Summary
In murky, crowded ponds in South Africa, female clawed frogs, Xenopus laevis (Daudin), vocalize to signal reproductive state. Female calls consist of acoustically similar clicks delivered in trains with characteristic rates. The rapping call of a sexually receptive female has a more rapid click rate [81 ms mean interclick interval (ICI)] than the ticking call of an unreceptive female (219 ms ICI). Rapping stimulates male advertisement calling, whereas ticking suppresses an already calling male. We examined how males label and discriminate female click rates. A labeling boundary experiment revealed that males perceive click rates between the means of rapping and ticking as lying on a continuum. They respond to 98 and 160 ms ICI as though to rapping and ticking, respectively. However, calling evoked by a click rate equally common to both calls (120 ms ICI) did not differ from the response to either rapping or ticking. A second experiment evaluated whether males discriminate click rates both labeled as ticking (180 and 219 ms ICI). Ticking suppresses advertising males, and suppressed males habituate (resume calling) to prolonged ticking. Both ticking stimuli suppressed males with equal effectiveness, and males habituated in equivalent amounts of time. When the stimulus was switched during habituation, no dishabituation occurred. We conclude that male labeling of click trains as rapping or ticking reflects an ambiguity resulting from the overlap in ICIs naturally occurring in the calls. Males do not respond differentially to click rates within the ticking category. Males thus combine discriminating and non-discriminating strategies in responding to the salient feature of female calls.
Keywords: anura, auditory discrimination, categorical perception, frog, vocal communication, Xenopus
Introduction
Vocal communication requires the decoding of spectral and temporal information from sounds. Acoustic features such as frequency and rhythm lie on continuous ranges, where neighboring regions can differ in behavioral salience. Dividing the continuum into regions that evoke distinct behavioral responses requires a perceptual strategy. In continuous perception – used, for example, in the discrimination of intermediate calls by female green tree frogs – females exhibit finely graded responses along the acoustic continuum between male advertisement and aggressive calls (Gerhardt, 1978). In categorical perception – used, for example, in the Polynesian field cricket’s identification of conspecific mating calls and predator bat echolocation calls – differences along a continuum are perceived with greater acuity at category boundaries than within the range of one category (Wyttenbach et al., 1996). Categorical perception can improve the speed and accuracy of discrimination between different classes of social communication signals (Gleason and Ratner, 1998). However, where there is an overlap between signals whose significance differs, a continuous strategy may be more appropriate.
The ticking and rapping calls (Tobias et al., 1998) of the South African clawed frog, Xenopus laevis (Daudin), provide an informative system in which to investigate the relative roles of continuous and categorical strategies in acoustic communication. X. laevis communicates underwater at night using eight vocal call types made up of clicks that differ in temporal patterns (Tobias et al., 2004). The two female calls are rapping, a rapid trill, which functions as an acoustic stimulant that increases male advertisement calling, and ticking, a slower trill, which suppresses male vocal production (Tobias et al., 1998). Sexually receptive females rap whereas unreceptive females tick.
Other X. laevis calls differ in click envelope, in relative amplitude between clicks, and/or in trill durations, but the most distinctive acoustic difference between female rapping and ticking is click rate (Tobias et al., 1998). The mean interclick interval (ICI) of rapping is 81 (±14 ms s.d.) and the mean of ticking is 219 (±71 ms), with some overlap in range (between 50 and 175 ms, Fig. 1A) (Tobias et al., 1998). Regardless of the female call type or the individual, spectral energy in the broadband clicks peaks at 1.2 kHz (Tobias et al., 1998). Rise-times and fall-times appear only slightly sharper in ticking clicks than in rapping clicks. A solitary female often raps at levels similar to ticking, 39 dB with reference to 1 µPa, but during a male–female duet in our 2 m laboratory tank the maximum rapping sound level reached 86 dB re 1 µPa (Tobias et al., 1998). Males use click rate to distinguish the sex of callers (Tobias and Kelley, 1987; Vignal and Kelley, 2007).
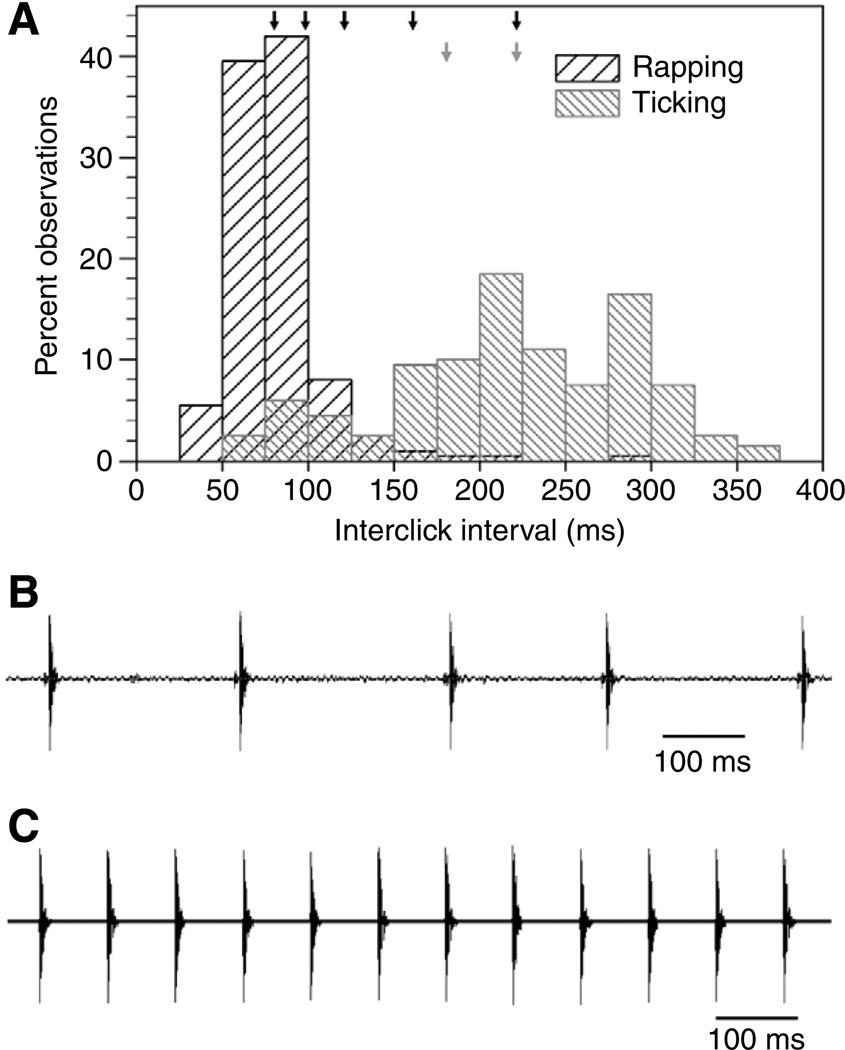
Fig. 1.
(A) Distribution of interclick intervals (ICIs) from natural bouts of ticking and rapping. Black arrows (top) indicate the click rates of stimuli used in the labeling experiment. Gray arrows show click rates used in the dishabituation experiment. (Modified from Tobias et al. 1998.) (B) Oscillogram showing a typical 1 s selection from the field recording of ticking that provided the clicks used to make playback stimuli. Scale bar, 100 ms. (C) Oscillogram of a 1 s sample of the fastest female call used as a playback stimulus. This stimulus consists of ticking clicks presented at the 81 ms ICI of rapping. Scale bar, 100 ms.
We began by characterizing the labeling boundary for rapping and ticking: the ICI range over which the male’s vocal response changes from excitation to suppression. For continuous perception we would expect a graded male response to ambiguous ICIs; for categorical perception, a discrete change in the response over a narrow range of ICIs. To examine the male’s response within a call-type category, we first established that males habituate to one female call. Receptive males call continually, pausing occasionally, as long as they hear rapping; we have not reliably observed habituation to rapping. However, preliminary observations suggested that males do habituate to ticking, by calling in a rebound from suppression. After suppression, we presented males with additional trills with ICIs within the characteristic range of ticking. A change in the frog’s vocal response (dishabituation) relative to the control condition would indicate that the frog had detected the difference in stimuli and was altering his calling accordingly (indicating continuous discrimination within click rates labeled as ticking). The absence of dishabituation would suggest that males do not discriminate among ticking stimuli (consistent with the prediction of categorical perception of within-category stimuli). Rapping and ticking thus provide a powerful system in which to study how temporal features of sound are identified and discriminated.
Materials and methods
Animals and recording set-up
Sexually mature males were obtained from Nasco (Ft. Atkinson, WI, USA) or Xenopus I (Ann Arbor, MI, USA), housed singly in polycarbonate aquaria under a 12 h:12 h L:D cycle, and fed frog brittle twice a week. Sixteen males were selected for robust calling in the labeling experiment, with a criterion of >15 s spontaneous calling over 30 min. In the second, dishabituation experiment, 20 males were included with a calling criterion of more than three fast advertisement trills over 30 min. On the day before and the morning of the experiments (at least 6 h before testing), males were injected with human chorionic gonadotropin (hCG; 100–300 IU ml−1; Sigma, St. Louis, MO, USA) subcutaneously into the dorsal lymph sac, to promote vocal behavior (Wetzel and Kelley, 1983). Labeling boundary playback experiments were performed in a fiberglass tank (1 m×2 m×0.5 m deep) at the beginning of the dark period, under dim indirect illumination from a red lamp. Dishabituation experiments were performed in an approximately 1 m3 fiberglass tank filled 3/4-full with dechlorinated, filtered water and resting on a layer of foam. Labeling boundary experiments took place during the springs of 2003 and 2004, and dishabituation experiments during the fall and winter of 2005–2006, at water temperatures from 19 to 23°C.
Stimuli
In the labeling boundary experiment, stimulus CDs were played on a Sony Walkman (D-EJ360; Tokyo, Japan) or a CD recorder (Marantz CDR300; Mahwah, NJ, USA). In the dishabituation experiment, the initiation and insertion of sound stimuli was instead controlled on-line on a laptop computer (Apple Titanium G2). Call stimuli were amplified (Realistic MPA30) and presented through an underwater loudspeaker (University Sound UW-30 Diatran underwater loudspeaker, frequency response 0.1–10 kHz; San Diego, CA, USA). The volume of playback was matched to recordings of a live female ticking in the same tank during pilot experiments. To create stimuli, we started with bouts of female ticking (selection in Fig. 1B) recorded in the field (Tobias et al., 1998), using a Cornell Bioacoustics Program hydrophone (output sensitivity −163±3 dB re 1 V/µPa, frequency sensitivity 0.015–10 kHz; Ithaca, NY, USA), into a Marantz cassette tape recorder (PMD430) and digitized. We edited periods of silence between the clicks to produce the following constant intervals: 81, 98, 120, 160, 180 and 219 ms ICIs (see Fig. 1A,C).
Playback procedures
Labeling boundary protocol
The click rates presented include the means for rapping (81 ms ICI) and for ticking (219 ms ICI). In addition, we presented three intermediate rates – 98, 120 and 160 ms ICIs – that represent log intervals between the means of rapping and ticking (Fig. 1A) (Tobias et al., 1998). Logarithmic spacing of the intermediate stimuli was chosen because human listeners and European starlings perceive rate logarithmically, with similar behavioral discrimination over logarithmically proportional intervals (Braaten and Hulse, 1993; Palmer and Krumhansl, 1990).
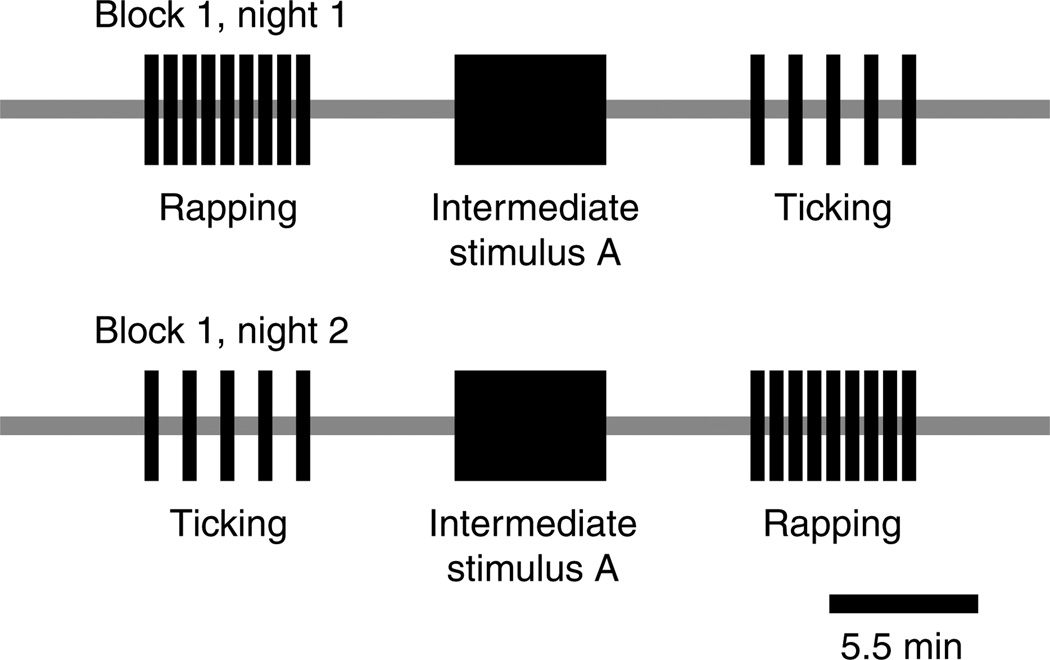
In pilot studies, the boundary between rapping and ticking click rates appeared to vary with presentation order (preliminary data). To control for presentation order, we presented the intermediate test stimulus between a rapping control and a ticking control stimulus each night (Fig. 2). The order of rapping and ticking controls was decided pseudorandomly by coin toss, with the requirement that both orders be heard at least twice in a block of 5 nights. Stimuli were 5.5 min in duration, separated by 5.5 min of silence and preceded by 5.5 min of silence during which baseline calling was recorded. To prevent a saturation effect in the amount of calling measured, this stimulus duration exceeded the typical calling bout duration for males exposed to female calls with different ICIs. As an example, on the first night of the 5-night block using the 120 ms ICI intermediate stimulus, the stimulus CD might contain in order: baseline silence, rapping, silence, the stimulus with 120 ms ICIs, silence, ticking and a final silence (Fig. 2).
Fig. 2.
Schematic design of the labeling boundary experiment. The example protocol shows one possible experimental sequence of a male tested on the first two nights of the block with the first intermediate test stimulus. Scale bar, 5.5 min.
Every subject was tested with all three intermediate stimuli. Each intermediate stimulus was presented nightly for a single block of 5 nights. The order of the three testing blocks was determined pseudorandomly. For most blocks, we analyzed data from nights 1–4. In some blocks, subjects did not call robustly during initial stimuli on the first night, possibly because of novelty-induced suppression. If time spent calling to the control stimuli (rapping and ticking click rates) on night 1 totaled <30 s, we analyzed nights 2–5. This occurred in 16 out of 48 blocks, involving nine subjects. Responses were averaged over the 4 nights. To confirm that nights 2–5 in the 16 blocks were equivalent to nights 1–4 in the other blocks, we performed a repeated-measures analysis of variance (RM-ANOVA) testing the total calling (responses and baseline) by night. Nights 4 and 5 each produced significantly more calling than night 1 (P<0.05 and P<0.01, respectively). No significant difference between nights 1 and 2 was found. A week without hormone injections preceded each of the three testing blocks.
Dishabituation protocol
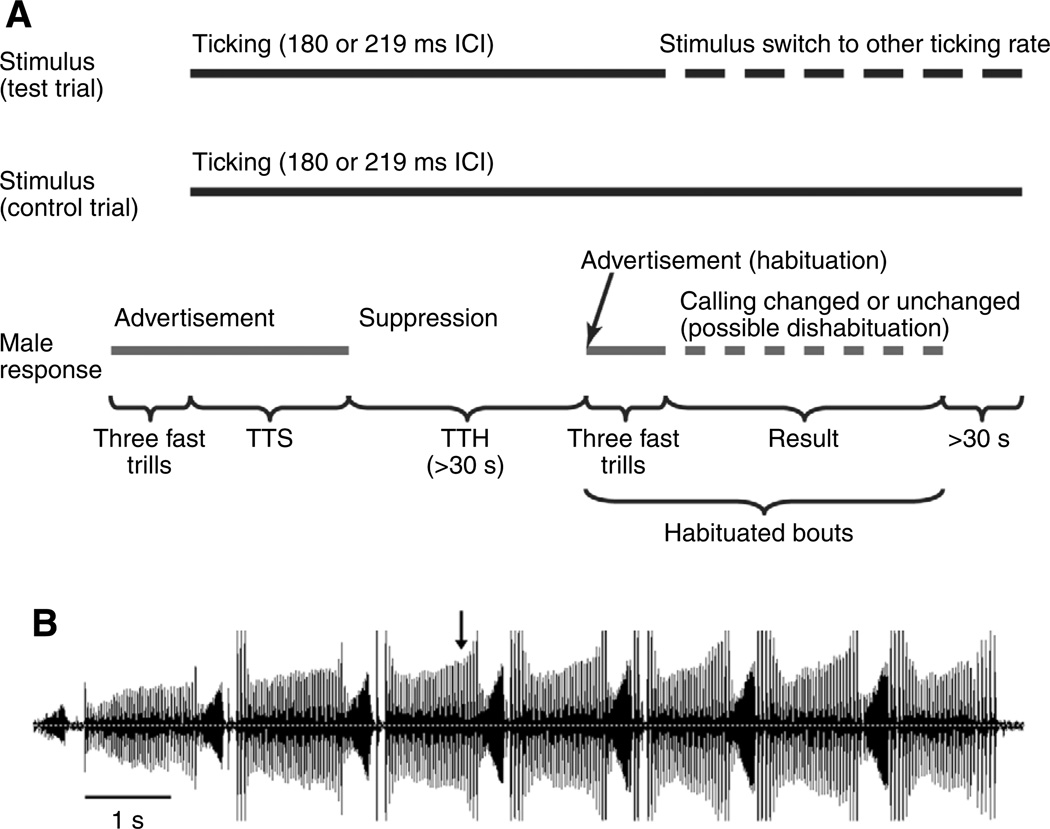
The phenomenon of dishabituation indicates discrimination between stimuli that lie nearby along the gradient of a perceptual parameter. We used a dishabituation paradigm (Fig. 3A) to test for discrimination between two click rates labeled as ticking (180 ms and 219 ms ICI). These two rates were chosen because 219 ms ICI is the mean and therefore likely to be an effective suppressor; 180 ms ICI is more similar to rapping but is well within the range labeled as ticking. After suppression, when frogs had habituated to one or the other of the ticking stimuli by beginning to call again with three fast trills of the advertisement call (Fig. 3B), we either switched the stimuli (test trial, Fig. 3A) or held the stimulus constant throughout the habituated calling (control trial). In either condition, time spent calling was counted until a pause of at least 30 s occurred. If the frog changed his calling behavior in any way – whether by suppressing more quickly, or by calling longer or with more rapid trill alternations – this change would constitute dishabituation and would indicate that the frog had detected the difference in stimuli and was altering his calling accordingly.
Fig. 3.
(A) Schematic protocol of the dishabituation experiment. Each night, two introductory trials (not shown) were terminated after 30 s of suppression of male calling by the initial ticking stimuli. The top dark gray bar shows the temporal extent of the stimulus, with either a continuation or a switch to another stimulus (broken line, test trial). Lower solid gray bars show the relative timing of male advertisement and subsequent calling. The second bar becomes broken to show the time during which habituated advertisement calling was evaluated for post-stimulus-switch change, or dishabituation. ICI, interclick interval; TTH, time to habituation; TTS, time to suppression. (B) Example of a habituated bout of advertisement calling under a test condition. The male had been suppressed for approximately 4 min prior to habituation. The arrow marks the time point that the 180 ms ICI stimulus was changed to the 219 ms ICI. The bout was followed by 30 s of silence (not shown) while the 219 ms ICI stimulus continued to play. The loudest slow-trill clicks are clipped because of the male moving close to the hydrophone. On this time scale, the peaks of the fast-trill clicks cannot be easily separated by eye because the clicks overlap.
Experiments were begun after the male started calling (either spontaneously or, more often, promoted by 30 s of clicks broadcast at the rapping rate after 15 min without calling). After the male had produced three fast trills of the advertisement call, we presented either the 180 or the 219 ms ICI stimulus chosen by coin flip (Fig. 3A). We measured time spent calling (all male calls) until there was a 30 s pause in calling, which was our criterion for suppression. Time from the stimulus onset until the beginning of the 30 s suppression period is the time to suppression (TTS; Fig. 3A).
After two trials measuring the TTS to the two stimuli, we began dishabituation trials (Fig. 3). These trials began in the same way as the introductory trials, with the exception that we continued to play the stimulus past the 30 s period of suppression. When a male again made three fast advertisement trills, we considered this calling as an instance of habituation. We calculated the time to habituation (TTH) as the time following TTS up until the male started calling again (Fig. 3A). (Habituation is defined as the decreased responsiveness to repeated stimulus exposure; in this case, the response that habituates is the suppression response, rather than the vocalization itself.) The TTS and TTH criteria were originally defined based on suppression and bout times in pilot experiments with three males (data not shown). In test trials (Fig. 3), after the three fast trills of habituated advertisement calling, the stimulus then playing was switched to the other stimulus (180 or 219 ms ICI) using the stimulus-controlling computer. A custom-made stimulus program used the audio environment Max/MSP 4.5 (Cycling ’74, San Francisco, CA, USA) to make a patch that looped broadcasts of habituation stimuli and switched smoothly between stimuli during dishabituation trials. In control trials, there was no stimulus change; the stimulus continued until the male stopped calling for 30 s. Measurements of the male’s response to test and control trials included the time spent calling, the total time until a 30 s period without calling, and the number and rate of fast advertisement trills. If the male responded differently to test trials than to control trials, this alteration in vocal behavior would constitute dishabituation and would indicate that the male had detected the change in click rate.
Conditions were presented in a pseudorandom order, balanced to include all four combinations that differed in which stimulus came first and whether the stimulus changed mid-bout upon habituation. Trials were aborted after 10 min of suppression. Males were tested on successive nights (1 to 3) until two sets of four-stimulus combinations had been tested; 19 males completed the stimulus set.
Data collection and analysis
Male vocal responses were recorded with two hydrophones (Cornell Bioacoustics Program, sensitivity as described above), one suspended in the center of the tank at a depth of 0.5 m and the other placed on the bottom of the tank slightly to one side of the speaker in the corner. The hydrophone channels were recorded to CD (Marantz CD recorder CDR300) or stored as stereo MP3 files on a digital recorder (Marantz PMD670).
Male vocalization recordings were analyzed using Goldwave on a PC (Hewlett Packard Pavilion). Time spent calling was determined to within 0.5 s by visual inspection of the files or, when the recording failed because of CD error, by using the clock on the CD recorder and transcribing calling times to the nearest second by ear. In the labeling boundary experiment, isolated clicks were rounded up to 0.5 s.
The male advertisement call alternates between short, fast trills that are strongly intensity-modulated, and long, slow trills that vary less in peak click amplitudes (Fig. 3B). Bouts of advertisement calling sometimes include an acoustically related call, male answer calling, that differs in the relative duration of fast and slow trills: fast trills are lengthened and slow trills are shortened (Tobias et al., 1998). Males answer call during duets with rapping and ticking females, as well as during interactions with other males (Tobias et al., 1998; Tobias et al., 2004). Because answer calls occur during acoustic stimulation or physical interactions between animals, whereas advertisement calling is also given by isolated males without stimulation, we analyzed the number of fast trills per unit of time as a possible indication of male changes in perception or motivation. For the dishabituation experiment, fast advertisement trills were tallied, and inter-fast-trill intervals (IFTIs) were measured to test for dishabituation.
To avoid observer bias in the test for dishabituation, measurement of IFTIs in the habituated bouts was assessed visually by a second observer unaware of the stimulus code. Although the stimulus changes are audibly apparent in the sound files, the much higher sound level of the male call prevented the observer from seeing stimulus changes during the visual inspection of the signal files in Goldwave (waveforms depict amplitude versus time in Fig. 3B). To determine whether a change had occurred in the rate of fast trills after the stimulus switch, the second observer compared the times between the first three habituated fast trills (two intervals) and the subsequent three fast trills (two intervals). In the case of control trials the latter two intervals occurred after the stimulus would have been switched had it been a test trial. The third fast trill interval was excluded because the stimulus switch occurred at different time points within this slow trill (Fig. 3B).
In both experiments, two-tailed RM-ANOVAs were used to test for significance at the P<0.05 level. Fisher’s probable least squares difference (PLSD) was used as a post-hoc test because the probability of type I error was deemed acceptable at α=0.05. All values given are means ± s.e.m.
Results
We probed the boundary across which male X. laevis label two female calls: the fertility call rapping and the release call ticking.
Labeling boundary experiment
Males respond differentially to click trains with ticking and rapping ICIs (50.0±36.0 versus 80.9±38.2 ms of calling, respectively). Rapping elicited significantly more calling than did ticking (P<0.0005, RM-ANOVA, Fisher’s PLSD). Baseline levels of calling during the introductory period of silence tended to be lower (35.4±26.7 ms), but only rapping evoked calling that was significantly elevated from baseline (P<0.0001, RM-ANOVA, Fisher’s PLSD). These results confirm previous observations from the field (Tobias et al., 1998). Whereas ticking suppresses a male when it is presented during a bout of advertisement calling (see dishabituation experiment, below), it does not suppress calling relative to the baseline condition of males alone in a tank without acoustic playback at the start of the experiment.
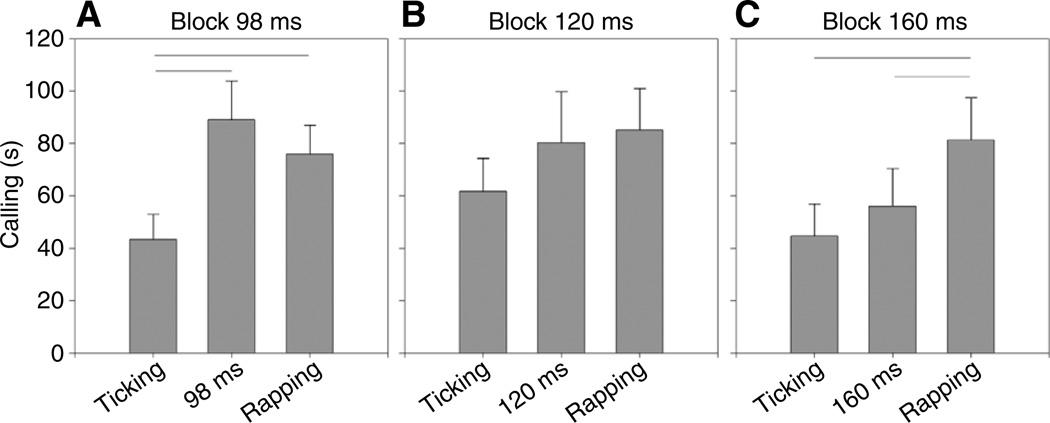
Of the three intermediate stimuli (Fig. 4), the one with a 98 ms ICI stimulated as much calling as the rapping stimulus (81 ms ICI), but significantly more calling than the ticking stimulus (219 ms ICI) presented on the same nights (P<0.0005, RM-ANOVA, Fisher’s PLSD, Fig. 4A). The response to the 160 ms ICI stimulus was significantly lower than the response to rapping on the same nights (P<0.05, Fisher’s PLSD, Fig. 4C). By contrast, calling during the 120 ms ICI stimulus did not differ significantly from calling during rapping or from calling during ticking in that block (P>0.05, RM-ANOVA, Fig. 4B). A significant effect of the call with 120 ms ICIs might have been masked by variability between individual males; for example, if half the animals responded ‘positively’ (as to rapping) and half ‘negatively’ (as to ticking or as in baseline calling). However, the variability in response across nights for each frog was equivalent to the variability between individuals (the by-subject analysis within the RM-ANOVA was not significant).
Fig. 4.
Mean and standard error of time spent calling in response to each playback stimulus during the three testing blocks (A–C). Horizontal lines indicate which columns are significantly different (P<0.05, RM-ANOVA, Fisher’s PLSD). (A) The response to the fastest intermediate stimulus (98 ms ICI) was significantly greater than the response to the ticking stimuli (219 ms ICI) presented during the same block. (B) Calling to the middle intermediate stimulus (120 ms ICI) was not significantly different from calling to the other two stimuli presented on the same nights (219 and 180 ms ICI). (C) The slowest intermediate stimulus (160 ms ICI) elicited calling that was significantly lower than calling to rapping stimuli (81 ms ICI) heard on the same nights.
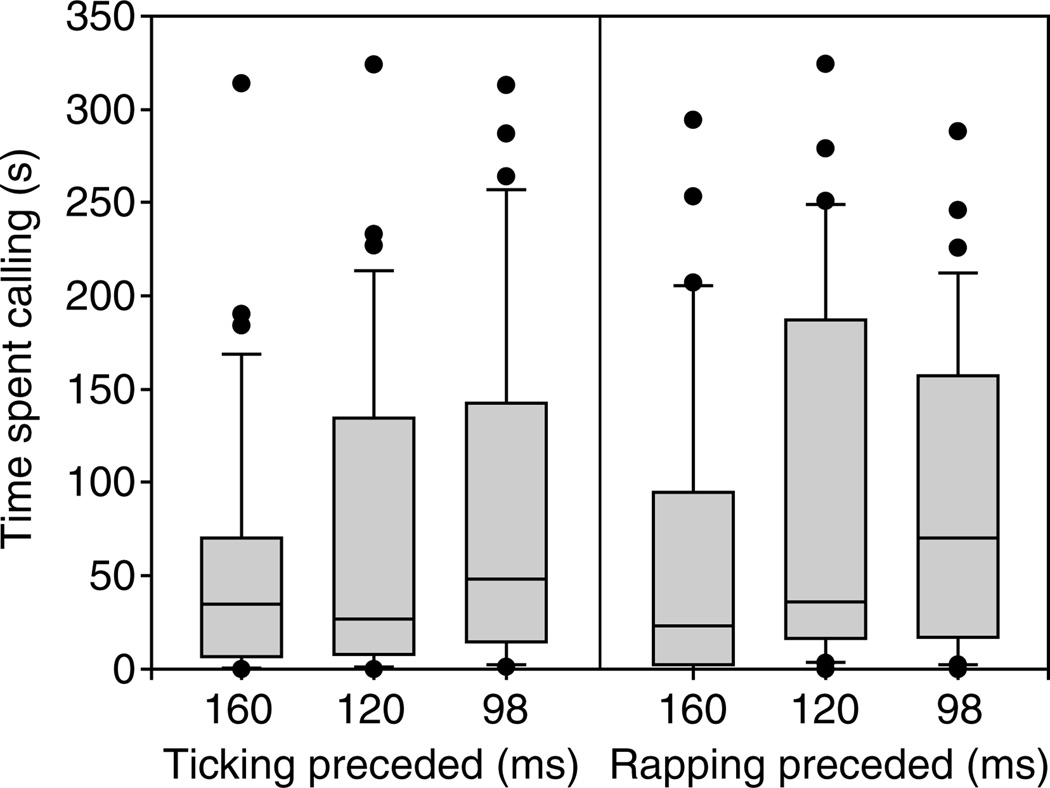
There was no significant stimulus order effect: the amount of time spent calling during the intermediate test stimuli (98, 120 or 160 ms ICI) did not depend on whether the 81 ms or the 219 ms ICI control stimulus preceded (t-test rejected at P>0.05, Fig. 5).
Fig. 5.
There was no significant effect of the preceding stimulus on time spent calling to the three intermediate click-rate test stimuli (t-test rejected at the P>0.05 level) in the labeling boundary experiment. Boxplots represent the median, interquartile range (box), 10th and 90th percentiles (whiskers), and outliers (dots).
Dishabituation experiment
Next we examined whether males discriminate between two click rates in the range of call overlap they label as ticking, 180 and 219 ms. We tested whether males that have habituated to one ticking rate react when the rate is switched to the other.
Male suppression and habituation to female ticking
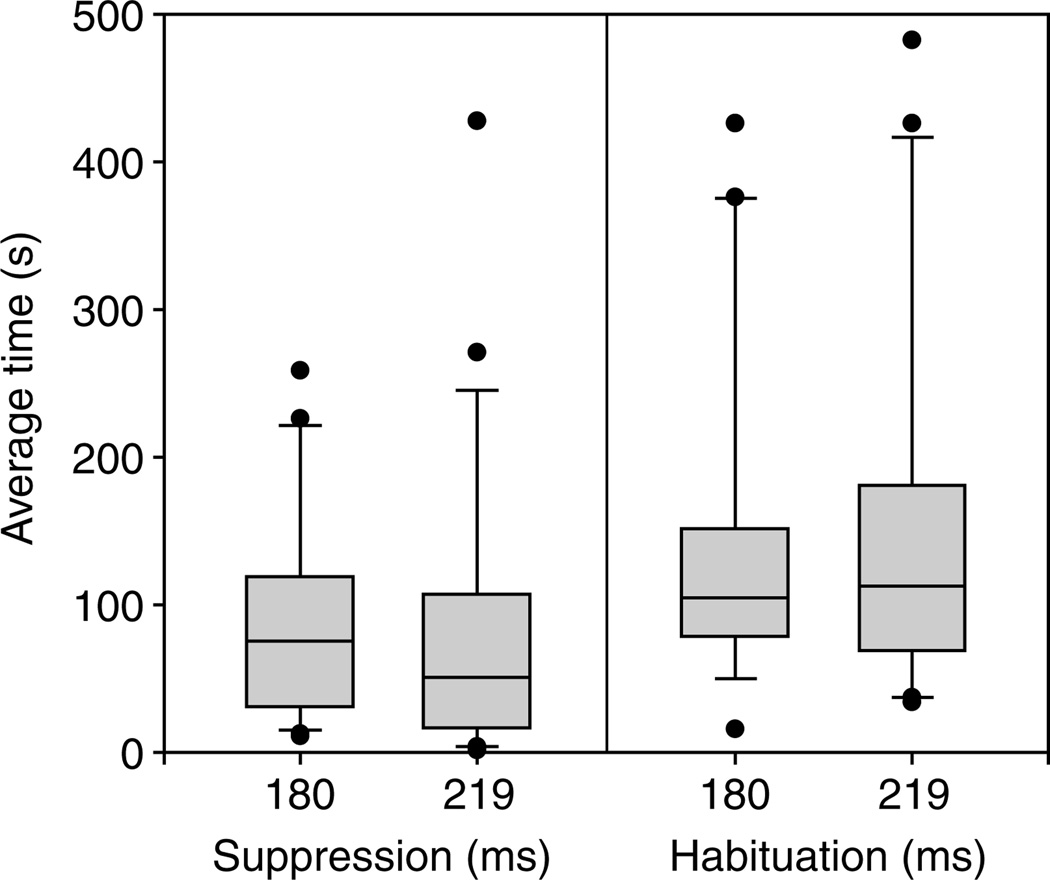
Both stimuli within the range of ticking ICIs effectively suppressed male calling (Fig. 6, left-hand panel), as measured by time to suppression or TTS (180 ms ICI: 91.3±72.0 s; 219 ms ICI: 80.5±101.5 s; N=20; RM-ANOVA rejected at P>0.05).
Fig. 6.
Time to suppression (TTS) and time to habituation (TTH) during the two stimuli 180 ms ICI and 219 ms ICI. Boxplots show median, interquartile ranges (boxes), and 10th to 90th percentile range (whiskers), with outliers (dots).
How long did suppression last (i.e. when did males habituate to the suppressive stimuli)? For both stimuli, the median time to habituation (TTH) was ~115 s (Fig. 6, right-hand panel). The median TTH was not significantly different between the two stimuli (RM-ANOVA rejected at P>0.05). An example of a male vocalization during habituation is shown in Fig. 3B.
Absence of dishabituation to different click rates of female ticking
There was no significant difference in total amount of time spent calling during the habituated bouts (Fig. 3B) whether the stimulus had changed or not, nor was there a difference in the bout lengths (all comparisons rejected at P>0.05, RM-ANOVA). Mean habituated bout lengths were 139±167 s to 180 ms ICI, 122±105 s to 219 ms ICI, 124±97 s when the stimulus was switched from 180 to 219 ms ICI, and 165±238 s when the stimulus was switched from 219 to 180 ms ICI. Neither was there a significant difference in the number of fast advertisement trills, which was similar across stimuli (median 35 trills in the control trials, and 36 trills in the test trials), or in the number of fast trills per total habituated calling (0.7 fast trills s−1 for both control and test trials). Was there any more immediate change in fast trill rates just before and after the time of stimulus change? The two IFTIs after the stimulus switch (or after a comparable time point in control trials) tended to be longer than the first two IFTIs in the habituated bout (2.2±0.8 versus 1.4±0.05 s IFTI), but there was no significant difference (P>0.05, RM-ANOVA). We also measured the IFTIs for spontaneous calling in the labeling boundary experiment. Across the first five trills in one bout of spontaneous calling the IFTI speeds up by 46 ms (903±200 ms mean and s.d. versus 857±161 ms; P<0.05). We would thus expect the IFTI to be sensitive to changes that could occur during a switch in stimuli in the dishabituation experiment. No such change was detected.
Discussion
The communicative significance of female calls is due primarily to differences in click rate. When a male is advertising, rapping stimulates whereas ticking suppresses calling (Tobias et al., 1998). For the isolated male, both ticking and rapping stimulate calling above baseline, but rapping is more effective (Vignal and Kelley, 2007), also shown in the present study. The average ICI of ticking is 219 ms but the range of ICIs during ticking bouts is broad (Fig. 1A). The average ICI of rapping is 81 ms and has a more narrow range of variation than ticking. The variability in ICIs produces ambiguous signals in the range of click rate values that overlap between rapping and ticking. We thus sought to determine, using the labeling boundary experiment, whether males identify intermediate stimuli (in the overlapping range) as rapping or ticking, using the amount of time spent calling as a measure.
Given the click rates that males clearly label as ticking, do males discriminate within the ticking range? We found that the male calling response to constant ticking suppresses. The suppression response eventually habituates, which is to say that with continued presentation of a ticking stimulus, males resume calling. We compared two ICIs within the ticking range for their effectiveness as suppressors and as habituating stimuli. Then we tested for dishabituation to determine whether males responded differentially to a change in ticking ICI.
The labeling experiment thus involved click rates in common between rapping and ticking; the dishabituation experiment used click rates males labeled as distinctly ticking. We will summarize these results and compare them to what is known about categorical partitioning of continuously varying stimuli by other species.
Labeling boundary between female calls
The fastest intermediate test stimulus, 98 ms ICI, was identified by the male as rapping, and the slowest stimulus, 160 ms ICI, was identified as ticking. The response to 120 ms ICI did not differ from either rapping or ticking responses. This result is not due to some males treating the 120 ms ICI stimulus consistently as rapping whereas others responded as though to ticking. In all other comparisons between rapping and ticking stimuli, males respond with significantly more calling in response to rapping (see also Tobias et al., 1998). Ticking may have elicited more calling than usual (and rapping less) on the nights including 120 ms ICI because prolonged exposure to an ambiguous stimulus may have reduced the archetypal quality of ticking and rapping rates in comparison.
Over the tested range from 81 to 219 ms ICI, there is no indication of a sharp boundary in the male responses to five click rates. Instead, the amount of time that the male spends calling appears to reflect the amount of overlap in the distribution of ICIs present in bouts of natural female rapping or ticking (Fig. 1B). Because 98 ms ICI is the upper limit of the standard deviation of natural rapping rates, whereas 160 ms is the lower limit of natural ticking, the male responds to 98 and 160 ms ICI accordingly. More intermediate rates should be ambiguous. In fact, when 120 ms ICI is played, the male’s response does not differ from his response to either ticking or rapping. Relatively few ICIs from either rapping or ticking contain this interval. It is a truly ambiguous signal and the male response is correspondingly ambiguous.
Suppression response and habituation to female ticking
When a male is calling, a broadcast of ticking can produce vocal suppression within 91 s. Within the ticking range, the two stimuli tested (180 and 219 ms ICIs) were equally effective in time to suppression; males do not respond preferentially to these intervals.
A vocally suppressed male will resume calling if the ticking stimulus is continued (habituation). Again, the time to habituation was equivalent for the two ICIs tested; males do not respond differentially to these click rates. When the stimulus producing habituation was exchanged for the other stimulus, the male’s response did not differ from the condition of no exchange. Thus, as far as can be determined from the male’s vocal response, there is no differential sensitivity to ICIs characteristic of ticking even though the two ICIs tested occur occasionally also in rapping (Fig. 1A).
Continuous and categorical perception
In categorical perception, differences along a continuum are perceived with greater acuity at category boundaries than within the range of one category. This perceptual phenomenon requires both (1) a sharp labeling boundary between classes and (2) a lack of discrimination between stimuli in the same class. Results with ICIs within the ticking class (including time to suppression, time to habituation and the absence of dishabituation) indicate that males do not discriminate among ticking stimuli (consistent with the second requirement for categorical perception). However, results of the labeling boundary experiments provide no evidence of a sharp boundary between rapping and ticking (failing the first requirement); males respond ambiguously at the labeling boundary between the two calls, around 120 ms ICI.
If the perceived boundary between rapping and ticking were sharp (i.e. if nearby click rates on opposite sides of some boundary rate produced significantly different responses), males might distinguish female calls more quickly and with fewer misjudgments. However, ambiguous labeling in situations of vocal ambiguity might have selective advantages. By calling to and clasping an unreceptive female, a male may, over the course of hours, induce her to become sexually receptive. The scarcity of ovipositing females on any given night during the breeding season could heighten the selective pressure on males to locate a female being clasped by another male, even when her calls are ambiguous. Perhaps accuracy could be sacrificed because refraining from advertising to a receptive female has more detrimental consequences than hazarding to pursue an unreceptive female. The energetic cost of mistakenly calling to a ticking female is presumably low, because males call in isolation for hours. Calling has the additional benefits of suppressing other males and possibly attracting another female.
Categorical perception has been described in the perception of communication and predator signals in a variety of species including crickets, birds and humans (Liberman et al., 1957; Liberman et al., 1961; Nelson and Marler, 1989; Wyttenbach et al., 1996). It is a perceptual strategy suited to situations in which identification of signals is more important than fine parameter discrimination, such as the Polynesian field cricket’s identification of conspecific mating calls and predator bat echolocation calls, which differ along a spectral continuum (Wyttenbach et al., 1996). Continuous perception, however, has been described in the discrimination of intermediate synthetic calls by female green tree frogs: a two-choice task revealed that auditory resolution is finer than necessary to distinguish between the principal modulation durations in discrete vocalizations (Gerhardt, 1978). Our results suggest that recognition of female calls by male X. laevis combines elements of both strategies: no discrimination within the ticking category and continuous discrimination across the overlapping range of rapping and ticking ICIs.
The ability of the male to discriminate different ICIs (and to categorize different intervals as belonging to the same class) must rely on the processing of temporal information within the central nervous system. Our electrophysiological recordings from auditory nerve fibers and cells in the first auditory nucleus, the dorsal medullary nucleus, reveal that all click rates within the vocal range are represented phasically, using a temporal code of synchronization to the envelope (Elliott, 2007). However, some cells in the auditory midbrain (the torus semicircularis) select for click rate, responding only to certain click rates, using an average spike rate code (Elliott, 2007). How tuning in the midbrain contributes to the differential behavioral responses of the male to female calls is a neuroethological question whose exploration can now be related to a more complete understanding of the male’s perception of behaviorally relevant temporal cues.
Acknowledgments
We are grateful to Claes U. J. Hansen for performing a blinded analysis of the IFTIs in habituated calling bouts. We thank Martha Tobias for help in designing and commenting on experiments and providing the female call recordings, and Ryan Blum for assistance in creating the Max/MSP playback patch. We would also like to thank Candace Barnard, Ulrik Nørum, Sonja Rakowski, Melissa Williams and Erik Zornik for practical assistance and helpful comments. Research was supported in part by a National Science Foundation graduate fellowship and by a National Institute of Deafness and Communication Disorders NRSA graduate fellowship, as well as National Institute of Health grant NS23684 to D.B.K.. Animal care and use for this study was approved by the Institutional Animal Care and Use Committee at Columbia University (AC-AAAA1586).
References
- Braaten RF, Hulse SH. Perceptual organization of auditory temporal patterns in European starlings (Sturnus vulgaris) Percept. Psychophys. 1993;54:567–578. doi: 10.3758/bf03211781. [DOI] [PubMed] [Google Scholar]
- Elliott TM. Doctor of Philosophy. New York: Columbia University; 2007. The neural basis of click rate coding in the auditory system. Executive Committee of the Graduate School of Arts and Sciences; p. 145. [Google Scholar]
- Gerhardt HC. Discrimination of intermediate sounds in a synthetic call continuum by female green tree frogs. Science. 1978;199:1089–1091. doi: 10.1126/science.628833. [DOI] [PubMed] [Google Scholar]
- Gleason JB, Ratner NB. Psycholinguistics. Orlando, FL: Harcourt Brace; 1998. [Google Scholar]
- Liberman AM, Harris KS, Hoffman HS, Griffith BC. The discrimination of speech sounds within and across phoneme boundaries. J. Exp. Psychol. 1957;54:358–368. doi: 10.1037/h0044417. [DOI] [PubMed] [Google Scholar]
- Liberman AM, Harris KS, Kinney JA, Lane H. The discrimination of relative onset-time of the components of certain speech and nonspeech patterns. J. Exp. Psychol. 1961;61:379–388. doi: 10.1037/h0049038. [DOI] [PubMed] [Google Scholar]
- Nelson DA, Marler P. Categorical perception of a natural stimulus continuum: birdsong. Science. 1989;244:976–978. doi: 10.1126/science.2727689. [DOI] [PubMed] [Google Scholar]
- Palmer C, Krumhansl CL. Mental representations for musical meter. J. Exp. Psychol. Hum. Percept. Perform. 1990;16:728–741. doi: 10.1037//0096-1523.16.4.728. [DOI] [PubMed] [Google Scholar]
- Tobias ML, Kelley DB. Vocalizations by a sexually dimorphic isolated larynx: peripheral constraints on behavioral expression. J. Neurosci. 1987;7:3191–3197. doi: 10.1523/JNEUROSCI.07-10-03191.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobias ML, Viswanathan SS, Kelley DB. Rapping, a female receptive call, initiates male-female duets in the South African clawed frog. Proc. Natl. Acad. Sci. USA. 1998;95:1870–1875. doi: 10.1073/pnas.95.4.1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobias ML, Barnard C, O’Hagan R, Horng SH, Rand M, Kelley DB. Vocal communication between male Xenopus laevis. Anim. Behav. 2004;67:353–365. doi: 10.1016/j.anbehav.2003.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vignal C, Kelley DB. Significance of temporal and spectral acoustic cues for sexual recognition in Xenopus laevis. Proc. R. Soc. Lond. B Biol. Sci. 2007;274:479–488. doi: 10.1098/rspb.2006.3744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetzel DM, Kelley DB. Androgen and gonadotropin effects on male mate calls in South African clawed frogs, Xenopus laevis. Horm. Behav. 1983;17:388–404. doi: 10.1016/0018-506x(83)90048-x. [DOI] [PubMed] [Google Scholar]
- Wyttenbach RA, May ML, Hoy RR. Categorical perception of sound frequency by crickets. Science. 1996;273:1542–1544. doi: 10.1126/science.273.5281.1542. [DOI] [PubMed] [Google Scholar]