridewood W. Linn Soc J Zool. 1897;26:53. [Google Scholar]; Rabb A. Copeia. 1960;1960-1V:369. [Google Scholar]; Yager D. personal communication.
- 15.Mesulam MM. J Histochem Cytochem. 1978;26:106. doi: 10.1177/26.2.24068. [DOI] [PubMed] [Google Scholar]; Adams J. Neurosci. 1977;2:141. [Google Scholar]
- 16.The percentage of HRP-labeled cells in N IX-X was determined by counting labeled and unlabeled cells on every other 50 μm neutral red counterstained section through the nucleus. Depending on injection size and survival time, the percentage of ipsilateral labeled cells ranged from 48 percent (small injection, 1 day survival, only ipsilateral cells labeled) to 97 percent (large injection, 2 days survival, many contralateral cells labeled). Autoradiograms (thickness, 10 μm) were analyzed at 50 μm intervals. Using the background criterion for labeled cells (10), between 57 and 63 percent of N IX-X cells were categorized as androgen concentrating.
- 17.Potter H. J Neurophysiol. 1965;28:1155. doi: 10.1152/jn.1965.28.6.1155. [DOI] [PubMed] [Google Scholar]
- 18.Sokoloff L. J Neurochem. 1977;29:13. doi: 10.1111/j.1471-4159.1977.tb03919.x.Studies of 14C-labeled 2DG in the rat demonstrated that occlusion of the external auditory meatus on one side decreased 2DG uptake in the contralateral inferior colliculus by 75 percent. The inferior colliculus is generally believed to represent the mammalian homolog of the torus semicircularis (17)
- 19.Sejnowski T, Kelley D, Paton J, Yodlowski M. Abstr Soc Neurosci. 1979;5:30. [Google Scholar]; Kelley D, Paton J, Sejnowski T, Yodlowski M. The maximum sound intensity of the taped vocalizations was 300 dyne/cm2 in preparation. [Google Scholar]
- 20.We do not yet know whether there are separate receptors for DHT and estradiol in the laminar nucleus of the torus semicircularis. Recent experiments in rats have shown that estradiol has a strong affinity for the DHT receptor, but that DHT binds very weakly to the estradiol receptor [ Chamness G, King T, Sheridan P. Brain Res. 1979;161:267. doi: 10.1016/0006-8993(79)90068-4.
- 21.Schmidt R. Am Zool. 1973;13:1169. [Google Scholar]; J Comp Physiol. 1974;92:229. [Google Scholar]


