Summary
The number of muscle fibers in the vocal organ of the adult male African clawed frog, Xenopus laevis, exceeds that of adult females. This sex difference is the result of rapid fiber addition in males between the end of metamorphosis, post-metamorphic stage 0 (PM0) and PM2. At PM0, male and female frogs have similar numbers of laryngeal muscle fibers. Males then add more muscle fibers than females and achieve an adult value that is 1.7 times the female number. Males castrated at PM0 have the same fiber number as females. Ovariectomy at PM0 does not alter muscle fiber addition in females. Gonadectomy at PM2 has no effect on fiber addition in either sex. Females attain masculine muscle fiber number if their ovaries are replaced with a testis at metamorphosis. Exogenous testosterone treatment at PM0 significantly increases fiber number in females but not in males. Exogenous testosterone given at PM2 has no effect on fiber number in females but decreases fiber number in males. We conclude that the testes are necessary for the marked addition of laryngeal muscle fibers seen in male X. laevis between PM0 and PM2. The masculine pattern of muscle fiber addition can be induced in females provided with a testis. Androgen secretion from the testes most probably accounts for masculinization of laryngeal muscle fiber number. After PM2, androgens are no longer necessary for muscle fiber addition and cannot increase fiber number in females.
Keywords: myogenesis, androgen, sexual differentiation
Introduction
Sex differences in the dilator laryngis muscles of Xenopus laevis contribute to the expression of vocal behaviors important for reproductive success (Kelley and Tobias, 1989). These dimorphisms include differences in laryngeal muscle fiber twitch type (Sassoon et al. 1987), muscle fiber physiology (Tobias and Kelley, 1988), dye coupling of muscle fibers (Tobias and Kelley, 1988), and gross morphology of both laryngeal muscle and cartilage (Ridewood, 1898; Patterson, 1939; Sassoon and Kelley, 1986). The adult male larynx has more and larger muscle fibers than the adult female larynx (Sassoon and Kelley, 1986). A greater number of laryngeal muscle fibers may contribute to the male’s ability to produce a loud and prolonged attraction call.
The sexually dimorphic fiber numbers present in adult larynges are established during post-metamorphic (PM) development (Sassoon and Kelley, 1986). At the end of metamorphosis, male and female frogs have the same number of muscle fibers. By adulthood, laryngeal muscle fiber number is higher in males than in females. Previous experiments suggest that sex differences in muscle fiber number result from greater androgen secretion in the male. Specifically, administration of exogenous androgen to post-metamorphic frogs induces laryngeal myoblast proliferation (Sassoon et al. 1986). Treatment with the anti-androgen flutamide during the first three months after metamorphosis prevents expected laryngeal muscle fiber addition in males (Sassoon and Kelley, 1986).
Prior studies also suggest that the ability of laryngeal cells to proliferate in response to testosterone decreases with age (Sassoon and Kelley, 1986). In these experiments, developing male and female frogs received testosterone implants and incorporation of tritiated thymidine into DNA from whole larynges was measured. Because this study (Sassoon and Kelley, 1986) used the entire larynx, and because testosterone induces chondrogenesis as well as myogenesis (Sassoon et al. 1986), we could not determine to what extent testosterone stimulated cell addition in muscle specifically. We undertook the present study to answer this question and to determine whether there is a specific hormone-sensitive period during which the presence of gonadal androgen is required for addition of muscle fibers in males. If so, this period could provide a window of opportunity for masculinization of muscle fiber number in females.
Three sets of experiments were carried out. First, laryngeal muscle fiber number was counted in both males and females throughout post-metamorphic development to determine when fiber addition diverged in the sexes. Next, we gonadectomized males and females and implanted females with testes in order to determine to what extent gonadal hormones are needed for muscle fiber development. Finally, we administered exogenous testosterone to intact males and females at two key developmental stages (PM0 and PM2) to determine whether testosterone can mimic the masculinizing effect of the testes and, if so, when during development this hormone is effective.
Materials and methods
Animals
Male and female post-metamorphic frogs were obtained from Nasco (Fort Atkinson, WI). Because maturation rates in Xenopus laevis are strongly affected by rearing conditions (Nieuwkoop and Faber, 1956), we have developed a staging scheme in which post-metamorphic growth is assigned to 7 stages characterized by the attainment of key developmental landmarks in the masculinization of the larynx (Tobias et al. 1990). The end of metamorphosis (tadpole stage 66 of Nieuwkoop and Faber, 1956) corresponds to stage PM0 and adulthood (males≈30g body weight; females≈90g body weight) to stage PM6. From PM0 to PM3, females were matched by age and body weight (bw) to males. After PM3, when body weight increases more rapidly in females, females were age matched to males. The body and larynx weights (at killing) of animals used in these studies are given in Table 1. Frogs were maintained on a 12:12 light:dark cycle in 0.4% saline in polycarbonate tanks at 20 °C and fed beef liver or frog brittle (Nasco) daily.
Table 1.
Average body and larynx weights for control and experimental frogs
| Males |
Females |
||||
|---|---|---|---|---|---|
| Group | Body weight (g) |
Larynx weight (mg) |
Group | Body weight (g) |
Larynx weight (mg) |
| 0 | 1.1±0.6 | 4.8±2.2 | 0 | 0.9±0.2 | 6.2±1.2 |
| 1 | 3.3±0.5 | 18.0±2.2 | 1 | 3.9±0.8 | 15.2±1.2 |
| 2 | 5.9±0.6 | 57.2±8.8 | 2 | 5.4 | 20.0±5.6 |
| 3 | 9.8±0.9 | 92.5±22.3 | 3 | 9.0±0.7 | 32.8±6.2 |
| 6 | 31.0±9.2 | 396.8±12.5 | 6 | 97.5 | 240.8±20.5 |
*0/2: 
|
7.4±2.0 | 29.2±8.1 | 0/2: 
|
4.9±0.3 | 19.0±2.9 |
0/2:  +FL +FL |
4.8±0.4 | 18.0±2.7 | 0/2:  + testis + testis |
6.6±0.8 | 112.7±35.4 |
2/6: 
|
22.8 | 117.5±52.5 | |||
| 0+ (control) | 1.6 | 5.8±1.4 | |||
| 0+ (control) | 1.9±0.5 | 15.3±12.7 | 2+ (control) | 7.2±1.3 | 20.3±2.3 |
| 2+ (control) | 6.6±0.5 | 42.2±7.1 | 0/0+: +TP | 2.8±0.9 | 107.8±16.7 |
| 0/0+: +TP | 2.7±1.5 | 129.8±16.1 | 2/2+: +TP | 6.5±0.4 | 173.8±4.8 |
| 2/2+: +TP | 6.6±0.6 | 236.0±20.3 | |||
0/2:  +testis +testis |
8.2 | 137.0 | |||
Values are given as means±S.D.
Single numbers indicate that body weight was only available for one animal within the group.
Slashes represent stage treatment begun/stage fibers counted. FL=flutamide. TP=testosterone propionate 0+ and 2+ represent animals grown for five weeks starting from PM0 and PM2, respectively.  =castrated male:
=castrated male:  =ovariectomized female.
=ovariectomized female.
Surgical procedures
At PM0, frogs were gonadectomized (castrated or ovariectomized) under anaesthesia induced by cold narcosis. A small incision was made in the abdominal wall just lateral to the ventral midline. The intestines and fat bodies were then extruded from the abdominal cavity to expose the ovaries or testes lying ventral to the interrenal glands. The posterior end of each gonad was gently lifted away from the interrenal glands and then excised along with its attached fat body. Intestines were replaced and muscle and skin were sutured shut (Supramid extra, 9-0 monofilament sutures; S. Jackson, Inc.). Juvenile (PM2) frogs were gonadectomized in a similar fashion except that anaesthesia was accomplished by immersion in 0.1% MS 222 (ethyl m-amino benzoate methane sulfonic acid, Aldrich Chemical Co.) and gonads with attached fat bodies were cauterized prior to excision.
For testicular implantation, a PM0 female was ovariectomized as described above except that one fat body was retained. The testis to be implanted had been removed from a PM0 male (testes can be maintained in cold 50 % Puck’s saline G, Gibco Co., for up to eight hours). The testis was placed in the ovariectomized female between the interrenal gland and the fat body (where the PM0 ovary is normally found) before the intestines were replaced. In addition to the five females with testis implants, one metamorphic male was castrated and received a testis implant.
The following criteria (which correlate reliably with sex assignment from stained, sectioned gonadal tissue; Witschi, 1971) served to distinguish testes from ovaries at the time of gonadectomy. Testes are more rigid and compact than ovaries and are not involuted. Testes do not extend as far posteriorly as ovaries (the posterior portion of the ovary is often obscured ventrally by the bladder). Finally, testes are usually more vascularized than ovaries (this criterion is not as reliable as the others). Gonadectomies were judged to be successful at the time of autopsy (PM2) if no gonadal tissue was evident after inspection under a dissecting microscope. Testis implants were judged to be successful if the testis was well vascularized and enveloped in the remaining fat body. The original sex of the animal was reconfirmed at the time of sacrifice by visual inspection of remaining oviducts or sperm ducts (oviducts are larger and more convoluted than sperm ducts at PM2).
In some experiments with castrated males, any remaining endogenous androgen was blocked using the anti-androgen, flutamide (kindly supplied by Schering Co.). Powdered flutamide (FL) was mixed with a silastic polymer (Dow Corning no. 738) and then extruded through a 1ml hypodermic syringe. Lengths of silastic containing 0.5mg flutamide/g body weight were implanted into the dorsal lymph sac of each frog. This dose of flutamide prevents muscle fiber addition in juvenile males (Sassoon and Kelley, 1986). Implants were replaced at PM1 to ensure a continual supply of flutamide over time. In other experiments, intact frogs received exogenous testosterone. Testosterone-treated frogs received compressed pellets of testosterone propionate (TP) 3 mm in diameter implanted into the dorsal lymph sac (2–3 mg at PM0 and 5–6 mg at PM2).
Histology
Animals were deeply anaesthetized by immersion in 0.1% MS 222. The frog was weighed and the larynx removed, cleaned, weighed, and placed immediately in fresh paraformaldehyde (4% in 0.1m phosphate buffer). Larynges were fixed for at least 12 h, rinsed in 0.2 m sodium cacodylate buffer, post-fixed in 1 % osmium tetroxide in cacodylate buffer (1–3 h) and embedded in epoxy resin (46% LX-112, 31% DDSA, 23% NMA, 1.6% DMP-30; Ladd). Sections 5–7 µm thick were cut using a rotary microtome (Spencer 820; AO) in a plane transverse to the longitudinal axis of the muscle fibers. As the region of the muscle from which counts were to be obtained was reached (Fig. 1, cross sections, rectangle), the angle of the block was carefully adjusted to ensure that sections were perpendicular to the longitudinal axes of the fibers for both inner and outer leaves of the bipennate muscle.
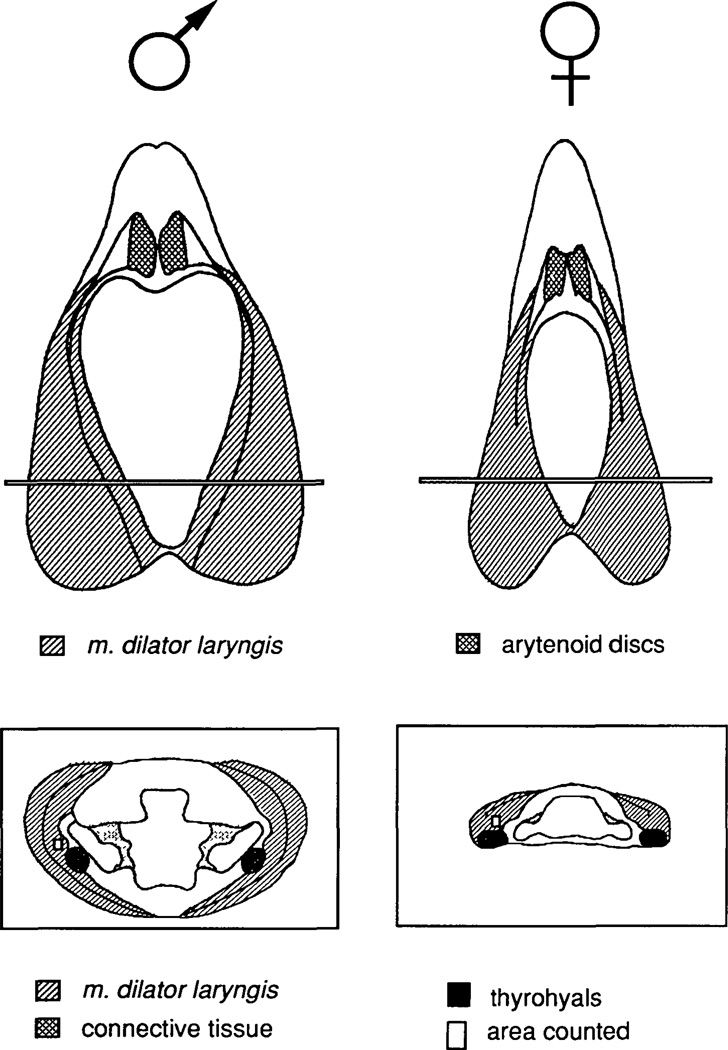
Fig. 1.
Dorsal views (top) and cross sections (bottom) of adult male and female larynges for a typical male and female larynx. For the dorsal views, anterior is up. For the cross sections, dorsal is up. Cross-sections are taken from the anterior-posterior level indicated in the laryngeal dorsal views. Fibers were counted from an area (small rectangle in cross sections) within the inner bipennate muscle just dorsal and lateral to the thyrohyal cartilage. The characteristic shape of the lumen of the larynx at this anterior-posterior level is illustrated in the cross sections.
Fiber counting
The Xenopus larynx consists of right and left bipennate muscles wrapped around a cartilaginous skeleton (Fig. 1). Since there is no difference in number of muscle fibers from the right and left halves of the larynx (Sassoon and Kelley, 1986), muscle fibers were counted on either side. From each larynx, three sections with the greatest muscle area were identified. In general, this region is located about 1/3 of the total laryngeal length from the posterior pole of the larynx and can be recognized by the shape of the cartilage lumen (illustrated in Fig. 1). However, because stage and hormone treatment can affect the correlation between greatest muscle area and lumen shape, many sections from each larynx were individually scanned to find the section with the largest muscle area. Three sections, all within 200 µm of each other along the anterior-posterior axis of the larynx and within the region of greatest muscle area, were chosen. A rectangular region, 8825 µm2 in area, of the inner leaf of the bipennate muscle just dorsal and slightly lateral to the thyrohyals (rods of cartilage that extend anterior to posterior within the larynx: Fig. 1) was chosen and muscle fibers within the region were counted using a camera lucida. Total muscle area for the section was determined by tracing the magnified section and measuring the area on a computer interfaced bit pad (Sigma Scan, Jandel Scientific). Fiber number for each section was calculated by multiplying the number of fibers per µm2 by the total muscle area of the section. Counts from the three sections were then averaged to give a mean muscle fiber number for that larynx. Muscle fiber numbers from different larynges of the same group were averaged to provide the group mean.
Fiber number was determined at 400× magnification and probably includes counts of both myotubes and myofibers since electron microscopy is required to distinguish between the two (Sassoon et al. 1986). Laryngeal myoblasts were not included in counts because they cannot be distinguished at the light microscope level (Sassoon et al. 1986). Because laryngeal muscle fibers are arranged as diagonal bands with insertion points that do not span the entire anterior-posterior length of the larynx, our muscle fiber counts do not represent total absolute muscle fiber number in the larynx. However, based on measurements of protein and DNA content in developing and adult laryngeal muscle (Sassoon and Kelley, 1986; Segil et al. 1987; Kelley et al. 1989) fiber counts are probably proportional to total fiber number.
The present muscle fiber counts differ somewhat from our previous study (Sassoon and Kelley, 1986) due to methodological differences. In the previous study, sections to be counted were chosen by lumen shape only. This criterion was not used in the present study because it varies with age, sex and hormone treatment. This study used lumen shape in combination with muscle area measurements, as described above. In the previous study, fibers were counted from 10 randomly chosen, small areas (150 µm2) of the bipennate muscles. This procedure resulted in greater variability between larynx counts than was obtained using a larger region and a consistent location across all larynges. Finally, in this study, fibers were examined at a higher magnification than that used previously (400×rather than at 250×) to ensure that no small fibers were missed. In our previous study, we missed some small muscle fibers (particularly in females) that cannot be distinguished at 250×. The smallest muscle fibers present in PM0 animals are 2 µm in diameter (Kelley and Dennison, 1990). Even these smallest muscle fibers could be easily distinguished at 400× under the light microscope.
Statistical analysis
For each group, the mean and standard deviation of the number of muscle fibers were calculated. The n for each group was four except testis implanted females (n=5) and one testis implanted male. Fiber number differences were analyzed using a two-way ANOVA (Model 1) which revealed significant differences by age and sex. Statistically different groups were distinguished using post-hoc Student t-tests. We made a strong prediction that if normal male and female fiber numbers differed at any stage, males would have more fibers than females. As a result, we chose a one-tailed t-test with a significance level of P≤0.05 for comparisons between males and females throughout normal development. Two-tailed t-tests with a significance level of P≤0.05 were used for all other comparisons.
Results
Normal development
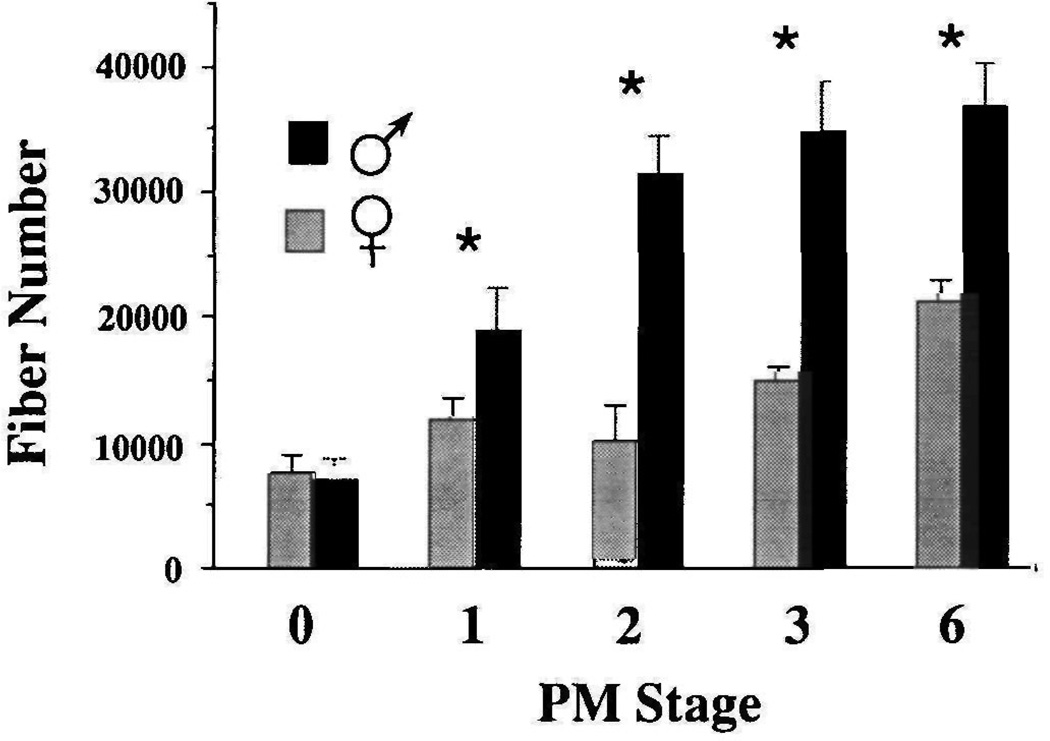
Laryngeal muscle fiber number in Xenopus laevis was determined for males and females throughout postmetamorphic development in order to determine when sex differences first appear (Fig. 2). At PM0, muscle fiber number does not differ in males and females (males=6969±1671, females=7589±1526; P>0.05). From PM1 on, laryngeal muscle fiber number is significantly larger in males than in females (PM1 male= 18896±3423, PM1 female=11814±1743; P≤0.01). Males continue to add fibers until PM3, after which fiber number does not differ significantly from adult values (PM3=34795±4073, PM6=36742±3435; P> 0.05). In females, fiber number increases gradually throughout post-metamorphic development and consistently exceeds PM0 values from PM3 on (PM0= 7589±1525, PM3=15 106±1102; P≤0.0005). At PM6, males have 1.7× as many fibers as females (PM6 males=36742±3435, PM6 females=21178±1634; P≤ 0.0005). Fiber addition continues in both sexes throughout post-metamorphic development.
Fig. 2.
Laryngeal muscle fiber numbers during PM development in males and females. Values represent the mean and standard deviation of muscle fiber number for each sex at each stage. Fiber number from four larynges were averaged to obtain each value. Asterisks indicate stages at which male and female values are significantly different (P≤0.05).
Sex differences in laryngeal muscle fiber number reflect differences in the rate of fiber addition during post-metamorphic development. In males, there is an early phase (between PM0 and PM2) during which fiber addition is rapid. After PM2, the rate of addition decreases. In females, the rate of fiber addition is less than that of males between PM0 and PM2 and roughly equivalent thereafter, when the rate of fiber addition in males has slowed. The greater number of muscle fibers in adult males is due to the first stage of rapid fiber addition between PM0 and PM2.
Gonadal manipulations
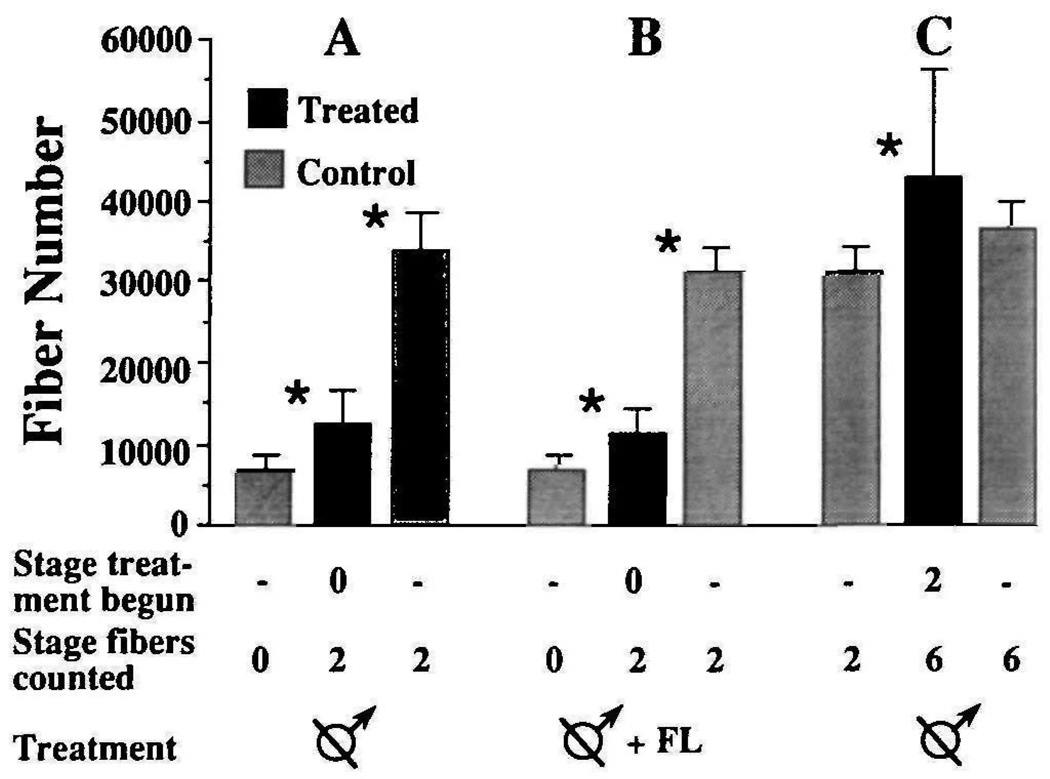
Is either stage of fiber addition in males dependent on gonadal hormones? To answer this question, we investigated the effect of castration on fiber number. Males were castrated at PM0 and laryngeal muscle fibers were counted at PM2 (Fig. 3A). Fiber number in castrated males (12555±4082) is significantly greater (P≤0.025) than in PM0 males (6969±1671), but significantly less (P≤0.0005) than in intact PM2 males (31439±3057). Thus, muscle fiber addition is severely reduced, but not arrested, by castration at PM0. To determine whether circulating androgens of non-testicular origin contributed to the observed increase in fiber number in gonadectomized males, males were castrated at PM0 and treated with the anti-androgen flutamide from PM0 until PM2 when fibers were counted (Fig. 3B). Flutamide-treated castrated males had the same number of laryngeal muscle fibers (11462±2733) as males that were only castrated (P>0.05). Fiber number in both groups of castrated males was comparable (P>0.05) to that of untreated females at the same stage (10175±2913). An additional four males were castrated at PM2 and laryngeal muscle fibers were counted at PM6 (Fig. 3C). Fiber number was not affected by castration at PM2 (PM6 castrates=43054±13174, PM6 controls=36742±3435; P>0.05). These results suggest that intact males experience two types of laryngeal muscle fiber addition. A rapid, testes-dependent rate of fiber addition occurs from PM0 to PM2. Castration unmasks a slow, testes-independent addition that occurs throughout postmetamorphic development.
Fig. 3.
Effect of castration on male laryngeal muscle fiber number. In each experiment (A,B,C), values obtained from gonadectomized males (middle bar) are compared to two controls: intact males at the stage that experimental males were castrated (bar at far left) and intact males at the stage that fibers in experimental males were counted (bar at far right). (A) Males were castrated at PM0 and fibers were counted at PM2. (B) Males were castrated and flutamide treated at PM0 and fibers were counted at PM2. (C) Males were castrated at PM2 and fibers were counted at PM6. Asterisks indicate significant differences (P≤0.05) between adjacent groups.
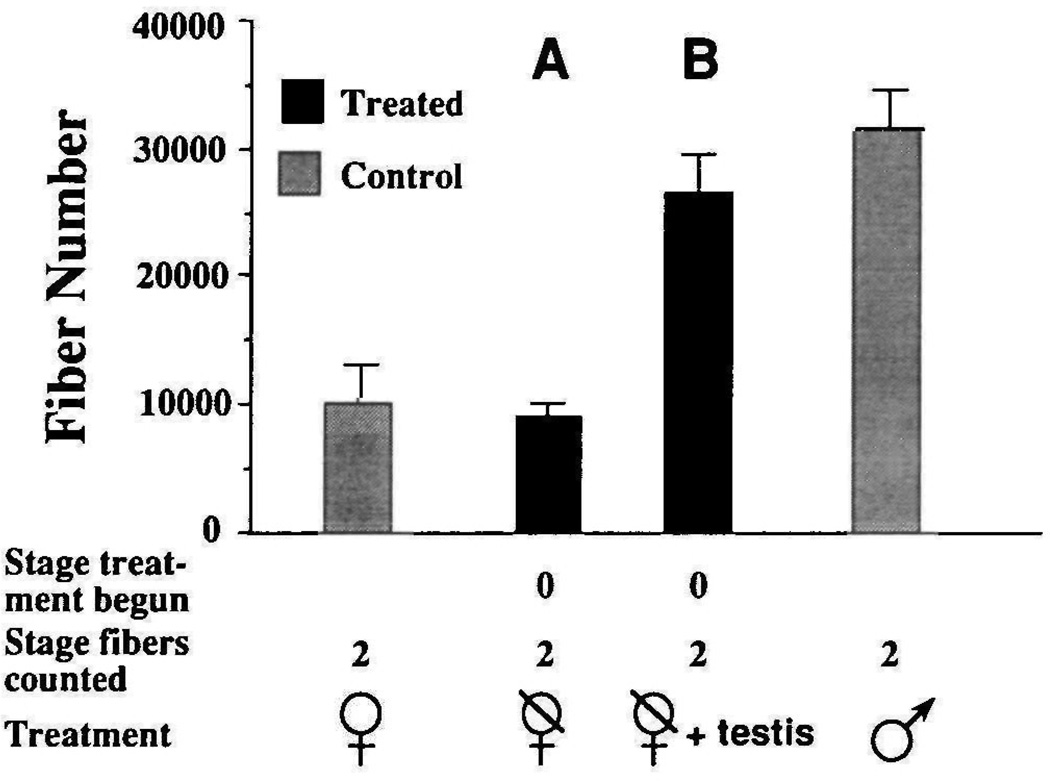
These data indicate that males must have testes to develop normal laryngeal muscle fiber number. Are gonads also required for the development of normal fiber number in females? Four females were ovariectomized at PM0 and reared to PM2, when fibers were counted (Fig. 4A). Fiber number was not significantly different from that of intact females (ovariectomized females=9068±1103, intact females=10175±2913; P>0.05). Thus the ovaries are not necessary for the normal development of female laryngeal fiber number between PM0 and PM2.
Fig. 4.
Effect of testicular implants on female laryngeal muscle fiber number. Fiber numbers are compared to normal PM values for males and females. (A) Ovariectomy at PM0 has no significant effect on female fiber number assessed at PM2 (P>0.05). (B) Testis-implanted females (n=3) have significantly more (P≤0.05) fibers than control females and significantly fewer (P≤0.05) fibers than control males.
In order to determine whether female laryngeal muscle fiber number could be increased by transplanted male gonads, five females were ovariectomized at PM0 and immediately received a testis from a stage matched male. Fiber number in testis-implanted females (Fig. 4B) was determined at PM2 (26427±3050; n=3) or PM3 (26818±1081; n=2). Females with testis implants had significantly more muscle fibers than control females (untreated PM2=10175±2913; P≤0.0005), and even more than adult females (untreated PM6=21178±1634; P≤0.05). However, fiber number in testis-implanted females does not attain values reached by males of a comparable developmental stage (PM2 males=31353±3524; P≤0.05). One possibility is that females could reach these levels if exposed to androgen for a longer period. This possibility is unlikely because fiber number in PM3 testis-implanted females was not any greater than that in PM2 testis-implanted females (P>0.05). It is also possible that two testes are needed for a frog to attain normal male laryngeal muscle fiber number. However, the control male implanted with one donor testis did achieve as many fibers (36530) as intact males with two testes. These results suggest that the testes are necessary and that one testis is sufficient for the expression of masculine laryngeal muscle fiber number between PM0 and PM2.
Exogenous androgen
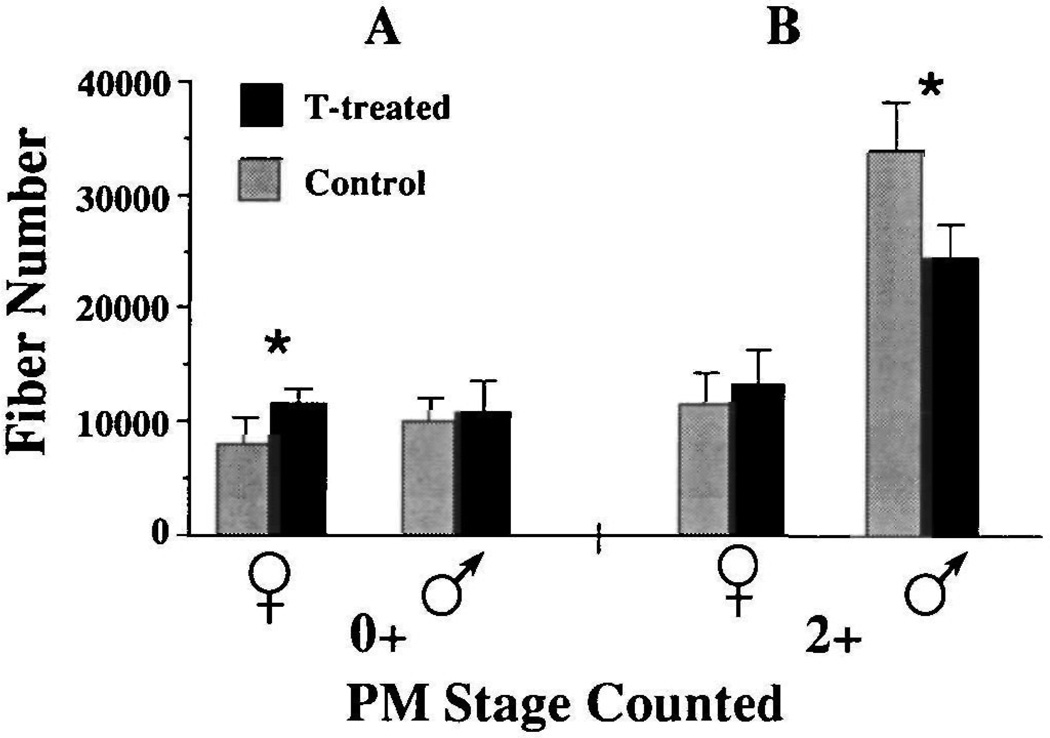
Our previous studies had implicated the secretion of testosterone in development of muscle fiber number in males (Sassoon and Kelley, 1986). We thus attempted to mimic testosterone secretion from the gonad by supplying exogenous testosterone to gonadally intact, developing females. Females treated for five weeks beginning at PM0 (Fig. 5A) had significantly more laryngeal muscle fibers than control females (treated females=11055±1233, controls=7839±2116; P≤0.025) and were not significantly different from control males (10139±1986; P>0.05). Fiber number in females was not affected by five weeks of testosterone treatment at PM2 (Fig. 5B). These results suggest that testosterone is an active agent in masculinizing laryngeal muscle fiber number and that the hormone must be present before PM2 to be effective.
Fig. 5.
Changes in laryngeal muscle fiber number in both males and females after treatment with exogenous testosterone (TP-treatment). Animals were treated for five week starting at PM0 (A) or at PM2 (B). Experimental and control animals whose fibers were counted after these five weeks are represented as PM0+ and PM2+, respectively. Note that the effects of TP-treatment depend on both age and sex of the animal. Females have significantly more fibers than control females when treated with TP at PM0 (P≤0.05) but there is no effect at PM2. Conversely, there is no effect in males treated with TP at PM0 while fiber number actually decreases in males treated at PM2 (P≤0.05).
As a control for the above experiment, we exposed gonadally intact males to exogenous testosterone during these stages. Five weeks of treatment at PM0 did not affect muscle fiber number in males (Fig. 5A). We interpret these results to suggest that the processes underlying fiber addition were already saturated by endogenous androgen. A surprising result was that testosterone treatment at PM2 (Fig. 5B) decreased fiber number compared to control males (treated= 24383±2977, controls=33855±4497; P≤0.01). We did not observe any cytological evidence consistent with androgen-induced muscle fiber death. A possible explanation for this finding is that high levels of exogenous androgen induced myotubes to fuse with pre-existing muscle fibers or with each other (effectively decreasing muscle fiber number) rather than inducing myoblast proliferation and fusion (which would have produced more muscle fibers).
Discussion
Male and female Xenopus laevis begin post-metamorphic development with the same number of laryngeal muscle fibers. While both sexes acquire more fibers throughout post-metamorphic development, males add more fibers more quickly than do females. The sex difference in fiber addition is established early in postmetamorphic development, by PM1. At adulthood, males have 1.7× as many fibers as females.
Two patterns of muscle fiber addition characterize early post-metamorphic development: rapid, testis-dependent addition in males and slow, gonad-independent addition in both sexes. Males gonadectomized at PM0 continue to add new muscle fibers but fiber addition is severely reduced compared to intact males. Muscle fiber addition in females does not depend on the presence of the gonads. Fiber addition in intact and ovariectomized females is similar to fiber addition in castrated males. In this respect, myogenesis in the larynx conforms to the classical vertebrate pattern of sexual differentiation in which the underlying developmental program (responsible for producing the feminine or ‘default’ phenotype) is present in both sexes but diverges in males under the influence of testicular secretions. Further support for this scheme comes from results of experiments in which females were implanted with a testis at PM0. Fiber addition in testis-implanted females reproduces the male typical pattern suggesting that, at PM0, the potential for testis-dependent fiber addition is present in females as well as in males.
The effect of testes on muscle fiber addition is probably due to secretion of an androgen: testosterone or one of its metabolites. The sex differences in muscle fiber addition described here parallel sex differences in blood androgen levels (Lambdin and Kelley, 1986). Both sexes have extremely low but equivalent androgen titers at PM0. Androgen levels in males then rise rapidly. Androgen titers in developing females increase slowly until adulthood. We have shown previously that the anti-androgen flutamide blocks muscle fiber addition in males if given early in post-metamorphic life (Sassoon and Kelley, 1986). We show here that flutamide does not affect muscle fiber addition in castrated males and conclude that the residual fiber addition seen in castrates does not depend on androgens of non-testicular origin. Taken together, these results suggest that rising levels of androgen in postmetamorphic males are responsible for testis-dependent increases in muscle fiber addition during this period. If so, we should be able to mimic the effect of an implanted testis in females by supplying androgen. Gonadally intact females treated with exogenous testosterone at PM0 do have more muscle fibers than control females. Treating intact males with additional androgen at this stage does not further augment fiber number suggesting that endogenous androgen saturates the underlying process of fiber addition.
Androgen-dependent muscle fiber addition operates under temporal constraints. In males, androgen deprivation at PM0 reduces muscle fiber addition but has no effect at PM2. Once attained, male fiber number is maintained even in the absence of androgens. There is no decrease in muscle fiber number for up to one year after castration at PM2. Females also lose the ability to add laryngeal muscle fibers in response to androgen sometime between PM0 and PM2. Testosterone treatment of females at PM0 increases fiber number over control values but has no effect at PM2. Thus, muscle fiber addition in the larynx has a ‘sensitive period’ similar to that described for other sexually differentiating systems in which exposure to androgen during a limited temporal window has permanent masculinizing effects (Kelley, 1988). We do not know when this temporal window opens. The failure of post-metamorphic androgen secretion to completely masculinize muscle fiber number in females suggests that the sensitive period may begin during tadpole life. Since androgen fails to increase muscle fiber number at PM2 in either sex, we know that this temporal window is closed by PM2.
The sexually dimorphic development of laryngeal muscle in X. laevis can be compared to another highly androgen-sensitive muscle, the levator ani/bulbocavernosus muscle (LA/BC) of rodents. A series of studies conducted in the 1960s concluded that the LA/BC muscle is present in both sexes at birth but disappears in females (due to death of existing muscle fibers) unless exogenous androgen is supplied (Venable, 1966; Gutmann et al. 1967; Cihak et al. 1970). If so, development of the LA/BC muscle would differ from the usual pattern of vertebrate sexual differentiation in that the masculine phenotype would be present in both sexes initially and feminization attributable to loss of masculine characteristics. However, a recent reinvestigation of this system has disputed this conclusion and produced results quite similar to our findings in the X. laevis larynx. Tobin and Joubert (1988) have demonstrated that the LA/BC muscle is present in adult females; fiber number is about 1/10 that of males. When testosterone is administered to females during the neonatal period, fiber number in the LA/BC muscle is increased (Tobin and Joubert, 1986). In adult (two month old) females, however, androgen increases myoblast (satellite cell) number without a concommitant increase in muscle fiber number (Joubert and Tobin, 1989). Thus, data from rodents and frogs suggest that sexual differentiation of androgen-sensitive muscle follows the pattern established for other somatic tissues: the default phenotype is feminine and the potential for masculinization in response to androgen secretion is initially present in both sexes but lost in females unless androgen is supplied.
What cellular processes account for rapid, androgen-dependent and slow, androgen-independent rates of fiber addition in X. laevis laryngeal muscle? In vertebrates, skeletal muscle fibers are believed to arise from myoblasts, which proliferate and then withdraw from the cell cycle to become myocytes. Myocytes fuse with each other to form terminally differentiated, multinucleated muscle fibers (Stockdale, 1982). In the male X. laevis larynx, the end of the sensitive period for androgenic control of muscle fiber number coincides with a developmental stage when fiber number is essentially complete (PM2) suggesting that androgen influences muscle fiber number by acting on a population of androgen-sensitive myoblasts. Once available myoblasts have proliferated, differentiated into myocytes and fused to form myofibers, androgen can no longer increase muscle fiber number.
The patterns of laryngeal muscle fiber addition seen in this study could be accounted for by the presence of androgen-dependent and androgen-independent myoblasts (Kelley et al. 1989). Both kinds of myoblasts would be present at PM0 in both sexes. In response to rising titers of androgen, androgen-dependent laryngeal myoblasts in males would be maintained, induced to proliferate and differentiate into myocytes, and finally to fuse and form myotubes. In females, levels of circulating androgen would not be sufficient to maintain or stimulate these myoblasts. The androgen-dependent rate would normally be confined to males but could be evoked in females by providing a source of androgen (a testis or exogenous hormone) while androgen-dependent myoblasts are still present at PM0. After females lose these cells, androgen would no longer be able to increase muscle fiber number. In this scenario, both sexes would lose the ability to respond to exogenous androgen with muscle fiber addition by PM2 but for different reasons. In females, androgen would no longer be effective because the responsive population of myoblasts had been depleted by death or, possibly, conversion to another cell type. In males, the responsive population of myoblasts would be depleted by differentiation into non-proliferating myocytes or myofibers.
In order for androgens to act, responsive cells must express specific intracellular receptor proteins (Wilson et al. 1981). The androgen receptor belongs to a family of steroid-binding proteins which can act as transcription factors for activating specific genes (Yamamoto, 1985; Evans, 1988; Chang et al. 1988; Lubahn et al. 1988). The ability of myoblasts to respond to androgen could be controlled by androgen receptor expression. The PM0 larynx demonstrates extremely high levels of androgen binding in both sexes (Kelley et al. 1989) attributable to the presence of androgen receptor (He et al. 1990). After PM0, the level of androgen binding declines as does the ability of androgen to evoke laryngeal cell proliferation (Sassoon and Kelley, 1986). However, we do not know if androgen receptor is expressed in myoblasts, as opposed to differentiated muscle cells.
Androgen may have different effects on developing muscle depending on the amount of androgen given and the state of differentiation of the muscle. Early in development, when androgen titers are low and myoblasts are abundant, androgens promote cell proliferation. Later in development when androgen titers are high and myoblast proliferation is nearly complete, androgens may promote cell differentiation (eg. myocyte fusion). In the LA/BC of adult female rats, androgen does increase the number of myonuclei apparently by promoting the proliferation of satellite cells which subsequently fuse with existing muscle fibers (Joubert and Tobin, 1989). In our experiments PM2 males exposed to supraphysiological levels of androgen demonstrate a decrease in muscle fiber number. Perhaps such high levels of androgen cause excess myotube fusion, producing the observed reduction in muscle fiber number. An alternative explanation is that androgen treatment causes muscle fiber death. This hypothesis is unlikely because there were no gross changes in muscle morphology that might be expected from necrosis of one-third of the muscle fibers.
One way in which androgen could control muscle fiber addition in the larynx is by acting on genes that control myogenesis. Some of these genes have been identified as members of a transcription factor family that includes MyoD, myogenin and others (Davis et al. 1987; Wright et al. 1989). Misexpression of MyoD in non-muscle cells causes conversion to the myogenic lineage; overexpression in vitro can result in premature myocyte fusion (Davis et al. 1987). Binding of androgen to its receptor in laryngeal myoblasts could affect the expression of myogenic determination genes and alter the myogenic program in these cells. If so, laryngeal myogenesis in X. laevis may provide an attractive experimental system in which to study the interaction of two classes of developmentally regulated transcription factors, steroid hormone receptors and myogenic determination factors. Such studies could yield molecular insights into how the process of sexual differentiation produces a masculine behavioral phenotype - the song of a male frog.
Acknowledgments
This research was supported by NIH grant NS 19949. We gratefully acknowledge the helpful comments of Dennis L. Gorlick, James T. Watson, Leslie L. Fischer and Roberta E. Bivins. We also thank Schering Corporation for kindly supplying us with flutamide.
References
- Chang C, Kokonitis J, Liao S. Molecular cloning of human and rat complementary DNA encoding androgen receptors. Science. 1988;240:324–326. doi: 10.1126/science.3353726. [DOI] [PubMed] [Google Scholar]
- Cihak R, Gutmann E, Hanzlikova V. Involution and hormone-induced persistence of the M. sphincter (levator) ani in female rats. J. Anat. 1970;106:93–110. [PMC free article] [PubMed] [Google Scholar]
- Davis RL, Weintraub H, Lassar AB. Expression of a single transfected cDNA converts fibroblasts to myoblasts. Cell. 1987;51:987–1000. doi: 10.1016/0092-8674(87)90585-x. [DOI] [PubMed] [Google Scholar]
- Evans RM. The steroid and thyroid hormone receptor superfamily. Science. 1988;240:889–895. doi: 10.1126/science.3283939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutmann E, Hanzlikova V, Cihak R. Persistence of the levator ani muscle in female rats. Experientia. 1967;23:852–855. doi: 10.1007/BF02146886. [DOI] [PubMed] [Google Scholar]
- He W-W, Fischer L, Sun S, Bilhartz D, Zhu X, Young C, Kelley D, Tindall D. Molecular cloning of androgen receptor from divergent species with the PCR technique: complete cDNA sequence of the mouse androgen receptor and isolation of cDNA probes from dog guinea pig and frog. Biochem. Biophys. Res. Comm. 1990 doi: 10.1016/0006-291x(90)91202-4. (in press). [DOI] [PubMed] [Google Scholar]
- Joubert Y, Tobin C. Satellite cell proliferation and increase in the number of myonuclei induced by testosterone in the levator ani muscle of the adult female rat. Devi Biol. 1989;131:550–557. doi: 10.1016/s0012-1606(89)80025-9. [DOI] [PubMed] [Google Scholar]
- Kelley D. Sexually dimorphic behaviors. Annual Review of Neuroscience. 1988;11:225–252. doi: 10.1146/annurev.ne.11.030188.001301. [DOI] [PubMed] [Google Scholar]
- Kelley DB, Dennison J. The vocal motor neurons of Xenopus laevis: development of sex differences in axon number. J. Neurobiol. 1990;21:869–882. doi: 10.1002/neu.480210605. [DOI] [PubMed] [Google Scholar]
- Kelley D, Sassoon D, Segil N, Scudder M. Development and hormone regulation of androgen receptor levels in the sexually dimorphic larynx of Xenopus laevis . Devi Biol. 1989;131:111–118. doi: 10.1016/s0012-1606(89)80042-9. [DOI] [PubMed] [Google Scholar]
- Kelley DB, Tobias ML. The genesis of courtship song: Cellular and molecular control of a sexually differentiated behavior. In: Carew TJ, Kelley DB, editors. Perspectives in Neural Systems and Behavior. New York: A. Liss; 1989. pp. 175–194. [Google Scholar]
- Lambdin L, Kelley D. Organization and activation of sexually dimorphic vocalizations: Androgen levels in developing and adult Xenopus laevis . Soc. Neurosci. Abstr. 1986;12:1213. [Google Scholar]
- Lubahn D, Joseph D, Sullivan P, Willard H, French F, Wilson E. Cloning of human receptor complementary DNA and localization to the X chromosome. Science. 1988;240:327–330. doi: 10.1126/science.3353727. [DOI] [PubMed] [Google Scholar]
- Nieuwkoop P, Faber J. Normal Table of Xenopus laevis. Amsterdam: North Holland; 1956. [Google Scholar]
- Paterson NF. The head of Xenopus laevis . Q. J. Microsc. Sci. 1939;81:161–230. [Google Scholar]
- Ridewood W. On the structure and development of the hyobranchial skeleton and larynx in Xenopus and Pipa; with remarks on the affinities of the aglossa. Linn. Soc. J. Zool. 1898;26:53–128. [Google Scholar]
- Sassoon D, Gray G, Kelley D. Androgen regulation of muscle fiber type in the sexually dimorphic larynx of Xenopus laevis . J. Neurosci. 1987;7:3198–3206. doi: 10.1523/JNEUROSCI.07-10-03198.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sassoon D, Kelley DB. The sexually dimorphic larynx of Xenopus laevis: development and androgen regulation. Am. J. Anat. 1986;177:457–472. doi: 10.1002/aja.1001770404. [DOI] [PubMed] [Google Scholar]
- Sassoon D, Segil N, Kelley D. Androgen-induced myogenesis and chondrogenesis in the larynx of Xenopus laevis . Devi Biol. 1986;113:135–140. doi: 10.1016/0012-1606(86)90115-6. [DOI] [PubMed] [Google Scholar]
- Segil N, Silverman L, Kelley DB. Androgen-binding levels in a sexually dimorphic muscle of Xenopus laevis . Gen. comp. Endocrinol. 1987;66:95–101. doi: 10.1016/0016-6480(87)90354-6. [DOI] [PubMed] [Google Scholar]
- Stockdale F. Introduction: Myoblast commitment and the embryogenesis of skeletal muscle. In: Pearson M, Epstein H, editors. Muscle Development. Cold Spring Harbor: Cold Spring Laboratory Press; 1982. pp. 339–344. [Google Scholar]
- Tobias ML, Kelley DB. Electrophysiology and dye-coupling are sexually dimorphic characteristics of individual laryngeal muscle fibers in Xenopus laevis . J. Neurosci. 1988;8:2422–2429. doi: 10.1523/JNEUROSCI.08-07-02422.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobias ML, Marin ML, Kelley DB. Post metamorphic masculination of the larynx in Xenopus laevis . 1990 In preparation. [Google Scholar]
- Tobin C, Joubert Y. Regulation du nombre et du diamètre des fibres dans un système neuro-musculaire hormono-dependant. Abstr. Colloque national des Neurosciences Bordeaux. 1986 [Google Scholar]
- Tobin C, Joubert Y. The levator ani of the female rat: a suitable model for studying the effects of testosterone on the development of mammalian muscles. Biol. Struct. and Morphogen. 1988;1:28–33. [PubMed] [Google Scholar]
- Venable J. Constant cell populations in normal, testosterone-deprived and testosterone-stimulated Levator ani muscles. Am. J. Anat. 1966;119:271–302. doi: 10.1002/aja.1001190205. [DOI] [PubMed] [Google Scholar]
- Wilson J, George F, Griffin J. The hormonal control of sexual development. Science. 1981;211:1278–1284. doi: 10.1126/science.7010602. [DOI] [PubMed] [Google Scholar]
- Witschi E. Mechanisms of sexual differentiation. In: Hamburg M, Barrington E, editors. Hormones in Development. New York: Appleton Century Crofts; 1971. pp. 601–618. [Google Scholar]
- Wright W, Sassoon D, Lin V. Myogenin - a factor regulating myogenesis - has a domain homologous to MyoD. Cell. 1989;56:607–617. doi: 10.1016/0092-8674(89)90583-7. [DOI] [PubMed] [Google Scholar]
- Yamamoto K. Steroid receptor regulated transcription of specific genes and gene networks. Ann. Rev. Genet. 1985;19:209–252. doi: 10.1146/annurev.ge.19.120185.001233. [DOI] [PubMed] [Google Scholar]