Abstract
Temporal constraints on androgen regulated masculinization of three sexually dimorphic laryngeal properties—tension, fiber type, and fiber recruitment—were examined in Xenopus laevis frogs. Endocrine state was manipulated at PM0 when the larynx is similar in males and females, at PM2 when the larynx begins sexual differentiation, and at PM6 when sexual differentiation is complete. Removing the testes in developing males (PM0 or PM2) completely arrests laryngeal masculinization. Masculinization resumes when testosterone is replaced later in development (PM2 or PM6, respectively). Thus, testicular secretions, in particular androgens, are required for laryngeal masculinization. The ability of androgens to masculinize tension, fiber type, and fiber recruitment in developing and adult larynges was also determined. Five weeks of testosterone treatment in PM0 or PM2 males and females completely masculinizes laryngeal tension and fiber type, but only partially masculinizes fiber recruitment. However, fiber recruitment can be fully masculinized in PM6 males castrated at PM2. We conclude that androgen induced masculinization of tension and fiber type are not temporally constrained but that androgen induced masculinization of fiber recruitment is. Prolonged androgen treatment can override the temporal constraints on masculinization of the larynx. Testosterone treatment for more than 6 months fully masculinizes fiber recruitment in developing (PM0 or PM2) females. In addition, prolonged treatment (>9 months) completely masculinizes tension, fiber type, and fiber recruitment in adult females; these properties were not fully masculinized by shorter (1–3 months) treatments in adult females. Testosterone induced masculinization in females is maintained for up to 8 months following testosterone removal; thus androgen effects are long lasting and possibly permanent.
INTRODUCTION
We have examined the pattern of sexual differentiation of laryngeal muscle tension, fiber type, and fiber recruitment in Xenopus laevis frogs (Tobias et al., 1991). These laryngeal properties contribute to the rate and amplitude modulation of male song (Tobias and Kelley, 1987; Sassoon et al., 1987). Laryngeal tension and fiber recruitment are the same in males and females at the beginning of postmetamorphic development. Subsequently, these characteristics remain virtually unchanged in females, while males undergo a radical transformation. Laryngeal fiber type is also sexually monomorphic at the start of postmetamorphic development. Fiber type then diverges in males and females; the number of slow twitch fibers increases in females and decreases in males (Sassoon et al., 1987; Tobias et al., 1991). Thus, sex differences in the adult larynx are primarily due to masculinization during postmetamorphic development. Masculinization is regulated by gonadal secretions at appropriate developmental stages. In this paper we determine when during development androgens are required for masculinization of laryngeal tension, fiber type, and fiber recruitment.
We have divided postmetamorphic development of males into seven stages identified by key events in laryngeal masculinization (Tobias et al., 1991). Laryngeal muscle fiber type begins to masculinize at postmetamorphic (PM) stage 2, while laryngeal muscle tension and fiber recruitment begin to masculinize at PM3. To determine when androgen is required for masculinization of these properties, we examined the effects of endocrine manipulation at PM0, when the larynx is sexually monomorphic, at PM2, when the larynx begins sexual differentiation, and at PM6, when sexual differentiation is complete. Two experimental paradigms were used. In one, males were androgen depleted by castration and subsequent masculinization was assessed with and without testosterone treatment later in development. In the other, males and females were androgen treated at PM0, PM2, or PM6 and masculinization was assessed following short (5 weeks) or long (>6 months) duration testosterone exposure. To determine if testosterone effects are permanent, masculinization of the female larynx was assessed up to 8 months after the cessation of testosterone treatment.
MATERIALS AND METHODS
Electrophysiological and Histochemical Procedures
Animal maintenance and procedures for electrophysiology and adenine triphosphatase (ATPase) histochemistry are described in the accompanying paper (Tobias et al., 1991). The larynx was removed, cleaned, weighed, and halved longitudinally. The left half was tested for electrophysiological properties. Muscle tension and electromyograms (EMGs) were recorded while stimulating the laryngeal nerve with trains simulating the fast portion of a male mate call (250 ms duration, 71 Hz). As in the accompanying paper, the percentage of transient tension was used to quantify masculinization of laryngeal tension; a fully masculinized larynx produces 100% transient tension. EMG potentiation was used to quantify masculinization of laryngeal fiber recruitment; EMG potentiation increases with masculinization. The right half of the larynx was rapidly frozen in liquid hitrogen and processed for ATPase histochemistry. Laryngeal muscles containing a heterogeneous fiber population are unmasculinized, and laryngeal muscles containing a homogeneous population of fast twitch fibers are fully masculinized (see Fig. 3 for examples). An immature larynx, which contains a heterogeneous fiber population, was used as a control for staining pattern in all histochemistry experiments.
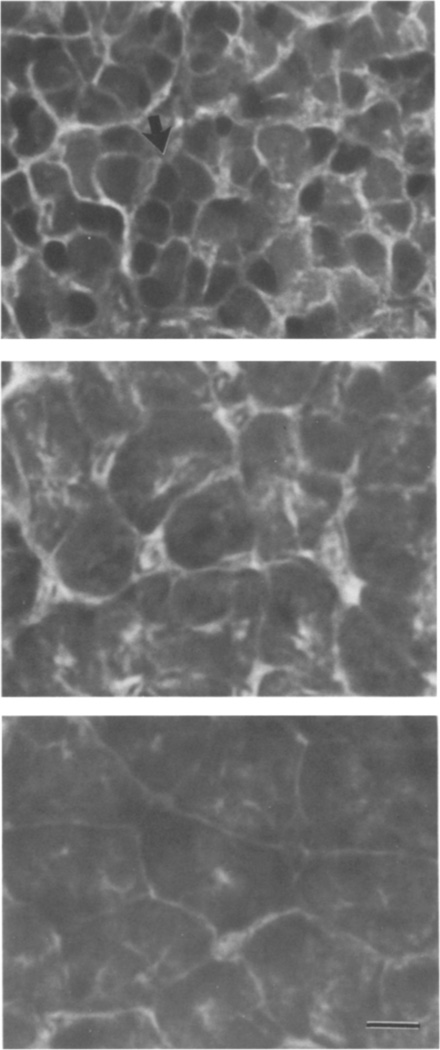
FIG. 3.
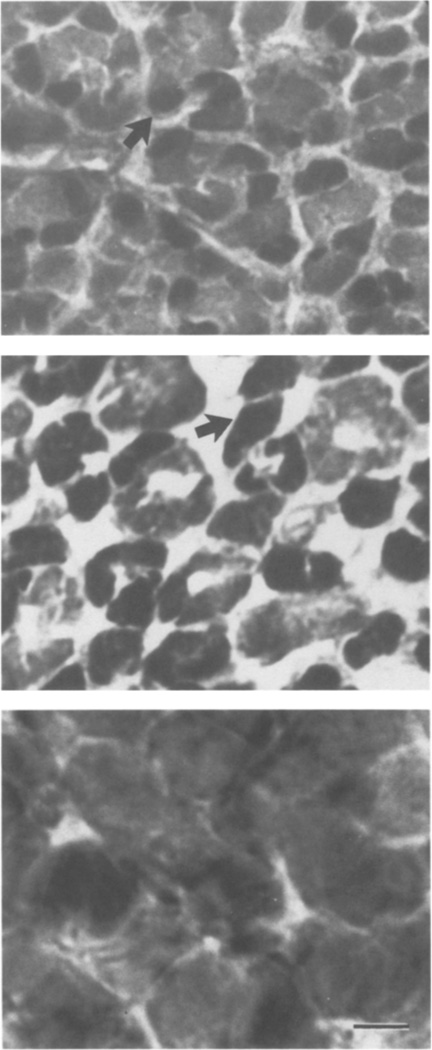
ATPase histochemistry of control, castrated, and androgen treated male laryngeal muscle. (Top) intact PM2 male; both fast and slow (arrow) twitch fibers are present. (Middle) PM6 male castrated at PM2; fiber composition similar to intact PM2 males (a slow twitch fiber is indicated by the arrow). (Bottom) androgen treated PM6 male castrated at PM2; no slow twitch fibers are present. Calibration bar: 10 µm.
Selection by Stage
Each stage in laryngeal masculinization is characterized by a range of larynx and body weights (see Table 1 of the accompanying paper, Tobias et al., 1991). In endocrine manipulated animals, it is not possible to obtain larynx weight prior to the experiment. Thus, male frogs at specific developmental stages were selected by body weight and age (see Tobias et al., 1991). The stages of laryngeal masculinization are not, by definition, applicable to females. The relation between body weight and age is similar in males and females until body weight reaches approximately 10 g (PM3 for males). Body weight then increases more rapidly in females than in males. To select females at developmental stages equivalent to male PM1-PM3, animals from different ovulation groups were obtained by age and body weight without regard to sex. Some male larynges from the group were weighed to identify their developmental stage; females in the group were then considered matched to males of that stage. To select females at developmental stages equivalent to male PM4 and PM5, female siblings of stage identified males were used. In many experiments, animals were treated at one stage in development and reared to a later stage. Maturation to the second stage was assessed by body weight alone. Body weights were obtained from all animals at the start of each experiment and both body and larynx weights were obtained at sacrifice. Because each experiment included a group of control males, their laryngeal weights at the end of the experiment could be used to ensure that the maturation of the group had progressed to the requisite stage. PM0 (end of metamorphosis) frogs are recognized when the tailbud is no longer visible from the ventral surface (Nieuwkoop and Faber, 1956). PM6 males weigh at least 25 g and PM6 females weigh at least 45 g.
TABLE 1.
Average Larynx Weights for Control and Experimental Frogs
| Stage treatment begun/ stage larynx weighed |
Larynx weight (mg) mean ± SD |
N | |
|---|---|---|---|
| I | ♀ PM0/PM0 + 5 weeks | 6 ± 2 | 4 |
| ♀ PM0 + T/PM0 + 5 weeks | 110 ± 19 | 10 | |
| ♂ PM0/PM0 + 5 weeks | 13 ± 10 | 7 | |
| ♂ PM0 + T/PM0 + 5 weeks | 119 ± 29 | 11 | |
| II |
|
29 ± 8 | 4 |
|
|
238 ± 24 | 3 | |
| III | ♀ PM2/PM2 + 5 weeks | 22 ± 4 | 6 |
| ♀ PM2 + T/PM2 + 5 weeks | 163 ± 25 | 10 | |
| ♂ PM2/PM2 + 5 weeks | 54 ± 36 | 7 | |
| ♂ PM2 + T/PM2 + 5 weeks | 233 ± 24 | 13 | |
| IV | ♂ PM2/PM2 | 57 + 21 | 3 |
|
|
147 + 53 | 11 | |
| ♂ PM2 sham/PM6 | 409 ± 54 | 10 | |
|
|
309 ± 47 | 8 | |
| V |
|
104 ± 63 | 4 |
|
|
119 ± 31 | 4 | |
|
|
222 ± 26 | 4 | |
| ♀ PM6 + T/PM6 + >9 months | 375 ± 102 | 5 |
Note. T, testosterone; ![]() , ovariectomized female;
, ovariectomized female; ![]() , castrated male; PMX, postmetamorphic stage X.
, castrated male; PMX, postmetamorphic stage X.
Hormone Treatments
A summary of experimental groups is given in Table 1.
Gonadectomy
Frogs were deeply anesthetized by cold narcosis (PM0) or immersion in 0.1% MS222 (ethyl m-amino benzoate methane sulfonic acid, Aldrich Chemical Co.; PM2). The gonads and their associated fat bodies were removed by excision (PM0) or cautery (PM2). In sham operated controls, visual examination of the gonads was used to determine sex but the gonads were not removed. The appearance of the gonads at these stages and criteria used to distinguish ovaries from testes are described elsewhere (Marin et al., 1990). The incised muscles and then the skin were sutured and the animal was allowed to recover in moist towels. Castrated animals were examined at sacrifice for any gonadal re-growth and were used only if none had occurred. Castration at PM0 has been shown to block androgen-dependent addition of muscle fibers (Marin et al., 1990). Castration has also been shown to reduce circulating testosterone in adult males to <1.0 ng/ml (5% of control values; Kelley, 1980). We thus assume that in these experiments on developing frogs castration reduced the level of circulating androgens.
Hormone implants
Frogs were anesthetized by cold narcosis. The hormone pellet was inserted through a small incision in the posterior portion of the dorsal lymph sac and positioned either just posterior to the eye in larger animals or in the posterior lymph sac in smaller animals. The incision was sutured and the animal allowed to recover. Control animals were obtained from the same initial group and reared under identical conditions but received no pellet.
In PM0 females exposed to prolonged androgen treatment, testosterone propionate was mixed with silastic (Dow Corning) and a length of polymer equivalent to 1–2 mg of hormone was implanted. For other experiments, testosterone propionate (Sigma Co.) was compressed into pellets (3-mm diameter) using a pellet press (Parr). PM0 frogs received 2- to 3-mg pellets, PM2 frogs received 5- to 6-mg pellets, and PM6 frogs received 16- to 20-mg pellets. Frogs were typically treated with hormone pellets for a 5-week period; this period is shorter than the duration of any one developmental stage (see Tobias et al., 1991). These androgen doses and durations of treatment have been shown to be effective in masculinizing other laryngeal properties (Sassoon et al., 1987; Kelley et al., 1989; Marinet al., 1990). Androgen pellets in adult frogs produce supraphysiological androgen levels: 5-mg pellets produce 15–25 times physiological levels, 10-mg pellets produce levels greater than the measurable range (Wetzel and Kelley, 1983). Thus, the pellets used in this study also produced supraphysiological levels of androgen.
In one experiment, a testis was used as the hormone source. PM0 female frogs received a single testis from an age-matched male. The frogs were ovariectomized and a donor testis was placed within the remaining fat body of one excised gonad (see Marin et al., 1990 for a more detailed description of the procedure). Such transplants become well vascularized and are effective in releasing hormone as judged by the induction of nuptial pads, an androgen-dependent characteristic.
Data Analysis
To determine the effects of castration at PM2, experimental animals were compared with intact PM2 males. Males castrated at PM2 that received subsequent androgen replacement were compared with PM6 males that had received a sham operation at PM2 to determine if they were fully masculinized. Males castrated at PM0 that received subsequent androgen treatment at PM2 were compared with intact PM6 males. To determine if a given hormone treatment effectively masculinized laryngeal muscle tension or EMG potentiation, groups of treated frogs were compared with control animals of the same stage and sex and with PM6 males. Testosterone treated males were compared with testosterone treated females to determine if androgens have equivalent effects on the sexes. A nonparametric test which uses ranked measurements from unequal group sizes (Mann–Whitney test; Snedecor and Cochran, 1967) was used for all comparisons. Of the measures we have used, EMG potentiation is the most variable; this variability makes assessment of the completion of masculinization particularly difficult. We have nonetheless retained this measure because it reflects the masculinization of the neuromuscular synapse (Tobias and Kelley, 1988). Masculinization of fiber type was assessed using ATPase histochemistry (Sassoon et al., 1987). Muscles containing a homogeneous population of fast twitch fibers were considered fully masculinized, while those containing a mixed population of fiber twitch types were considered unmasculinized.
RESULTS
Castration at PM2/Testosterone Treatment at PM6
In intact males, masculinization of laryngeal tension, EMG potentiation, and fiber type occurs between PM2 and PM6 (Tobias et al., 1991). To determine if masculinization requires the testes after PM2, males were castrated at PM2, reared to PM6 (when the larynx is fully masculinized in intact males), and then tested. To determine if the capacity for masculinization is restricted to the developmental period when masculinization normally occurs, an additional group of males was castrated at PM2 and reared to PM6. These males then received a testosterone pellet for 5 weeks and were tested.
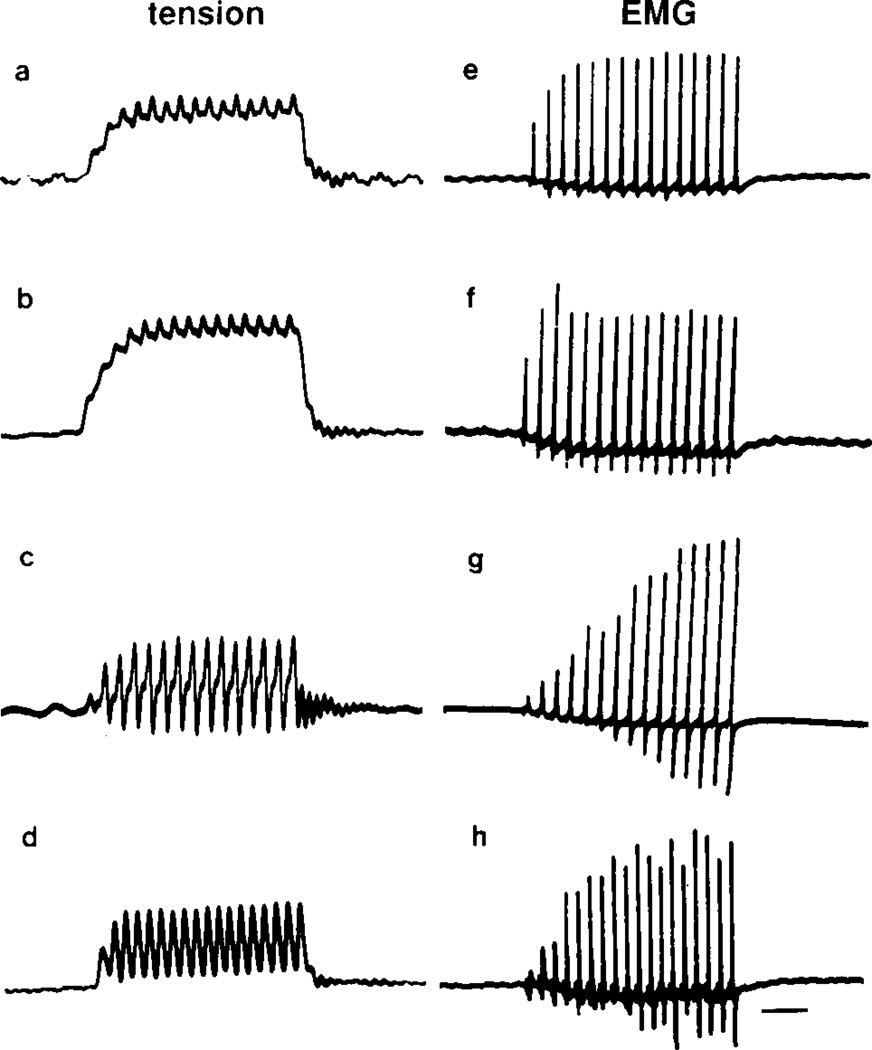
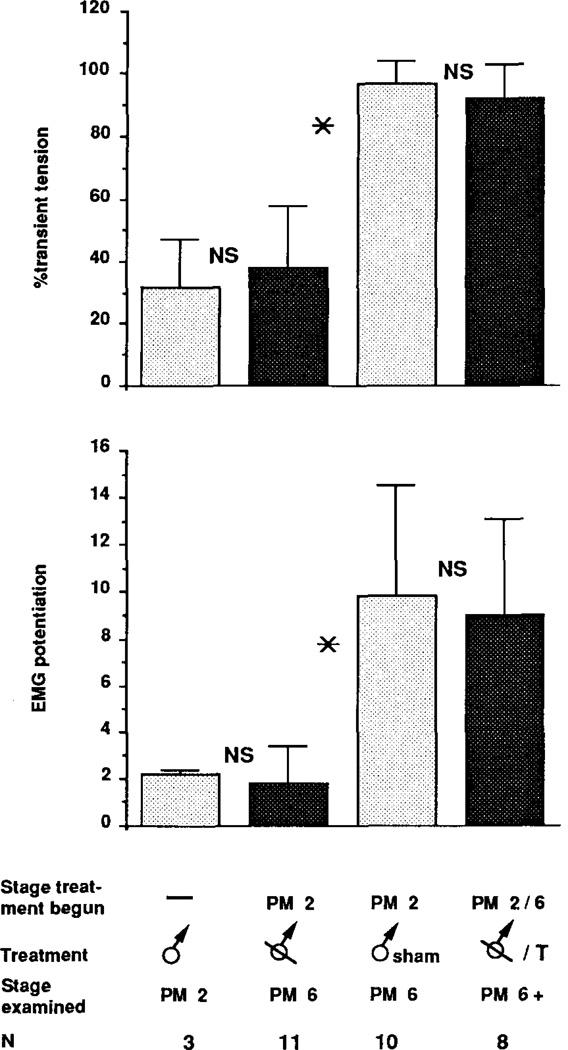
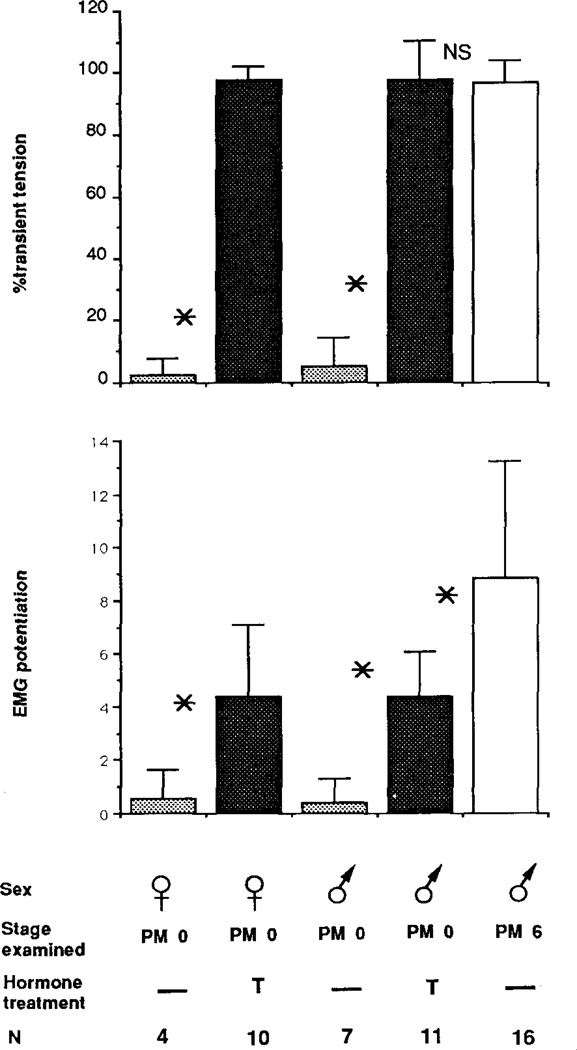
Castration at PM2 arrested masculinization of laryngeal muscle tension, EMG potentiation, and fiber type when assessed at PM6. Larynges from intact PM2 males and PM6 males castrated at PM2 produced primarily maintained tension with smaller transients superimposed (Figs. 1a, 1b). The amplitude of the EMG was nearly constant throughout the stimulus train for both (Figs. 1e, 1f). In contrast, larynges from PM6 males that had received sham operations at PM2 produced entirely transient tension (Fig. 1c) and showed marked EMG potentiation (Fig. 1g); these records were indistinguishable from those of intact PM6 males (not shown). Group means for the percentage of transient tension and EMG potentiation did not increase after castration compared with those of intact PM2 males (Fig. 2). Values for intact and castrated males were significantly lower than those for sham operated males (P < .01). The transformation of laryngeal muscle fiber type, from slow to fast twitch, was also prevented by castration at PM2 (Fig. 3, middle panel); larynges from both intact PM2 males (Fig. 3, upper panel) and PM6 males castrated at PM2 contained a heterogeneous population of slow and fast twitch fibers.
FIG. 1.
Laryngeal tension and EMG records from control, castrated, sham operated, and androgen treated males. (a, e) Intact PM2 males; (b, f) PM6 males castrated at PM2; (e, g) PM6 males that had received sham operations at PM2; (d, h) androgen treated PM6 males castrated at PM2. Castration at PM2 prevents masculinization (compare top two traces); larynges from both groups produce mostly maintained tension and little EMG potentiation. Androgen treatment in PM6 castrates reinitiates masculinization (compare bottom two traces); larynges from both groups produce entirely transient tension and marked EMG potentiation. Calibration bar: 50 ms.
FIG. 2.
Effect of castration at PM2 on male laryngeal masculinization with and without testosterone treatment at PM6. Means ± standard deviations for the percentage of transient tension and EMG potentiation are shown for intact PM2 males, PM6 males castrated at PM2, PM6 males that had received a sham operation at PM2, and androgen treated PM6 males castrated at PM2. Asterisks indicate a statistically significant difference between groups; NS indicates that there is no statistical difference between groups.
Androgen treatment of PM6 males castrated at PM2 completely masculinized laryngeal tension, EMG potentiation, and fiber type. Laryngeal tension and EMG records from androgen treated castrated males (Figs. 1d, 1h) were indistinguishable from sham operated males (Figs. 1c, 1g); both produced entirely transient tension and marked EMG potentiation. Values for the percentage of transient tension and EMG potentiation from androgen treated castrates were significantly greater than those for untreated castrates (P < .01) and did not differ significantly from sham operates (Fig. 2). Larynges from androgen treated castrated males contained no slow twitch fibers (Fig. 3, lower panel).
Together, these data indicate that the testes are required after PM2 for subsequent masculinization of laryngeal tension, EMG potentiation, and fiber type. Further, testicular secretions prior to PM2 are not sufficient for subsequent masculinization of these properties. However, androgen regulated masculinization is not restricted to the period of development in which masculinization normally occurs (PM2 to PM6) since androgen treatment at PM6 can compensate for androgen depletion between PM2 and PM6. Thus, the larynges of males castrated at PM2 maintain a developmentally dormant state in which masculinization can be reactivated, even in adulthood, if androgen is supplied.
Testosterone Treatment at PM2
We next addressed the possibility that an androgen-independent phase of maturation between PM2 and PM6 is required for laryngeal masculinization by determining whether male larynges can be masculinized by androgen treatment at PM2. The ability of testosterone to masculinize the female larynx was also examined.
Testosterone treatment for 5 weeks at PM2 completely masculinized laryngeal muscle tension but only partially masculinized EMG potentiation in both sexes (Fig. 4). The percentage of transient tension was significantly greater in testosterone treated than in untreated PM2 males and females (P < .01) and was not significantly different from that in intact PM6 males. EMG potentiation was significantly greater in testosterone treated than in untreated PM2 males and females (males, P < .05; females, P < .01) but was significantly less than in intact PM6 males (P < .01). Testosterone treatment at PM2 induced conversion from a heterogeneous slow and fast twitch muscle to an all fast twitch muscle as assessed by ATPase histochemistry (Fig. 5). Since laryngeal muscle tension and fiber type can be completely masculinized by testosterone treatment at PM2, we conclude that no androgen-independent phase of maturation between PM2 and PM6 is necessary for masculinization. Since EMG potentiation cannot be completely masculinized at PM2, but can be completely masculinized in PM6 males castrated at PM2, EMG potentiation requires a phase of testes-independent maturation between PM2 and PM6 for complete masculinization.
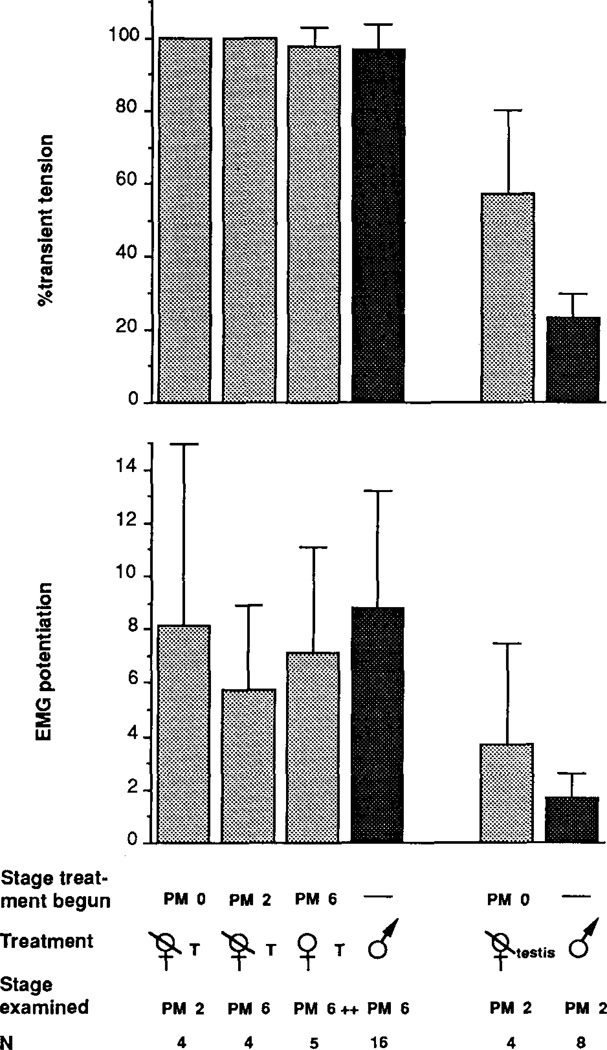
FIG. 4.
Effect of 5 weeks testosterone treatment on male and female laryngeal tension and fiber recruitment at PM2. Testosterone treated groups can be compared with PM6 male values (column at far right) and untreated frogs of the same age and sex. The percentage of transient tension is fully masculinized by testosterone treatment in both sexes. EMG potentiation is only partially masculinized by testosterone treatment in both sexes. Asterisks indicate a significant difference between groups. NS indicates no significant difference between groups.
FIG. 5.
Effect of 5 weeks testosterone treatment on male fiber type at PM2. (Top) intact PM2 male; heterogeneous population of fast and slow (arrow) twitch fibers. (Middle) testosterone treated PM2 male; homogeneous population of fast twitch fibers. (Bottom) intact PM6 male; homogeneous population of fast twitch fibers. Calibration bar: 10 µm.
Effect of Testosterone Treatment in PM2 Males Castrated at PMO
We next determined if the presence of the testes prior to PM2 is required for testosterone induced masculinization at PM2. Males were castrated at PM0, and the ability of testosterone to masculinize the larynx at PM2 was examined.
Testosterone treatment of PM2 males castrated at PM0 fully masculinized laryngeal muscle tension and fiber type. The percentage of transient tension in testosterone treated castrates was significantly greater than that in untreated castrates (100 ± 0 vs 15 ± 16%; P < .01). Tension values from testosterone treated castrates did not differ significantly from those for intact PM6 males. Testosterone treatment also completely masculinized fiber type in PM2 males castrated at PM0 (data not shown). Only two testosterone treated castrates were analyzed for effects on EMG potentiation; one was partially (4.1) and the other fully (7.0) masculinized. We conclude that the presence of the testes between PM0 and PM2 is not required for androgen induced masculinization of laryngeal tension and fiber type at PM2.
Testosterone Treatment at PMO
The testes are not required between PM0 and PM2 for androgen induced masculinization of laryngeal muscle tension or fiber composition at PM2. We thus wished to determine whether laryngeal properties could be prematurely masculinized by androgen at PM0. Five weeks of testosterone treatment at PM0 fully masculinized the percentage of transient tension (Fig. 6) and fiber type (data not shown) in males and females. Thus, testosterone treatment induced masculinization of laryngeal tension and fiber type before either of these properties begins to masculinize during normal development.
FIG. 6.
Effect of 5 weeks testosterone treatment on male and female laryngeal tension and fiber recruitment at PM0. Testosterone treated groups can be compared with PM6 male values (column at far right) and untreated frogs of the same age and sex. Testosterone treatment fully masculinizes the percentage of transient tension and partially masculinizes EMG potentiation in both sexes. Asterisks indicate a significant difference between groups. NS indicates no significant difference between groups.
Testosterone treatment at PM0 resulted in partial masculinization of EMG potentiation in both sexes (Fig. 6). Values for EMG potentiation were significantly greater in testosterone treated than in untreated males and females (P < .05) and significantly less than in intact PM6 males (P < .05).
Permanence of Testosterone Effects
Are the effects of testosterone on larynx masculinization permanent? Seven females received implants of testosterone propionate at PM2, the pellet was removed 5 weeks later, and the frogs were reared an additional 3, 4, or 8 months before testing. No difference was observed among any of the treatment groups and the results are averaged. There was a small but significant decrease in the percentage of transient tension produced by female larynges before and after pellet removal (PM2 + T = 94 ± 14%; PM2 + T removed = 89 ± 7 %; P < .01). The percentage of transient tension, however, clearly did not revert to untreated values (PM2 controls = 19 ± 7%). Muscle fibers were typed histochemically in two females 8 months after pellet removal; the laryngeal muscle contained a homogeneous population of fast twitch muscle fibers. In addition, laryngeal muscle fiber type was examined in two females that had received testosterone implants for 5 weeks at PM0 and then had had the pellet removed for 9 months; these larynges also contained a homogeneous population of fast twitch fibers. Thus, fiber twitch characteristics of laryngeal muscle do not change after testosterone removal. There was no significant difference in female laryngeal EMG potentiation before and after testosterone removal (PM2 + T = 5.9 ± 2.9; PM2 + T removed = 3.2 ± 1.8; P > .05). Thus, female larynges remain highly masculinized for periods up to 8 months following the cessation of testosterone treatment.
Long Term Androgen Treatment in Females
Five weeks of testosterone treatment did not fully masculinize EMG potentiation in developing larynges. Three months of testosterone treatment did not fully masculinize laryngeal tension or fiber type in adult females (Sassoon et al., 1987; Tobias and Kelley, 1987). We wished to determine whether long duration testosterone treatment could more effectively masculinize the female larynx than short duration treatments. Females were ovariectomized at PM0 or PM2, at which time they received a testosterone implant. The larynges were examined at PM2 or PM6, respectively. In addition, sexually mature females (PM6) were testosterone treated for 9 to 14 months and their larynges examined.
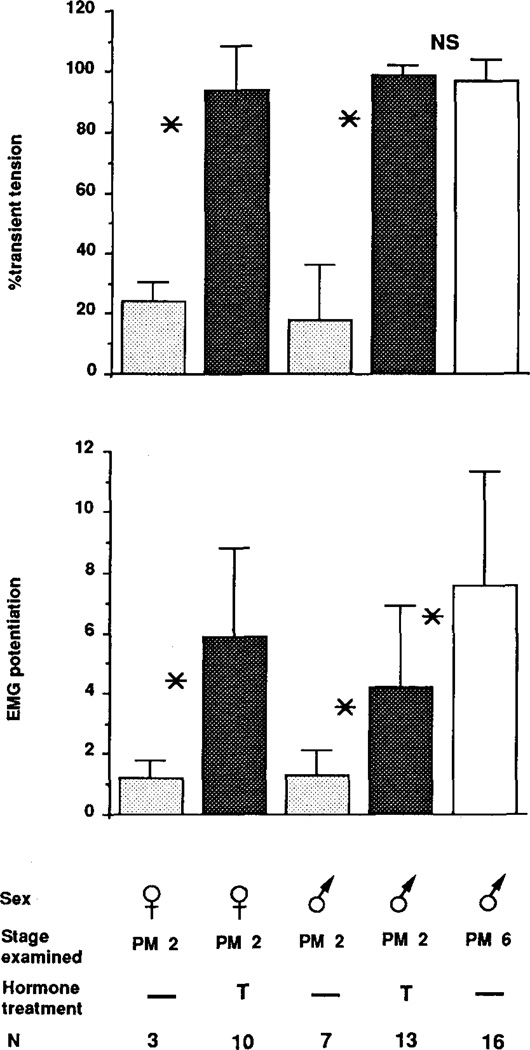
Long duration testosterone treatment fully masculinized the percentage of transient tension (Fig. 7) and fiber type (not shown) in developing (PM0, PM2) and adult (PM6) females. Long duration testosterone treatment also fully masculinized EMG potentiation at all stages; values did not differ significantly from those for PM6 males (Fig. 7). Thus, very long duration testosterone treatments can masculinize laryngeal properties which short duration treatments cannot. Females retain androgen sensitivity even into adulthood, but require very long exposures to fully masculinize laryngeal tension, fiber type, and EMG potentiation.
FIG. 7.
Effect of long duration testosterone treatment on laryngeal tension and EMG potentiation in females. Means ± standard deviations are given for the percentage of transient tension and EMG potentiation for females ovariectomized at PM0 and given testosterone until PM2, females ovariectomized at PM2 and given testosterone until PM6, and PM6 females that received testosterone pellets for 9 months or longer. No error bar for the percentage of transient tension indicates that all animals tested produced 100% transient tension. Values for PM6 males (solid bar) are included for comparison. Means ± standard deviations are also shown for females ovariectomized at PM0 and given one testis until PM2; values for PM2 males (solid bar) are included for comparison.
We then asked whether long duration physiological doses of androgen would have the same effect on masculinization as the supraphysiological doses achieved with hormone implants. Four females were ovariectomized at PM0 at which time they received a testicular implant from a male of the same stage. They were reared to PM2 and their larynges examined (Fig. 7). The percentage of transient tension was significantly greater (P < .05) in testis-implanted females than in intact PM2 males, but was significantly lower (P < .01) than in intact PM6 males. Values for EMG potentiation were not significantly different from those for intact PM2 males. The laryngeal muscle was heterogeneous in fiber composition as would be a PM2 male larynx at that stage (data not shown). In contrast to the partial masculinization induced by a testis implant, PM2 females given long term testosterone treatments were fully masculinized in all measures. Thus, the physiological effects of an implanted testis masculinizes the female larynx less effectively than does the supraphysiological dose of androgen supplied by a hormone pellet.
Effect of Androgen Manipulation on Larynx Weight
Laryngeal weights of all experimental and control animals are presented in Table 1. Male larynx weight continues to increase, but at a slower rate, in the absence of testes (II, IV). For example, males castrated at PM0 had greater laryngeal weights at PM2 than intact PM0 males (29 ± 8 vs 5 ± 2 mg) but lower laryngeal weights than did intact PM2 males (49 ± 10 mg). Testosterone treatment accelerated larynx weight gain in intact (I, III) and castrated (II, IV) males. Testosterone treatment also accelerated larynx weight gain in females (I, III, V). An increase in female larynx weight was also produced by a testis implant (V).
During normal postmetamorphic development in males, laryngeal weight is an excellent predictor of the extent of masculinization of tension, fiber type, and fiber recruitment (Tobias et al., 1991). This study indicates that the extent of laryngeal masculinization can be predicted by larynx weight in hormone manipulated animals as well.
Larynx weight is not correlated with body weight in hormone manipulated animals. For example, castrated males have developmentally immature larynges (while the body weight and age were characteristic of PM2 males, the larynx weight was characteristic of PM1 males). Conversely, androgen treated frogs have developmentally precocious larynges (while the body weight and age were characteristic of PM2 males, the larynx weight was characteristic of PM5 males). Thus, developmental stages assessed by age and body weight were not the same in intact frogs as they were in hormone manipulated frogs.
DISCUSSION
Androgen Sensitive Periods for Masculinization
Our previous studies established that different laryngeal properties become masculinized at different stages in postmetamorphic development (Tobias et al., 1991). Masculinization is directed by androgen secretion from the testes. Do the naturally occurring phases of masculinization reflect strict limitations on androgen sensitive periods? For laryngeal fiber type and tension the answer is clearly no. In intact males, laryngeal muscle fiber type and the percentage of transient tension begin masculinization at PM2 and PM3. respectively. Testosterone treatment for 5 weeks fully masculinizes both properties earlier than (PM0), at the same time as (PM2), or later than (PM6, in the absence of testes) normally occurring masculinization. Thus, in males, provided sufficient androgen is supplied, tension and fiber type can be masculinized at any point during postmetamorphic development. The cellular and molecular events underlying masculinization must be androgen sensitive at the end of metamorphosis (PM0) and remain androgen sensitive, even in the absence of the testes, through adulthood. However, because the amount of androgen supplied in these experiments was supraphysiological, it is possible that the larynx loses sensitivity to lower, physiological amounts of androgen during this period.
The temporal constraints on androgen regulated masculinization of fiber recruitment are more stringent than those for tension and fiber type. Testosterone treatment at PM0 or PM2 only partially masculinizes EMG potentiation. This partial masculinization resembles the intermediate masculinization seen during development from PM3 to PM5 (Tobias et al., 1991). Males castrated at PM2 and reared to PM6 can be fully masculinized by testosterone treatment at PM6. Again, this full masculinization resembles the full attainment of masculinized EMG values seen late in normal development at PM6. We can infer from the present data that the two-stage process of normal EMG masculinization (Tobias et al., 1991) is not controlled simply by an increase in exposure to androgen. Were this so, exposure to high androgen levels early in postmetamorphic development would have completely masculinized EMG potentiation as it did fiber type and tension. Instead, full masculinization of EMG potentiation depends on a maturation process that is independent of androgen exposure between PM2 and PM6. We do not know whether androgen exposure prior to PM2 is required for full masculinization.
Although androgens are required to masculinize laryngeal muscle fiber type, tension, and fiber recruitment, they are not required to maintain the masculine phenotype. Castrated adult males continue to exhibit a homogeneously fast twitch laryngeal muscle 6 months after castration (Sassoon et al., 1987) and continue to produce male typical tension and EMG potentiation 3 months after castration (Tobias and Kelley, 1987). Androgens are also not required to maintain masculine phenotype in female laryngeal muscle; androgen induced masculinization in female larynges is maintained for up to 8 months following androgen removal (this study). These data imply that effects of androgen on laryngeal muscle fiber type, tension, and fiber recruitment are permanent.
Permanence of androgen action has also been assessed in the androgen-dependent sexually dimorphic temporalis muscle of guinea pigs (Lyons et al.. 1986). However, in this system, androgen is required for both conversion to and maintenance of masculine fiber type. Fiber type in the temporalis muscle reverts to the female phenotype following castration.
Androgen Regulation of Laryngeal Muscle Fiber Type and Tension
Androgen treatment can convert laryngeal muscle fibers to fast twitch as early as PM0, before sexual differentiation of fiber type would normally have occurred. No previous postmetamorphic androgen exposure is required for androgen induced masculinization of fiber type; males castrated at PM0 or PM2, and tested after androgen treatment at PM2 or PM6, respectively, are fully masculinized. Testosterone induces full masculinization of fiber type and tension in 5 weeks, a process which takes approximately 5 months during normal development. Exposure to high doses of androgen can thus both accelerate and induce fiber type conversion during early postmetamorphic development. The gradual fiber type conversion in untreated males during normal development is probably the response to physiological androgen secretion at levels lower than those supplied experimentally.
We presume that the absence of fiber type conversion from slow to fast in females is due to differences in androgen exposure between males and females during development. We show here that complete fiber type conversion can be achieved in females exposed to high levels of androgen during early postmetamorphic development (PM0 and PM2). Thus, females are capable of male-like fiber type conversion if sufficient androgens are supplied. The ability to transform laryngeal muscle fiber type decreases with maturation in females. While 5 weeks of androgen exposure is sufficient to fully masculinize fiber type in PM0 or PM2 females (as shown here), 5 months of androgen treatment only partially masculinizes adult females (Sassoon et al., 1987). Adult females retain the ability to convert laryngeal fiber type but prolonged androgen exposures (>9 months) are required.
As the number of muscle fibers expressing fast myosin ATPase increases, the percentage of transient tension recorded from whole muscle also increases (Tobias et al., 1991). We have suggested that the ability of male laryngeal muscle to produce strong, rapid tension transients (100% transient tension at 71 Hz) relies on a homogeneous population of fast twitch muscle fibers (Tobias and Kelley, 1987). This hypothesis is supported by the result that androgen manipulation during post-metamorphic development has the same effect on tension and fiber type. Both properties develop under the same temporal constraints. We have never observed a larynx capable of producing male-typical tension which did not also have a homogeneously fast twitch laryngeal muscle. Masculinization of fiber type is necessary for masculinization of tension. However, masculinization of fiber type may not be sufficient for masculinization of tension. At PM0, larynges frequently produce 0% transient tension even though nearly half the laryngeal muscle fibers are fast twitch. Similarly, larynges of adult males occasionally produce <100% transient tension even though the laryngeal muscle is homogeneously fast twitch. It is likely that some additional property of the muscle, besides the ATPase activity of myosin, contributes to generating rapid tension transients.
Androgen Regulation of Laryngeal Muscle Fiber Recruitment
Intracellular recordings from individual muscle fibers have revealed that the marked EMG potentiation characteristic of male laryngeal muscle is due to weak, facilitating neuromuscular synapses (Tobias and Kelley, 1988). During nerve stimulus trains, muscle fibers are progressively recruited and the EMG progressively increases in amplitude (potentiates), as does sound amplitude during the male call. In the female, neuromuscular synapses are strong. In response to nerve stimulus trains, all fibers respond together and the EMG shows little potentiation. During early postmetamorphic development, both sexes show little EMG potentiation (Tobias et al., 1991). Thus, synaptic modifications in male neuromuscular junctions occur late in postmetamorphic development. We show here that androgens are required to complete this process, although some androgen-independent maturation may also be required.
Females, like males, do not fully masculinize EMG potentiation in response to 5 weeks of androgen treatment during early postmetamorphic development. Adult females also cannot fully masculinize EMG potentiation in response to 5 weeks of androgen treatment. However, long duration androgen treatments (>6 months) can fully masculinize EMG potentiation if begun at PM0, PM2, or PM6. Thus, the cellular events underlying EMG potentiation must be present but dormant during normal development. Presumably, female laryngeal synapses do not experience modifications similar to those of the male because they are not exposed to prolonged androgen exposure during development.
The efficacy of laryngeal neuromuscular synapses may be controlled during development by the laryngeal motor neuron, the laryngeal muscle fiber, or some combination of both elements. Of particular interest are the changes in synaptic efficacy which occur in males during postmetamorphic development to produce the primarily weak synapses of the adult. The male laryngeal motor neuron could contribute to masculinization by maintaining multiple nerve terminals on male muscle fibers. Such multiple terminals have been shown to be individually weak at frog neuromuscular junctions (Trussell and Grinnell, 1985). Approximately one-fourth of the laryngeal muscle fibers are multiply innervated in adult males (Tobias and Kelley, 1988). The male laryngeal motor neuron could also restrict calcium entry into the presynaptic terminal thus preventing acetylcholine release sufficient in quantity to generate an action potential in the muscle fiber. The laryngeal muscle fiber might also contribute to weak synapses via modification of the ion channels on which the acetylcholine acts. In either case, masculinization must be effected via response to secreted androgen which, as we have shown here, controls laryngeal EMG potentiation. Both motor neuron and muscle express androgen receptor during postmetamorphic development (Gorlick and Kelley, 1987; Kelley et al., 1989). Androgen secretion has been shown to maintain multiple synapses in another developing neuromuscular synapse, the levator ani of rats (Jordan et al., 1990). Synaptic remodeling, both elimination and sprouting, occurs throughout postmetamorphic development and adulthood in frogs (Herrera and Werle, 1990; Herrera et al., 1991). In cultured laryngeal muscle of X. laevis, androgen can modify acetylcholine receptor channel properties, even in the absence of innervation (Erulkar and Wetzel, 1989). It is thus from among these pre- and postsynaptic candidates that we seek the androgen regulated cellular changes responsible for the masculinized vocal synapse.
Acknowledgments
We thank Diana Catz, Leslie Fischer, Maria Moschella, and James Watson for helpful comments on the manuscript. We are indebted to John Robertson for expert assistance with the photomicrographs. This research was supported by NS 23684.
REFERENCES
- Erulkar S, Wetzel D. 5α-Dihydrotestosterone has non-specific effects on membrane channels and possible genomic effects on ACh-activated channels. J. Neurophysiol. 1989;61:1036–1052. doi: 10.1152/jn.1989.61.5.1036. [DOI] [PubMed] [Google Scholar]
- Gorlick D, Kelley D. Neurogenesis in the vocalization pathway of Xenopus laevis. J. Comp. Neurol. 1987;254:614–627. doi: 10.1002/cne.902570409. [DOI] [PubMed] [Google Scholar]
- Herrera A, Werle M. Mechanisms of elimination, remodelling, and competition at frog neuromuscular junction. J. Neurobiol. 1990;21:73–98. doi: 10.1002/neu.480210106. [DOI] [PubMed] [Google Scholar]
- Herrera A, Banner L, Werle M, Regnier M, Nagaya-Stevens N. Postmetamorphic development of neuromuscular junctions and muscle fibers in the frog cutaneous pectoris. J. Neurobiol. 1991;22:15–28. doi: 10.1002/neu.480220103. [DOI] [PubMed] [Google Scholar]
- Jordan C, Letinsky M, Arnold A. Critical period for the androgenic block of neuromuscular synapse elimination. J. Neurobiol. 1990;21:760–767. doi: 10.1002/neu.480210509. [DOI] [PubMed] [Google Scholar]
- Kelley D. Auditory and vocal nuclei of frog brain concentrate sex hormones. Science. 1980;207:553–557. doi: 10.1126/science.7352269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley D, Sassoon D, Segil N, Scudder M. Development and hormone regulation of androgen receptor levels in the sexually dimorphic larynx of Xenopus laevis. Dev. Biol. 1989;131:111–118. doi: 10.1016/s0012-1606(89)80042-9. [DOI] [PubMed] [Google Scholar]
- Lyons G, Kelly A, Rubinstein N. Testosterone-induced changes in contractile protein isoforms in the sexually dimorphic temporalis muscle of the guinea pig. J. Biol. Chem. 1986;261:13,278–13,284. [PubMed] [Google Scholar]
- Marin M, Tobias ML, Kelley DB. Hormone sensitive stages in the sexual differentiation of laryngeal muscle fiber number in Xenopus laevis. Development. 1990;110:703–711. doi: 10.1242/dev.110.3.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieuwkoop P, Faber J. Normal Table of Xenopus laevis (Daudin) North-Holland: Amstersdam; 1956. [Google Scholar]
- Sassoon DA, Gray GE, Kelley DB. Androgen regulation of muscle fiber type in the sexually dimorphic larynx of Xenopus laevis. J. Neurosci. 1987;7:3198–3206. doi: 10.1523/JNEUROSCI.07-10-03198.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snedecor G, Cochran W. Statistical Methods. Ames, IA: Iowa State Univ. Press; 1967. pp. 130–131. [Google Scholar]
- Tobias ML, Kelley DB. Vocalizations by a sexually dimorphic isolated larynx: Peripheral constraints on behavioral expression. J. Neurosci. 1987;7:3191–3197. doi: 10.1523/JNEUROSCI.07-10-03191.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobias ML, Kelley DB. Electrophysiology and dye-coupling are sexually dimorphic characteristics of individual laryngeal muscle fibers in Xenopus laevis. J. Neurosci. 1988;8:2422–2429. doi: 10.1523/JNEUROSCI.08-07-02422.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobias ML, Marin M, Kelley DB. Stages in laryngeal masculinization of Xenopus laevis. Dev. Biol. 1991;147 doi: 10.1016/s0012-1606(05)80023-5. 000–. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trussell L, Grinnell A. The regulation of synaptic strength within motor units of the frog cutaneous pectoris muscle. J. Neurosci. 1985;5:243–254. doi: 10.1523/JNEUROSCI.05-01-00243.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetzel D, Kelley D. Androgen and gonadotropin control of the mate calls of male South African clawed frogs, Xenopus laevia. Harm. Behav. 1983;17:388–404. doi: 10.1016/0018-506x(83)90048-x. [DOI] [PubMed] [Google Scholar]