Abstract
Computation of rate in auditory signals is essential to call recognition in anurans. This task is ascribed to a group of central nervous sytem nuclei in the dorsal midbrain or torus semicircularis, homologous to the inferior colliculus of mammals. We have mapped the connections of the subnuclei of the torus semicircularis in Xenopus laevis to determine which receive auditory and which receive lateral line information. Relative to terrestrial anurans, the torus of X. laevis is hypertrophied and occupies the entire caudal, dorsal midbrain. Auditory input to the torus, that arising directly from the dorsal medullary nucleus, is present only in the laminar nucleus. The principal and magnocellular nuclei receive their input from the lateral line nucleus of the medulla. All three nuclei of the torus also have reciprocal connections with the superior olive and the nucleus of the lateral lemniscus. Ascending efferents from all three nuclei of the torus innervate central and lateral thalamic nuclei, and all have a weak reciprocal connection with the posterior thalamus. The laminar and magnocellular nuclei have reciprocal connections with the ventral thalamus, and all three nuclei of the torus receive descending input from the anterior entopeduncular nucleus. The laminar and magnocellular nuclei also receive descending input from the preoptic area. Based on our identification of toral nuclei and these results we assign a major function for the detection of water-borne sounds to the laminar nucleus and a major function for the detection of near field disturbances in water pressure to the principal and magnocellular nuclei.
Indexing terms: torus semicircularis, temporal processing, vocalizations, inferior colliculus
The torus semicircularis of anurans, which is homologous to the inferior colliculus in mammals, is an important area for processing auditory information. In particular, the torus is implicated in the ability to discriminate between different frog calls, which typically vary in beat or click rate (Rose and Capranica, 1983, 1984, 1985; Alder and Rose, 1998). Because neural processing of the temporal features of sounds is poorly understood, the torus provides an attractive target system from both computational and behavioral standpoints. The neuroanatomy of the torus has been extensively studied in terrestrial anurans (Wilczynski, 1981; Wilczynski and Neary, 1983; Feng and Lin, 1991), but in the aquatic anuran Xenopus laevis, data are sparse and conflicting (Paton et al., 1982; Nikundiwe and Nieuwenhuys, 1983; Will et al., 1985b; Lowe, 1986). Unlike adult terrestrial frogs, aquatic anurans maintain an intact lateral line system and this modality is also processed in the torus (Elepfandt, 1996); how lateral line and acoustic information interact is not known.
By what pathway does auditory information arrive at the torus? Xenopus has a modified tympanum, located just under the skin on the side of the head, into which the middle ear bones insert (Wever, 1985). Anuran acoustic end organs include the amphibian and basilar papillae, the saccule, and the lagena (Feng et al., 1975; Caston et al., 1977; Baird and Lewis, 1986). Auditory information derived from movement of the sensing membranes and hair cells within the auditory end organs is conveyed to the medulla by means of axons of the 8th nerve, which projects to the dorsal medullary nucleus, or DMN (Matesz, 1979; Wever, 1985; Will et al., 1985a; Will and Fritzch, 1988). The DMN projects to the superior olive and the nucleus of the lateral lemniscus; these together with the DMN, in turn, project to the midbrain auditory region, the torus semicircularis (Wilczynski, 1981; Wilczynski and Capranica, 1984).
The anuran torus itself includes three subnuclei: the principal, laminar, and magnocellular (Potter, 1965). The organization of the torus semicircularis in X. laevis superficially resembles that of terrestrial frogs. Neuroanatomic and electrophysiological studies suggested that auditory information goes primarily to the principal nucleus (Will et al., 1985b; Lowe, 1986). However, a 2-deoxyglucose study using deafened and hemideafened male X. laevis suggested instead that auditory information is restricted to the laminar nucleus (Paton et al., 1982). This subnucleus is further distinguished by the presence of cells that concentrate gonadal steroid hormones (Morrell et al., 1975; Kelley, 1980).
The torus semicircularis also processes a second sensory modality, the detection of near field changes in water pressure sensed by the lateral line system (Elepfandt, 1996). This input enters the central nervous system by means of the lateral line nerves, which innervate the lateral line nucleus (n.LL) located medial to the DMN (Will et al., 1985a). Cells of the n.LL project to the torus directly (Plassmann, 1980; Altman and Dawes, 1981; Lowe, 1986). In Xenopus, the lateral line system remains intact into adulthood, whereas in terrestrial frogs, the lateral line system degenerates during metamorphosis and is absent in the adult. Which subnuclei of the torus receive lateral line input has not been studied extensively; Lowe’s results (1986) suggest input to the magnocellular nucleus (as per Will et al., 1985b). In any case, the maintenance of a functional lateral line system in adults complicates the organization of the torus in Xenopus relative to terrestrial Anura. Whether the laminar nucleus receives any direct input from either sensory system is also unclear.
To determine which subnuclei of the torus receive auditory input and lateral line input, respectively, in Xenopus laevis, we carried out a detailed neuroanatomic study of the torus. We injected tracers into each subnucleus of the torus specifically and mapped their respective afferents and efferents. In addition, we used the method of Golgi to characterize the dendritic arborization patterns of neurons in each subnucleus. The information thus obtained will be helpful in understanding temporal processing of auditory and lateral line information in Xenopus laevis.
MATERIALS AND METHODS
Animals
Adult Xenopus laevis males were purchased from Nasco (Fort Atkinson, WI). Frogs were housed in polycarbonate tanks in dechlorinated water and maintained on a 12/12 light dark cycle with ad libitum feeding (frog brittle) three times a week. In all, 70 frogs were examined during the course of this study. The optimal survival time (one in which both anterograde and retrograde labeling were maximal) was determined in a series of pilot experiments. Although 2–3 days permitted the visualization of anterograde transport, this interval was inadequate for retrograde transport. A survival time of 7–10 days after injection revealed transport in both directions; anterograde labeling was not reduced relative to shorter survival intervals. All procedures involving the frogs conformed to the requirements of the NIH and Columbia’s Institutional Animal Care and Use Committee.
Neuroanatomic tracers
For in vivo injections, Fluoro-Ruby (Molecular Probes, 10,000 MW) was prepared as a 10% solution in 0.01 M phosphate buffer and front-loaded (by means of immersion of the tip) into a micropipette. For in vitro injections, we also used Fluoro-Ruby crystals; a saturated solution in double-distilled water (ddH2O) was allowed to dry onto the tip of a minuten pin (FST #26002-10). Small crystals were more effective in placing tracer into nuclei that lie just below the surface of the brain. With Fluoro-Ruby, retrograde connections are characterized by the labeling of cell bodies. Anterograde connections are characterized by synaptic boutons as well as a diffuse rhodamine labeling. Although some Fluoro-Ruby from the injection site occasionally crossed subnuclear boundaries, the discrete nature of the anterograde and retrograde labeling suggested that transport only occurred from the tissue that was disrupted by the pressure injection, e.g., injections into the principal nucleus yielded retrograde labeling in the lateral line nucleus and none in the DMN, and vice versa for injections into the laminar nucleus. For biocytin injections, 4% biocytin (Sigma) was front-loaded into micropipettes. Micropipettes (Sutter instruments; catalog no. BF100-78-10; borosilicate with filament: O.D. 1.0 mm, I.D. 0.78 mm) were pulled on a P-87 Brown/Flaming micropipette puller (Sutter instruments) and broken back manually to 24–30 µm O.D. for Fluoro-Ruby injections and 15–24 µm O.D. for biocytin injections.
In vivo injections
Frogs received intraperitoneal injections of 1.0 ml of a 1.3% MS-222 solution (3-aminobenzoic acid ethyl ester; Sigma). When deeply anesthetized (15 to 20 minutes), the skin of the dorsal surface of the head was incised, retracted and the bone covering the dorsal midbrain exposed. This portion of the skull was removed with a drill (Dremel variable speed model 395 type 5 with 106 Dremel engraving bit), the dura and pia were retracted and the dorsal surface of the left midbrain exposed.
For all injections, the micropipette was positioned just anterior to the cerebellum with a micromanipulator (Narishige). For injections into the laminar and principal nuclei, the pipette was placed approximately midway between the medial and lateral margins of the torus; the position for the injections into the magnocellular nucleus was approximately two-thirds the distance from the medial margin. For injections into the laminar nucleus, the micropipette was advanced ventrally approximately 0.8 mm deep. For principal injections, the micropipette was inserted into the same spot but was angled 30 degrees caudally and advanced 1.0 mm deep. For the magnocellular injection the pipette was angled 5 degrees caudally and advanced 1.1 mm deep. The Fluoro-Ruby solution was ejected by using a picosprizter (Picosprizer II; General Valve Co.) set at 15 msec (pulse duration) and 30 psi (pulse pressure). Three to four injections were made, each separated by 1 minute. The skin overlying the injection site was sutured, and the animal was allowed to recover for 7–10 days. The animal was then very deeply anesthetized by injecting 1.0 ml of 1.3% MS-222 intraperitoneally, and perfused transcardially with approximately 60 ml of a 0.6% NaCl and 100 ml of a 4% paraformaldehyde solution in phosphate buffered saline (PBS; pH 7.4). The brain was removed, placed in a 20% sucrose in 4% paraformaldehyde solution overnight, frozen in embedding medium (Tissue-Tek 4583), and sectioned at 20–30 µm on a cryostat (Bright Instrument Company). After dehydration, sections were cover-slipped with cytoseal (VWR); alternate sections were cresyl violet stained to facilitate identification of brain nuclei.
Characterization of the strength of anterograde and retrograde connections was as follows: If 10–30 synaptic boutons were present per section, the finding was designated as a weak anterograde connection. If 100–200 synaptic boutons were found per section, the finding was considered a strong anterograde connection. The area around the synaptic boutons in a nucleus with strong anterograde inputs exhibits higher intensity fluorescence than the rest of the neuropil. If 1–3 cell bodies were present per section, the retrograde connection was designated as weak. If 10–40 cells were found per section, the retrograde connection was considered strong.
Biocytin injections
The dorsal midbrain was exposed and biocytin injected by means of a micropipette, as described above. Animals recovered for 8–10 hours and were then perfused as above. Brains were sectioned on a sliding microtome (American Optical) at 100 µm. Sections were collected into PBS (10 mM), washed, peroxide quenched (0.5 ml of 30% H2O2 in 29.5 ml of PBS) for 20 minutes, washed again, and reacted by using a biotin-avidin system (Vectastain ABC kit) overnight. The sections were then washed in 0.02 M phosphate buffer (PB) and reacted by using diaminobenzidine (10 mg of DAB dissolved in 10 ml of ddH2O, to which 15 ml of 0.2 M PB and 0.35 ml of 8% nickel ammonium sulfate is added) for 10 minutes. To start the color reaction, two drops of 1% H2O2 were added to each well and left until sections turned gray. The sections were then washed in 0.02 PB 3× for 3 minutes, mounted onto Super Frost Plus–subbed slides (Fisher), dehydrated, and cover-slipped by using Permount.
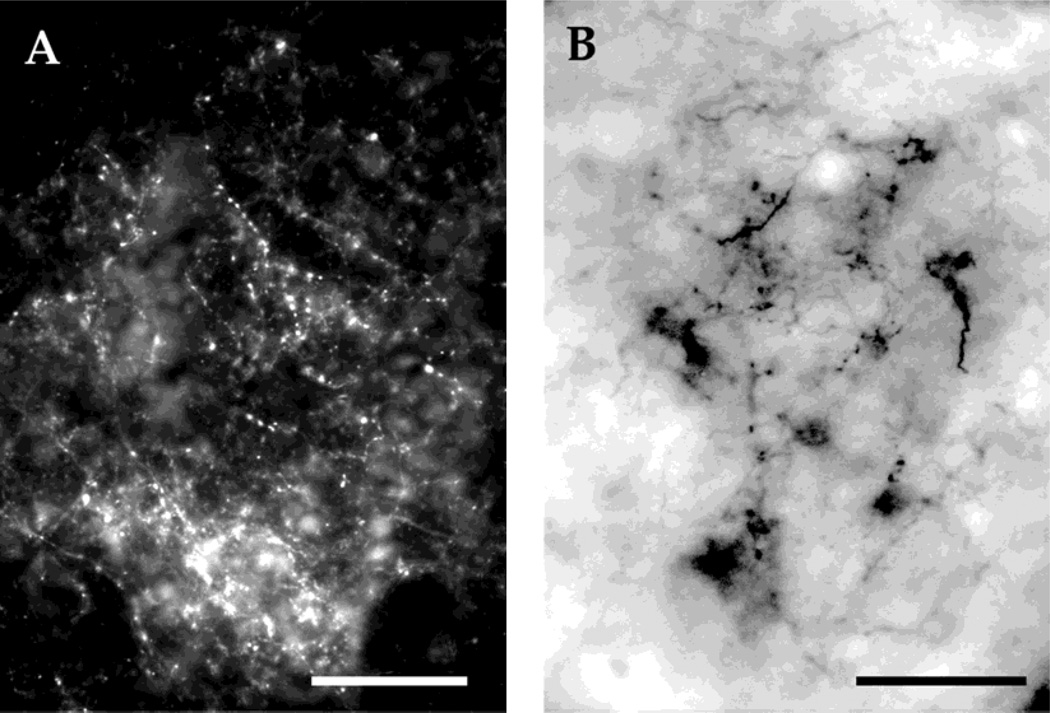
Fluoro-Ruby injections were found to yield many more synaptic boutons than biocytin, thus permitting a more substantive assessment of the strength of connections between nuclei (Fig. 1A,B). In addition, retrograde labeling was absent in the biocytin injections. Anterograde connections to the central thalamus in Fluoro-Ruby injections were confirmed with large biocytin injections into the torus.
Fig. 1.
A: Synaptic boutons in the central thalamus after a Fluoro-Ruby injection into the torus. B: Synaptic boutons in the central thalamus after a biocytin injection into the torus. Scale bars = 200 µm in A, 50 µm in B.
In vitro injections
The dorsal thalamus and medulla are very difficult to inject in vivo due to proximity to the choroid plexus; we thus adopted an isolated, perfused brain preparation (Luksch et al., 1996). Xenopus laevis males were injected with 1.0 ml of 1.3% MS-222, and, once deeply anesthetized (~15 minutes), were perfused on ice with approximately 60 ml of oxygenated saline (11 mM glucose, 75 mM NaCl, 25 mM NaHCO3, 2 mM KCl, 0.5 mM MgCl2, and 2 mM CaCl2). After perfusion, for thalamic injections, the brain was removed and injected with 15% tetramethylrhodamine dextran amine (3,000 molecular weight, lysine fixable; Molecular Probes D-3308) dissolved in 0.01 M phosphate buffer. The three or four injections were each separated by 1 minute. For DMN injections, tetramethylrhodamine dextran amine was dissolved in water and dried onto the tip of a minuten pin. This pin was advanced into the DMN with a micromanipulator. Brains were maintained at 4°C in continuously oxygenated saline for 3 to 4 days. After fixation overnight in a 20% sucrose in 4% paraformaldehyde solution, brains were cryostat sectioned as for in vivo tracing. Strong and weak connections were characterized as above.
Golgi staining
Each frog was killed by decapitation, and its brain was immediately dissected out and processed with a variant of the Golgi-Cox method described by Ramon-Moliner (1970). Fresh nervous tissue was impregnated in a solution of mercuric chloride, potassium chromate, potassium dichromate, and sodium tungstate for approximately 3 weeks. After impregnation, the tissue was washed, alkalinized in a lithium hydroxide, potassium nitrate solution, washed in a weak acetic acid solution, and the dehydrated and embedded in soft Epon for sectioning (120 µm in the transverse, horizontal, and sagittal planes). Sections were then mounted on clean glass slides and cover-slipped with Permount. As is typical for this Golgi method (Ramon-Moliner, 1970), we observed only Golgi-stained dendrites and not axons in this study. Three brains were processed and sectioned in this manner.
Neuroanatomic designations
To identify toral nuclei, we relied on the neuroanatomic nomenclature of Kelley et al. (1975). To identify diencephalic nuclei, we used the designations of Neary and Northcutt (1983). For medullary nuclei, we used the designations of Will et al. (1985a). In particular, we follow their usage for the nucleus receiving lateral line input—lateral line nucleus—rather than “medial octavolateralis nucleus” because in Xenopus laevis, neither lateral line nerve enters the rhombencephalon with the eight nerve.
Photomicrography
Photomicrographs were taken with a digital camera (Spot; Diagnostic Instruments, software version 3.02) and prepared for publication by using Adobe Photoshop. The color images were desaturated, and brightness and contrast were adjusted to preserve outlines of the section and cellular detail. Uneveness of illumination was adjusted by increasing the brightness of peripheral dark regions of the image to match the rest of the section; the borders between illumination zones were smoothed by using the “smart blur” function (radius and threshold: 15.8).
RESULTS
The nuclear organization of the torus semicircularis
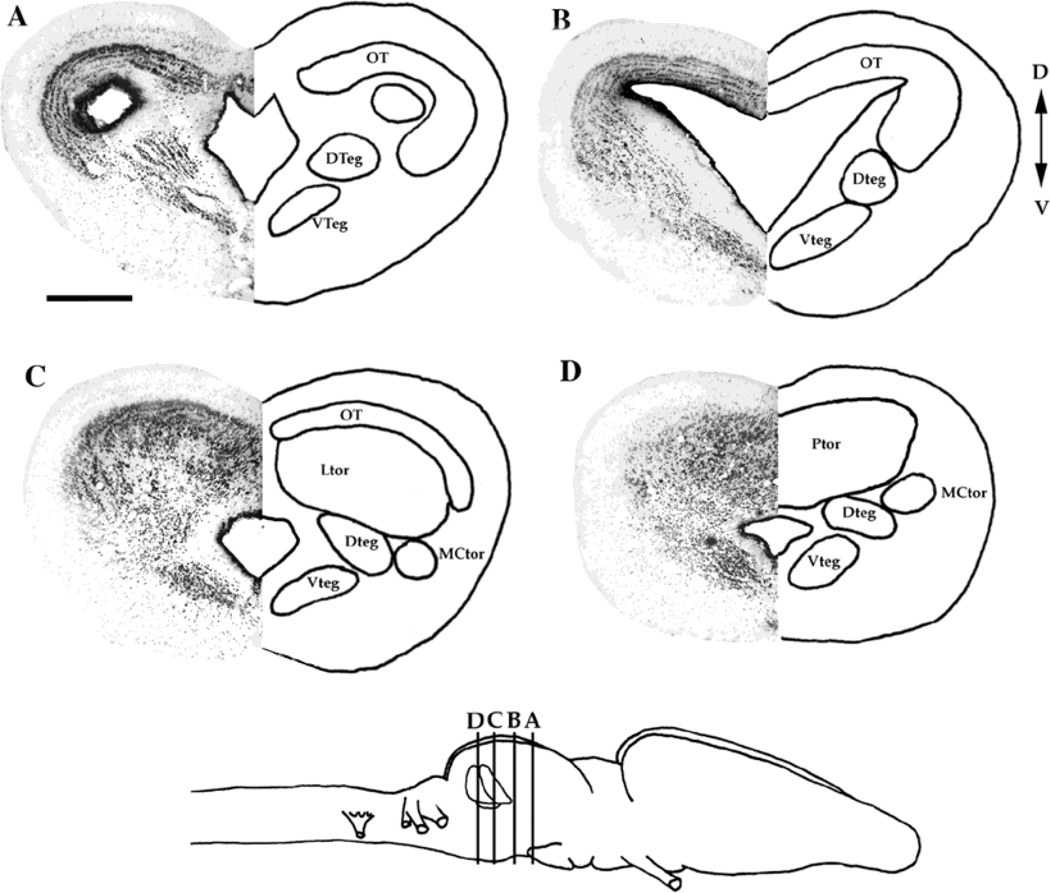
The torus semicircularis’ three major divisions include the laminar, principal, and magnocellular nuclei (Potter, 1965). These nuclei are readily identified in transverse section in most terrestrial frogs. However, in Xenopus laevis, the principal and laminar nuclei are hypertrophied and lie caudal to the tectal ventricle (Figs. 2, 3). The laminar nucleus consists of parallel sheets of cells that extend dorsoventrally directly caudal to the tectal ventricle; the nucleus is most easily visualized in horizontal or sagittal sections (Fig. 3). The principal nucleus lies caudal to the laminar nucleus, and its cells are more diffusely arranged than the laminar nucleus (Figs. 3, 4). In transverse section, it is difficult to distinguish the border between the principal and laminar nuclei, because the laminar arrangement of cells is not apparent as it is in horizontal section (compare Fig. 2C with 3B). The laminar nucleus occupies the dorsal mesencephalon just underneath the caudal extent of the optic tectum (Fig. 2C). Proceeding rostral to caudal in transverse section, the laminar nucleus is present just posterior to the caudal pole of the tectal ventricle. The principal nucleus is found caudal to the laminar nucleus in the dorsal mesencephalon posterior to the caudal pole of the optic tectum (Fig. 2D). The magnocellular nucleus is characterized by large cells that are lateral and ventral to the rest of the torus, and can be easily visualized, for this reason, in any plane (Fig. 2C,D).
Fig. 2.
A–D: Nissl-stained transverse sections of the mesencephalon, flanked by a schematic diagram of nuclei locations. Bottom: Schematic diagram (side view) of the Xenopus laevis brain, showing the locations of the sections in A–D. Anterior is to the right. D, dorsal; V, ventral. For abbreviations, see list. Scale bar = 500 µm in A (applies to A–D).
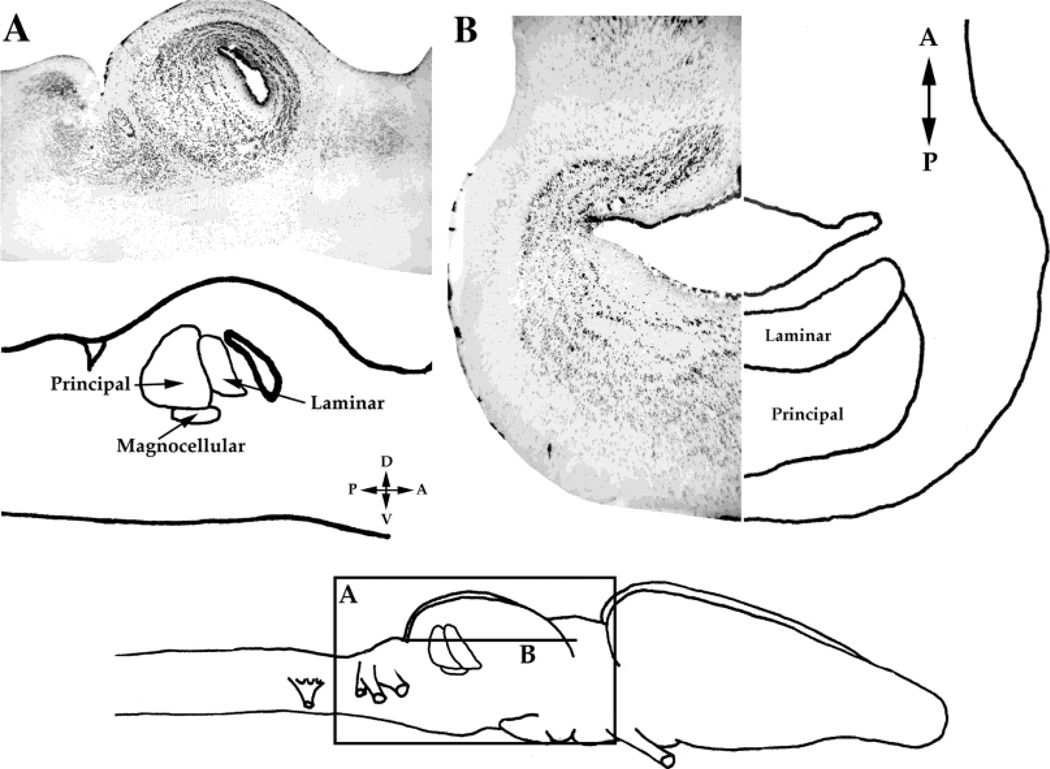
Fig. 3.
A: Nissl stain of a sagittal section through the midbrain with a schematic diagram of the toral nuclei. B: Nissl stain of a horizontal section, flanked by a schematic diagram of the toral nuclei. Bottom: Schematic diagram of the locations of the sections in A and B. A, anterior; P, posterior; D, dorsal; V, ventral.
Fig. 4.
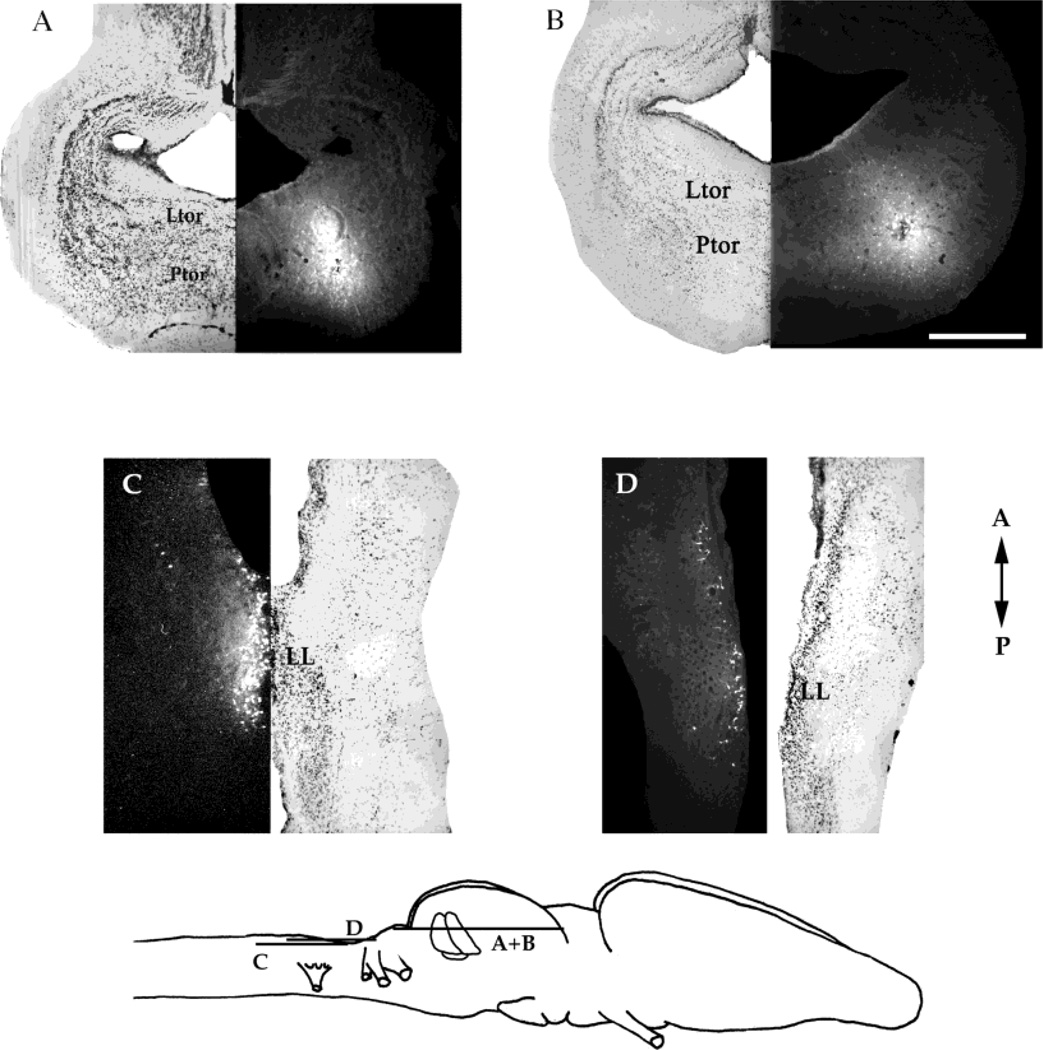
Injections into the laminar nucleus. A,B: Horizontal sections through the mesencephalon, with the locations of the injections and the corresponding Nissl-stained section. C: Horizontal section through the medulla in the same brain as A, with a corresponding Nissl-stained section. Apparent labeling in the cerebellum is autofluorescence. D: Transverse section through the medulla from another brain that received a laminar nucleus injection together with the corresponding Nissl-stained section. E: Schematic diagram of the locations of the sections in A–D. A, anterior; P, posterior; D, dorsal; V, ventral. For other abbreviations, see list. Scale bar = 500 µm in A (applies to A–D).
Ascending afferents to the torus
Four injections were made into the laminar nucleus of the torus (Fig. 4A,B). Labeling in the medulla included weak anterograde and strong retrograde signals (as defined in Materials and Methods section) in the DMN, which is the auditory nucleus of the medulla (Fig. 4C,D). This labeling is much stronger in the contralateral than in the ipsilateral DMN. Thus, we conclude that the laminar nucleus is reciprocally connected to the DMN; the projection from the DMN to the contralateral laminar nucleus is a strong one. In vitro injections into the DMN support this conclusion (data not shown).
Four injections were limited to the principal nucleus (Fig. 5A,B). Strong retrograde and weak anterograde labeling in the contralateral lateral line nucleus of the octavolateralis area of the medulla were present (Fig. 5C,D), along with weaker labeling of this nucleus on the ipsilateral side. We conclude that the principal nucleus is reciprocally connected to the contralateral, lateral line nucleus of the medulla; the ipsilateral projections are weak.
Fig. 5.
Injections into the principal nucleus. A,B: Horizontal sections through the mesencephalon with the locations of the injections and the corresponding Nissl-stained sections. C,D: Horizontal sections of the medulla in the same brains as A and B respectively, with the corresponding Nissl-stained sections. Bottom: Schematic diagram of the locations of the sections in A–D. A, anterior; P, posterior. For other abbreviations, see list. Scale bar = 500 µm.
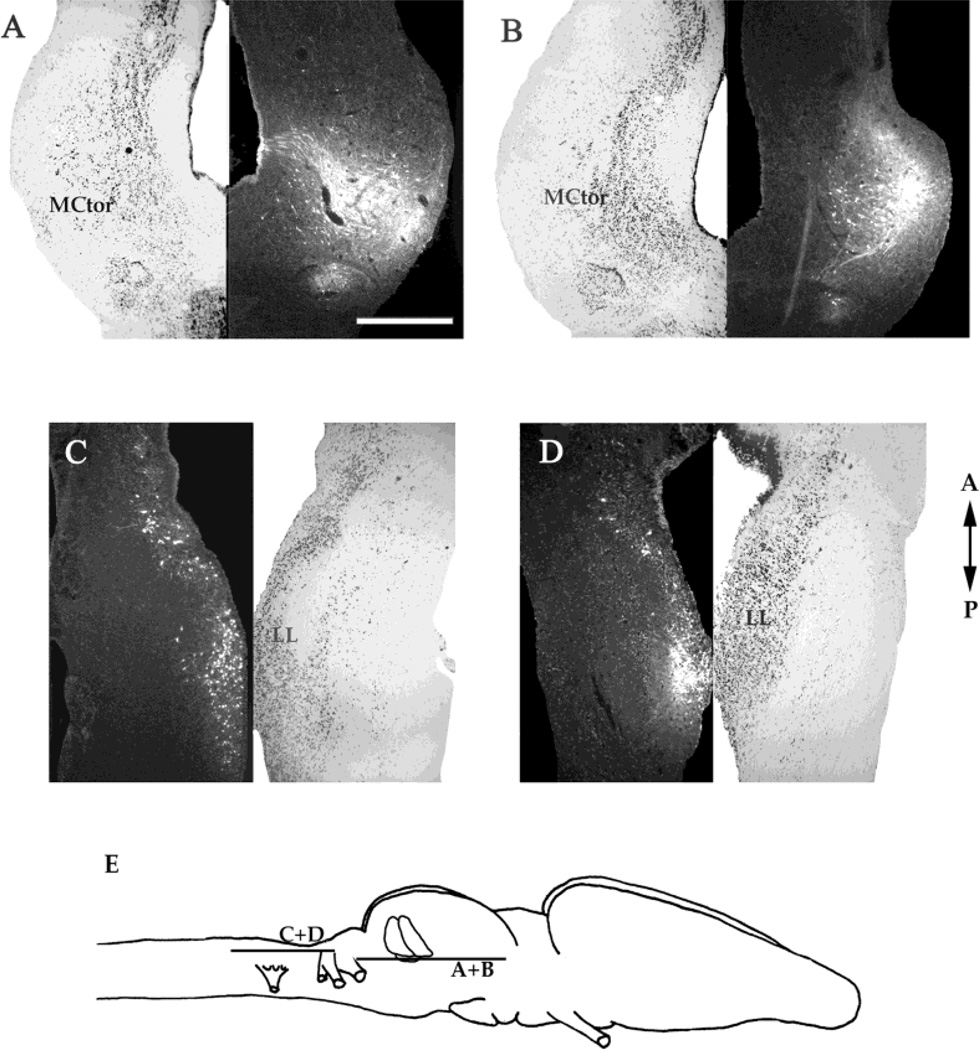
Three injections were limited to the magnocellular nucleus (Fig. 6A,B). Strong retrograde and weak anterograde labeling was observed in the contralateral lateral line nucleus. Weaker labeling in both directions was present on the ipsilateral side. We conclude that the major input to the magnocellular nucleus is the contralateral medullary lateral line nucleus.
Fig. 6.
Injections into the magnocellular nucleus. A,B: Horizontal sections through the mesencephalon with the locations of the injections and the corresponding Nissl-stained sections. C,D: Horizontal sections through the medulla in the same brains as A and B, respectively, with the corresponding Nissl-stained sections. E: Schematic diagram of the locations of the sections in A–D. A, anterior; P, posterior. For other abbreviations, see list. Scale bar = 500 µm.
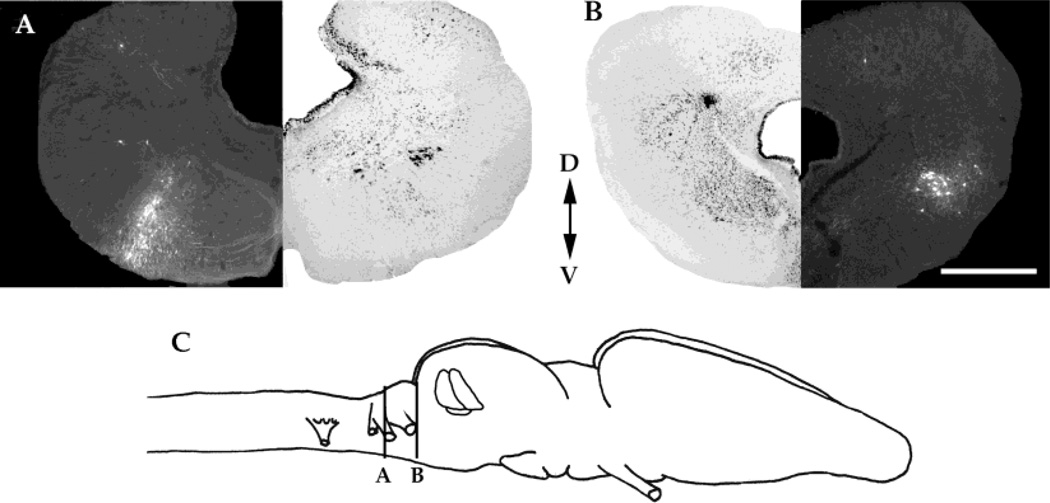
All three toral nuclei have strong reciprocal connections with the ipsilateral superior olivary nucleus (SON) (Fig. 7A). All nuclei project to the ipsilateral nucleus of the lateral lemniscus and receive projections from the contralateral nucleus of the lateral lamniscus (Fig. 7B). In addition, all three nuclei receive weak input from a vestibular nucleus of the medulla, the lateral octavus nucleus (data not shown).
Fig. 7.
A: Transverse section through the medulla of a brain that received a laminar nucleus injection with a corresponding Nissl-stained section. B: Transverse section through the caudal mesencephalon of the same brain as A with a corresponding Nissl-stained section. C: Schematic diagram of the locations of the sections in A and B. D, dorsal; V, ventral. Scale bar = 500 µm in B (applies to A,B).
Connections between nuclei of the torus
Strong reciprocal labeling among the different subnuclei of the torus was observed; all subnuclei exhibited both anterograde and retrograde labeling. Injection sites were confined not only to particular subnuclei but also to one side of the torus (e.g., Figs. 4–6). Anterograde labeling was characterized by synaptic boutons and varicosities (see Fig. 1 for illustration), ensuring that labeling was not due to diffusion of tracer from the injection site. Labeling is strong between ipsilateral partners and weak between contralateral partners. For example, the laminar nucleus projects to the principal and magnocellular nuclei on the ipsilateral side more strongly than the contralateral side. However, the strongest intratoral connections are between subnuclei of the same type, e.g., ipsilateral laminar and contralateral laminar.
Ascending efferents from the torus
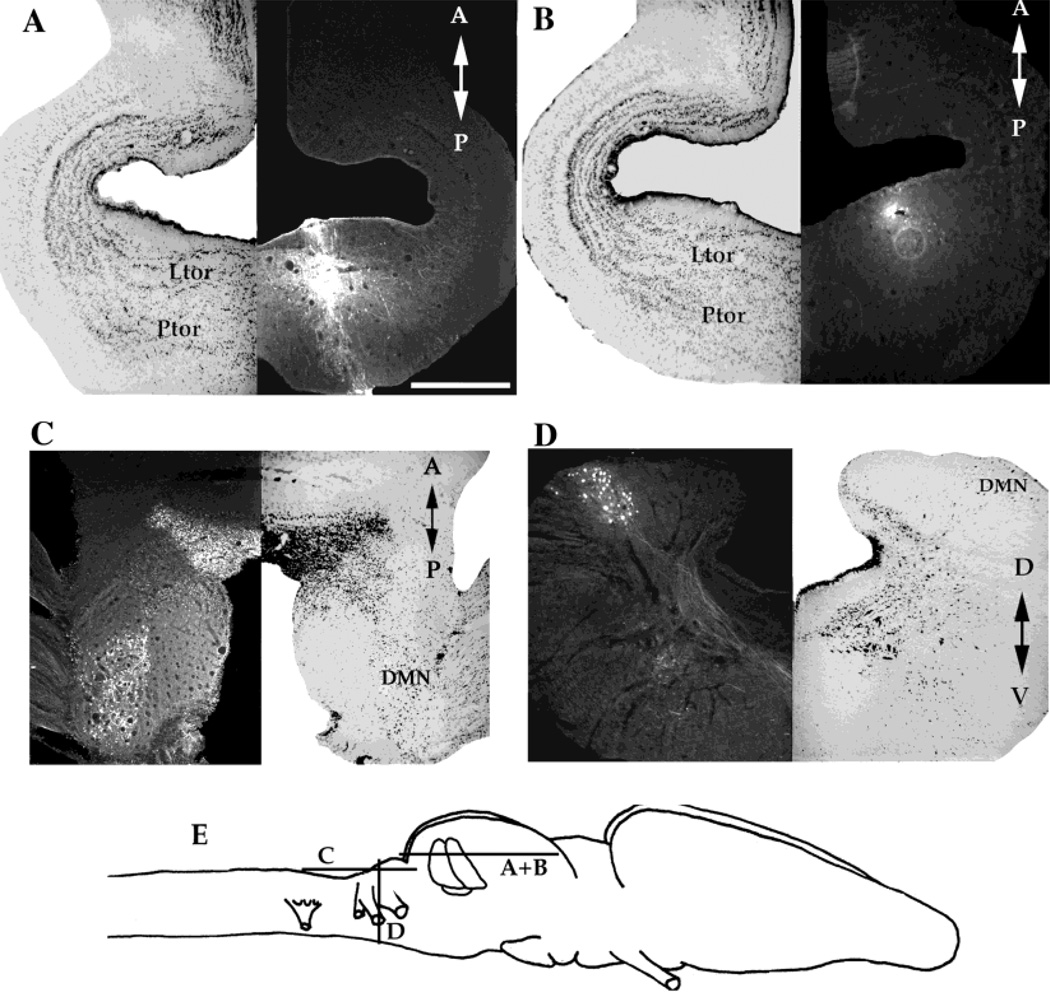
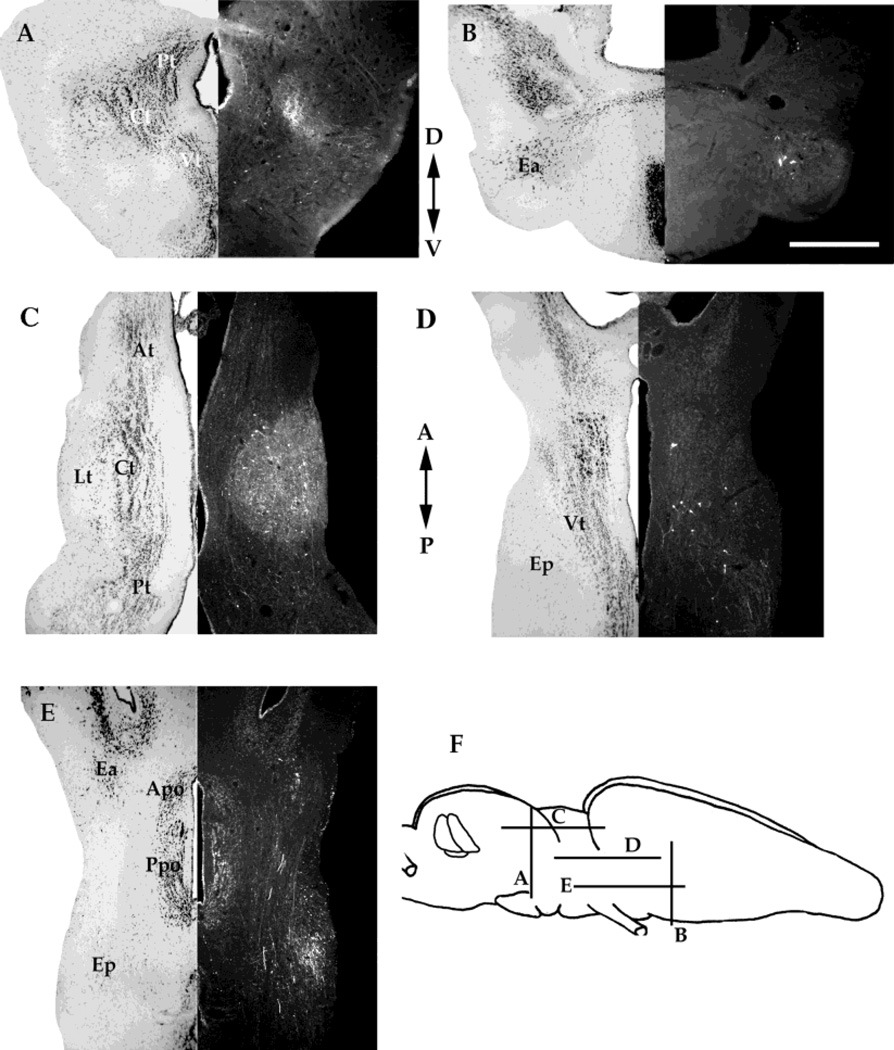
The pattern of labeling in the thalamus after principal, laminar, and magnocellular nuclei is similar. Injections in all three nuclei yielded extremely heavy anterograde labeling in the central (Fig. 8A,C) and lateral (Fig. 8C) thalamic nuclei. There were also many retrogradely labeled neurons in the central and lateral thalamic nuclei after magnocellular injections (Fig. 8C), a few in the laminar injections, and none after injections into the principal nucleus (Fig. 8A). All three toral nuclei evince a weak reciprocal connection with the posterior thalamus (Fig. 8A,C). In the laminar and magnocellular injections, a reciprocal connection with the ventral thalamus (Fig. 8D) is apparent; the retrograde signal is more prominent than the anterograde. The ventral thalamus is not labeled after injections of the principal nucleus. All labeling in thalamic nuclei is stronger on the ipsilateral side. Also present after the laminar and magnocellular injections is retrograde labeling in the preoptic area of the hypothalamus (Fig. 8E). Injections in all three nuclei also yielded a strong retrograde signal in the ipsilateral anterior entopeduncular nucleus (Fig. 8B,E). The magnocellular injections also yielded both anterograde and retrograde labeling in the posterior entopeduncular nucleus (Fig. 8E). The anterior entopeduncular nucleus is located laterally and ventrally in the rostral end of the diencephalon, just below the caudal extent of the telencephalon. The posterior entopeduncular nucleus is located ventrally and laterally in caudal diencephalon.
Fig. 8.
A: Transverse section through the diencephalon of a brain that received an injection into the principal nucleus. B: Transverse section through the caudal telencephalon and rostral diencephalon of a brain that received an injection into the laminar nucleus. C: Horizontal section through the diencephalon of a brain that received an injection into the magnocellular nucleus. D: Horizontal section through the diencephalon of a brain received a magnocellular nucleus injection. E: Horizontal section through the hypothalamus of a brain that received a magnocellular nucleus. F: Schematic diagram of the locations of sections in A–E. A, anterior; P, posterior; D, dorsal; V, ventral. For other abbreviations, see list. Scale bar = 500 µm.
Dendritic orientation of neurons in nuclei of the torus
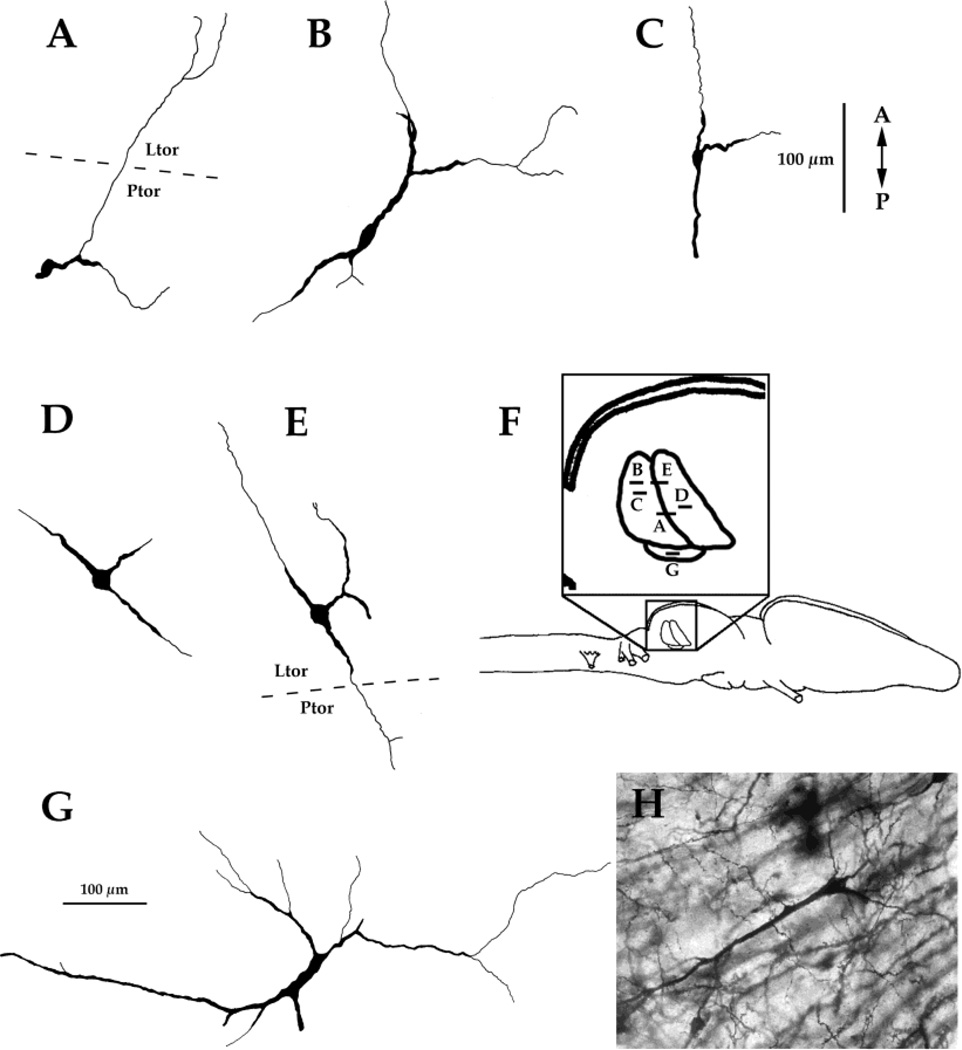
Because neurons can receive input on distal dendrites, which may extend beyond the subnuclear borders occupied by their cell bodies, we used the Golgi method to characterize the dendritic arborization patterns of neurons from each subnucleus of the torus. Many cells observed in the principal nucleus have dendrites that extend into the laminar nucleus (Fig. 9A). Reciprocally, cells observed in the laminar nucleus often have dendrites that extend into the principal nucleus (Fig. 9E). A cell in the magnocellular nucleus with a long dendrite that extended into the principal nucleus is illustrated in Figure 9G. A characteristic cell type, found in both the laminar and principal nuclei, has dendrites which project both rostrally and caudally, and an additional dendrite that projects laterally (Fig. 9C–E).
Fig. 9.
Golgi drawings. A,B,C: Cells from the principal nucleus. D,E: Cells from the laminar nucleus. G: A cell from the magnocellular nucleus. F: Schematic diagram of the locations of these cells. H: Example of a Golgi-stained neuron. A, anterior; P, posterior. For other abbreviations, see list.
DISCUSSION
Our results indicate that, in Xenopus laevis, the major source of afferents to the principal and magnocellular nuclei of the torus is the medullary lateral line nucleus. The only toral nucleus to receive direct projections from the DMN, the major medullary acoustic nucleus, is the laminar nucleus. The medullary connections of the laminar and principal nuclei are stronger contralaterally than ipsilaterally and are reciprocal; only contralateral connections were observed for the magnocellular nucleus. All three toral nuclei are reciprocally connected; this connection is predominantly ipsilateral. Dendrites of neurons in the laminar nucleus extend into the principal nucleus and vice versa; dendrites of neurons in the magnocellular nucleus extend into the principal nucleus. All three nuclei of the torus receive weak input from the lateral octaval nucleus, which receives input from all four acoustic end organs (Will and Fritzch, 1988) in addition to vestibular input. These projections present the possibility that the principal and magnocellular nuclei receive indirect auditory input in addition to their input from the lateral line system.
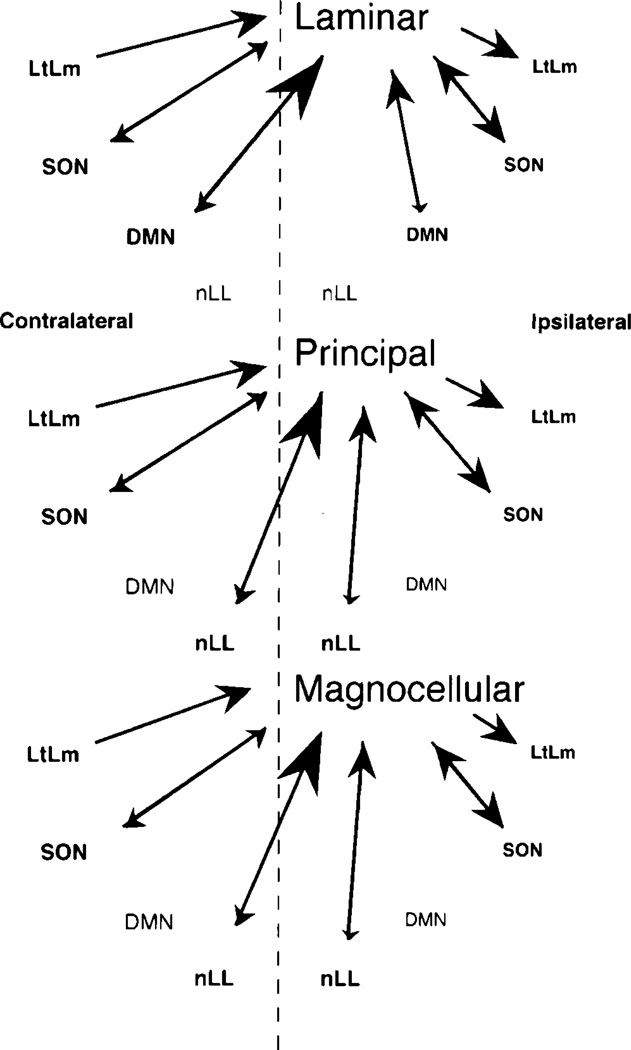
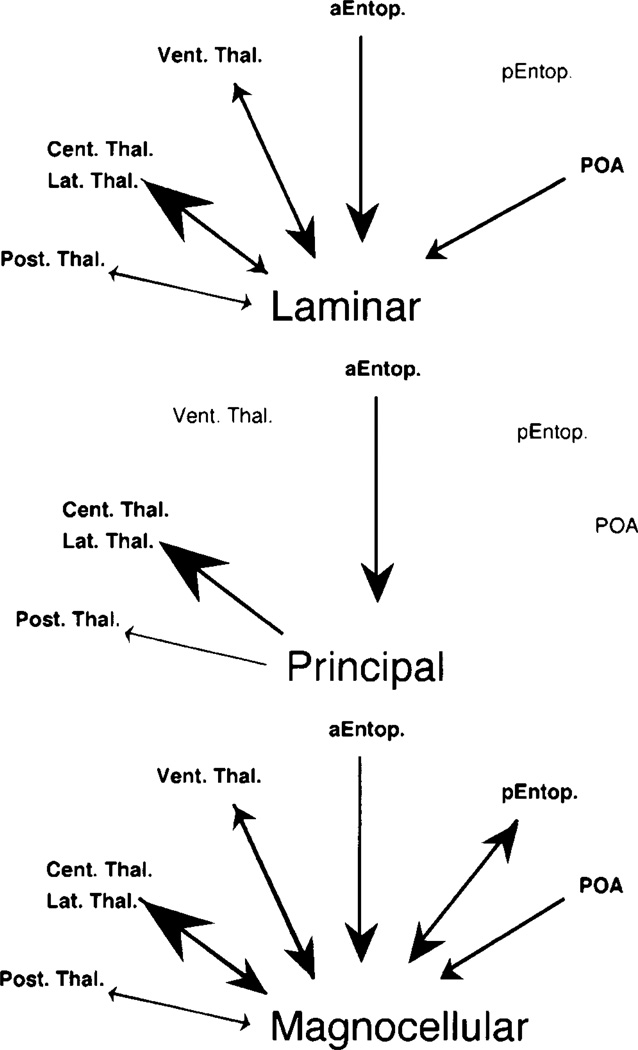
Caudal to the midbrain, all three toral nuclei are reciprocally connected with the superior olivary nucleus and the nucleus of the lateral lemniscus. Rostrally, all three nuclei send a major projection to ipsilateral central and lateral thalamic nuclei and receive strong input from the anterior entopeduncular nucleus. The principal nucleus is distinguished from the laminar and magnocellular nuclei in that its thalamic connections are not reciprocal, it does not project to the ventral thalamus, nor does it receive a projection from the preoptic area. The pattern of connections described here is diagrammed in Figures 10 and 11.
Fig. 10.
Schematic diagram of the medullary afferents and efferents to each subnucleus of the torus. The size of the arrow indicates the strength of the connection. nLL, lateral line nucleus. For other abbreviations, see list.
Fig. 11.
Schematic diagram of telencephalic, diencephalic and mesencephalic connections of the toral subnuclei. The size of the arrow indicates the strength of the connection. aEntop., anterior entopeduncular nucleus; pEntop. posterior entopeduncular nucleus; POA, preoptic area; Vent. Thal., ventral thalamus; Cent. Thal., central thalamus; Lat. Thal., lateral thalamus; Post. Thal., posterior thalamus.
Relation to previous studies in Xenopus laevis
Laminar nucleus of the torus semicircularis
In general, the results described here are similar to those reported for other anurans, including terrestrial frogs, and confirm that the basic pattern of auditory processing is not substantially altered in Xenopus laevis, despite its entirely aquatic habitat (a secondary adaptation in the genus; Trueb, 1996). These similarities are discussed in more detail below. However, our results do conflict with those of Will et al. (1985b) and Lowe (1986) in Xenopus laevis. Will et al. (1985b) focuses primarily on projections of the medullary nuclei (studied with HRP); these were confirmed by injections into the torus, and no ascending projections from the torus are described. Unfortunately, the only illustration of the torus is a schematic sagittal section, apparently somewhat lateral to that shown here in Figure 3, in which three regions in the torus are outlined; it is unclear which correspond to which toral nuclei. The illustrated injection into the magnocellular nucleus seems also to include what we would consider the principal nucleus. In the schematic indication, the injection site is very caudal and medial and not located in a position where we observe the large cells characteristic of the magnocellular nucleus. This study describes a projection from the DMN to the “rostroventral principal nucleus;” we believe the “rostroventral principal nucleus” is actually the laminar nucleus.
The confusion in nomenclature in both this study and in that of Lowe (1986) arises from adopting the designations of Nikundiwe and Nieuwenhuys (1983) for toral nuclei. This latter work, a descriptive study, relied heavily on the neuroanatomy of terrestrial frogs in the identification of toral nuclei from transverse sections. As described originally in Morrell et al. (1975) and in Kelley et al. (1975), the nuclear organization of the torus in Xenopus differs substantially from terrestrial anurans. What the above authors label as the principal nucleus is actually the ventral extent of the laminar nucleus, which in Xenopus is oriented dorsoventrally instead of rostrocaudally, as it is in terrestrial anurans. The region of the torus that, in Lowe (1986), shows the strongest field potentials in response to auditory stimulation is, we believe, in the laminar rather than in the principal nucleus. This conclusion is supported by 2-deoxyglucose studies of auditory-evoked activity in the torus (Kelley, 1980; Paton et al., 1982). The laminar nucleus (the rostral nucleus in horizontal sections) shows heavy labeling in response to auditory stimulation. This labeling is absent contralaterally when the animals are deafened unilaterally and is absent on both sides when the animal is bilaterally deafened. The response in the principal nucleus (the caudal nucleus in horizontal sections; Kelley, 1980) is unaffected by deafening. Because, as we show here, the laminar nucleus receives the only direct input from the DMN, and because the DMN is very well established as the first order target of input from the acoustic end organs (Will et al., 1985a), we conclude that the laminar nucleus is the primary auditory nucleus of the torus semicircularis in Xenopus laevis.
Principal nucleus of the torus semicircularis
What are the inputs to the principal nucleus? We demonstrate here that the principal nucleus is strongly and reciprocally connected to the medullary lateral line nucleus. Golgi impregnations reveal that neurons in the laminar nucleus extend dendrites into the principal nucleus and vice versa. Thus, it is likely that neurons in each of these nuclei have access to information from both their principal sensory modality and from the secondary modality; sensory interactions between these modalities have been described in X. laevis torus (Zittlau et al., 1985). In addition, these toral nuclei receive input from neurons in the superior olive and nucleus of the lateral lemniscus.
In their study, Will et al. (1985b) describe strong connections between the lateral line nucleus and the magnocellular nucleus but not the principal nucleus. However, the injection into the magnocellular nucleus illustrated probably included a large portion of the principal nucleus as described here so that, not withstanding a difference in nomenclature, our results agree. In their study of the octavo-lateralis system, Will et al. (1985b) did not describe any n.LL input to the superior olive; medullary olivary afferents were restricted to the DMN. Injections into the olive resulted in a strong ipsilateral projection “to the Tp [principal nucleus] …. the terminal field of superior olivary fibers lies caudal to that of secondary octavus fibers, i.e., in the central part of the Tp.” This more caudal region corresponds to the region we have designated as the principal nucleus. Taken together, these results suggest that the principal nucleus receives a direct projection from the lateral line nucleus and two indirect projections from the DMN, one by means of the extension into the laminar nucleus of principal neuron dendrites and a second by means of DMN projections to the superior olive. A third input to the principal nucleus arises from the contralateral lateral octaval nucleus and was also described by Will et al. (1985b).
Magnocellular nucleus of the torus semicircularis
In this study, injections of the magnocellular nucleus yielded labeled cells in both the anterior and the posterior lateral line nucleus. In addition, a weak contralateral projection from the lateral octaval nucleus was present. Dendrites of magnocellular neurons extend into the principal nucleus. These results indicate that activity in the magnocellular nucleus is mostly subject to input from the lateral line nucleus; direct or indirect input from auditory nuclei of the medulla was not observed, aside from putative auditory input from the lateral octaval nucleus. Lowe (1986) reports some auditory responses from two animals in the lateral torus; absent histologic verification, it is not clear whether this activity was in the lateral aspect of the laminar nucleus or the magnocellular nucleus; the former seems more likely.
Toral nuclei of terrestrial frogs
A study by Feng and Lin (1991) in Rana pipiens differentiates the connections of these three nuclei of the torus. In R. pipens it is the principal nucleus, and not the laminar nucleus, which receives most of the direct input from the DMN (Feng and Lin, 1991). The magnocellular nucleus also receives a projection from the DMN and acoustically evoked single and multiunit activity was readily recorded in both nuclei. The laminar nucleus receives input from the ipsilateral superior olive and nucleus of the lateral lemniscus. The input to toral nuclei from the superior olive in X. laevis is also mostly ipsilateral but, in contrast, input from the nucleus of the lateral lemniscus is contralateral.
In X. laevis, the laminar nucleus projects to the central and lateral thalamic nuclei and to ventral thalamus; a similar set of projections was described for the laminar nucleus of Rana pipiens (Feng and Lin, 1991). In X. laevis, the principal nucleus projects robustly to central and lateral thalamic nuclei but not to ventral thalamus; in R. pipiens, the projection to thalamic nuclei is very sparse and no projection to ventral thalamus was reported (Feng and Lin, 1991). The projections of the magnocellular nucleus may differ between these two species. In X. laevis, the connections are very similar to those of the laminar nucleus, whereas in R. pipiens, more extensive projections to thalamus were described (Feng and Lin, 1991); other studies, however, suggest that the central nucleus is the major thalamic target of all toral nuclei in Rana (Wilczynski, 1981). In both genera, retrograde labeling patterns mirror anterograde patterns, suggesting that connections are reciprocal. Toral afferents from the anterior entopeduncular nucleus are common in anurans (Wilczynski, 1981).
Given the very different afferents to the laminar nuclei of X. laevis and Ranids, it is reasonable to ask whether these nuclei are, in fact, homologous. Might what we describe as the laminar nucleus actually be a subnucleus of the principal, for example, or might the nuclei be interchanged in position? Without a careful series of developmental studies on the origins of the nuclei, their birthdates and patterns of cell migration, the question cannot be addressed definitively. The information available to us is cytoarchitectonic and connectional. In X. laevis three major toral nuclei can readily be distinguished in Nissl-stained sections, provided that appropriate planes of section are used. The magnocellular nucleus contains large cells and is located laterally and caudally; the principal nucleus extends caudad to the laminar nucleus, and the latter is distinguished by a clear laminar appearance (in horizontal section). In Rana, the laminar nucleus is located immediately ventral to the tectal ventricle with the principal nucleus just below it. Taking into account the relative hypertrophy of the torus in general in Xenopus (relative to Rana), we envisage that both nuclei are shifted caudally and somewhat dorsally. In X. laevis, the optic tectum overlies the torus only in its most anterior extent; caudally, the torus is the only component of the dorsal mesencephalon. In Ranids, the torus is, instead, tucked under the tectum.
The relative hypertrophy of the torus in Xenopus is very likely related to the maintenance of lateral line input and to the central role of this modality and the auditory modality in a completely aquatic and nocturnally active species with a rich vocal repertoire (Kelley and Tobias, 1999). An analogy is the hypertrophy of the inferior colliculus of echolocating bats relative to terrestrial mammals. It is not clear whether lateral line input to the principal nucleus is a primitive or a derived character in the Anura; because all anuran tadpoles have a lateral line system, the question can be tackled experimentally. What is clear is that, in X. laevis, the principal nucleus is devoted largely to the lateral line system, whereas the laminar nucleus is auditory. These findings suggest the laminar nucleus as a prime candidate for the sorts of temporal processing neurons (rate sensitive) that form the initial motivation for this study.
Functional implications
The torus semicircularis integrates many modalities of sensory information including lateral line (Plassmann, 1980; Altman and Dawes, 1981; Lowe, 1986) auditory, and somatosensory (Munoz et al., 1995). In addition, all three nuclei of the torus project to the optic tectum (Zittlau et al., 1988). This integration is similar to that seen in midshipman fish, a species that also exhibits extensive reciprocal interactions between the auditory and lateral line regions of the midbrain (Weeg and Bass, 2000). Integration across modalities could help the frog orient to the source of a call. Male frogs can use several different senses to find a nearby female (Aronson and Noble, 1945), and the torus semicircularis is well placed to perform such a task.
The torus semicircularis has also been implicated in temporal processing of auditory signals, an important computational task in anurans. Temporal processing is necessary to discriminate different frog calls, which are typically amplitude modulated at different rates. A large proportion of cells in the torus are sensitive to particular rates of amplitude modulation (Rose and Capranica, 1983, 1985). If the same holds true for Xenopus laevis, it is most likely that cells of this type would be found in the laminar nucleus, which we have shown to be the main auditory processing nucleus of the midbrain. Interestingly, the laminar nucleus has also been shown to concentrate steroid hormones (Kelley et al., 1975; Morrell et al., 1975; Kelley, 1980, 1981). This observation raises the possibility that hormones affect the rate sensitivity of laminar neurons, providing endocrine, and perhaps sex-specific, modulation of responses to male and female calls. In fact, Yovanof and Feng (1983) do report an estradiol-induced enhancement of auditory-evoked activity in the torus of Rana pipiens. In addition the ventral thalamus, which is reciprocally connected to the laminar nucleus, also concentrates steroid hormones in Xenopus laevis. Therefore, this nucleus can also be considered a candidate for eliciting sexually specific responses to different calls.
How the central nervous system encodes rate is a central question in auditory cognition. Xenopus laevis calls use a click rate code to convey information on location, sex, and sexual state. The relative unimportance of spectral cues and the importance of rate suggest that the system will provide an informative preparation in which to address this issue.
ACKNOWLEDGMENT
D.B.K. received support from the NIH (NS2 3684).
Grant sponsor: NIH; Grant number: NS23684.
Abbreviations
- Apo
anterior preoptic area
- At
anterior thalamus
- Ct
central thalamus
- DMN
dorsal medullary nucleus
- DTeg
dorsal tegmentum
- Ea
anterior entopeduncular nucleus
- Ep
posterior entopeduncular nucleus
- LL
lateral line nucleus
- LtLm
lateral lemniscus
- Ltor
laminar torus
- MCtor
magnocellular torus
- OT
optic tectum
- Ppo
posterior preoptic area
- Pt
posterior thalamus
- Ptor
principal torus
- SON
superior olivary nucleus
- Vt
ventral thalamus
- VTeg
ventral tegmentum
LITERATURE CITED
- Alder TB, Rose GJ. Long-term temporal integration in the anuran auditory system. Nat Neurosci. 1998;1:519–523. doi: 10.1038/2237. [DOI] [PubMed] [Google Scholar]
- Altman JS, Dawes EA. Mapping of lateral line and auditory input to the brain of Xenopus laevis. J Physiol (Lond) 1981;317:78–79. [Google Scholar]
- Aronson LR, Noble GK. The sexual behavior of Anura. II. Neural mechanisms controlling mating in the male leopard frog, Rana pipiens. Bull Am Mus Nat Hist. 1945;86:83–139. [Google Scholar]
- Baird RA, Lewis ER. Correspondences between afferent innervation patterns and response dynamics in the bullfrog utricle and saccule. Brain Res. 1986;369:48–64. doi: 10.1016/0006-8993(86)90512-3. [DOI] [PubMed] [Google Scholar]
- Caston J, Precht W, Blanks RHI. Response characteristics of frog’s lagena afferents to natural stimulation. J Comp Physiol. 1977;118:273–289. [Google Scholar]
- Elepfandt A. Sensory perception and the lateral line system in the clawed frog, Xenopus. In: Tinsley RC, Kobel HR, Tinsley RC, Kobel HR, Tinsley RC, Kobel HR, editors. The biology of Xenopus. Oxford: The Zoological Society of London; 1996. pp. 97–120. [Google Scholar]
- Feng AS, Lin WY. Differential innervation patterns of three divisions of frog auditory midbrain (torus semicircularis) J Comp Neurol. 1991;306:613–630. doi: 10.1002/cne.903060407. [DOI] [PubMed] [Google Scholar]
- Feng AS, Narins PM, Capranica RR. Three populations of primary auditory fibers in the bullfrog (Rana catesbeiana): their peripheral origins and frequency sensitivities. J Comp Physiol. 1975;100:221–229. [Google Scholar]
- Kelley DB. Auditory and vocal nuclei in the frog brain concentrate sex hormones. Science. 1980;207:553–555. doi: 10.1126/science.7352269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley DB. Locations of androgen-concentrating cells in the brain of Xenopus laevis: autoradiography with 3H-dihydrotestosterone. J Comp Neurol. 1981;199:221–231. doi: 10.1002/cne.901990206. [DOI] [PubMed] [Google Scholar]
- Kelley DB, Tobias ML. The vocal repertoire of Xenopus laevis. In: Hauser M, Konishi M, editors. The design of animal communication. Cambridge: MIT Press; 1999. pp. 9–35. [Google Scholar]
- Kelley DB, Morrell JI, Pfaff DW. Autoradiographic localization of hormone-concentrating cells in the brain of an amphibian, Xenopus laevis. I. Testosterone. J Comp Neurol. 1975;164:47–59. doi: 10.1002/cne.901640105. [DOI] [PubMed] [Google Scholar]
- Lowe DA. Organisation of lateral line and auditory areas in the midbrain of Xenopus laevis. J Comp Neurol. 1986;245:498–513. doi: 10.1002/cne.902450406. [DOI] [PubMed] [Google Scholar]
- Luksch H, Walkowiak W, Munoz A, Ten Donkelaar HJ. The use of in vitro preparations of the isolated amphibian central nervous system in neuroanatomy and electrophysiology. J Neurosci Methods. 1996;70:91–102. doi: 10.1016/S0165-0270(96)00107-0. [DOI] [PubMed] [Google Scholar]
- Matesz C. Central projections of the VIIIth cranial nerve in the frog. Neuroscience. 1979;4:2061–2071. doi: 10.1016/0306-4522(79)90078-2. [DOI] [PubMed] [Google Scholar]
- Morrell JI, Kelley DB, Pfaff DW. Autoradiographic localization of hormone-concentrating cells in the brain of an amphibian, Xenopus laevis. II. Estradiol. J Comp Neurol. 1975;164:63–77. doi: 10.1002/cne.901640106. [DOI] [PubMed] [Google Scholar]
- Munoz A, Munoz M, Gonzalez A, Ten Donkelaar HJ. Anuran dorsal column nucleus: organization, immunohistochemical characterization, and fiber connections in Rana perezi and Xenopus laevis. J Comp Neurol. 1995;363:197–220. doi: 10.1002/cne.903630204. [DOI] [PubMed] [Google Scholar]
- Neary TJ, Northcutt RG. Nuclear organization of the bullfrog diencephalon. J Comp Neurol. 1983;13:262–278. doi: 10.1002/cne.902130303. [DOI] [PubMed] [Google Scholar]
- Nikundiwe AM, Nieuwenhuys R. The cell masses in the brainstem of the South African clawed frog Xenopus laevis: a topographical and topological analysis. J Comp Neurol. 1983;213:199–219. doi: 10.1002/cne.902130207. [DOI] [PubMed] [Google Scholar]
- Paton JA, Kelley DB, Sejnowski TJ, Yodlowski ML. Mapping the auditory central nervous system of Xenopus laevis with 2- deoxyglucose autoradiography. Brain Res. 1982;249:15–22. doi: 10.1016/0006-8993(82)90164-0. [DOI] [PubMed] [Google Scholar]
- Plassmann W. Central neuronal pathways in the lateral line system of Xenopus laevis. J Comp Physiol. 1980;136:203–213. [Google Scholar]
- Potter DH. Mesencephalic auditory region of the bullfrog. J Neurophysiol. 1965;28:1132–1154. doi: 10.1152/jn.1965.28.6.1132. [DOI] [PubMed] [Google Scholar]
- Ramon-Moliner E. The Golgi-Cox technique. In: Nauta W, Ebbesson S, editors. Contemporary research methods in neuroanatomy. NY: Springer-Verlag; 1970. pp. 35–52. [Google Scholar]
- Rose G, Capranica RR. Temporal selectivity in the central auditory system of the leopard frog. Science. 1983;219:1087–1089. doi: 10.1126/science.6600522. [DOI] [PubMed] [Google Scholar]
- Rose G, Capranica RR. Processing amplitude-modulated sounds by the auditory midbrain of two species of toads: matched temporal filters. J Comp Physiol [A] 1984;154:211–219. [Google Scholar]
- Rose GJ, Capranica RR. Sensitivity to amplitude modulated sounds in the anuran auditory nervous system. J Neurophysiol. 1985;53:446–465. doi: 10.1152/jn.1985.53.2.446. [DOI] [PubMed] [Google Scholar]
- Trueb L. Historical constraints and morphological novelties in the evolution of the skeletal system of pipid frogs (Anura:Pipidae). In: Tinsley R, Kobels H, editors. The biology of Xenopus. London: Zool Soc; 1996. pp. 351–377. [Google Scholar]
- Weeg MS, Bass AH. Central lateral line pathways in a vocalizing fish. J Comp Neurol. 2000;418:41–61. [PubMed] [Google Scholar]
- Wever EG. The amphibian ear. Princeton: Princeton University Press; 1985. [Google Scholar]
- Wilczynski W. Afferents to the midbrain auditory center in the bullfrog, Rana catesbeiana. J Comp Neurol. 1981;198:421–433. doi: 10.1002/cne.901980304. [DOI] [PubMed] [Google Scholar]
- Wilczynski W, Capranica RR. The auditory system of anuran amphibians. Prog Neurobiol. 1984;22:1–38. doi: 10.1016/0301-0082(84)90016-9. [DOI] [PubMed] [Google Scholar]
- Wilczynski W, Neary TJ. Descending projections from the torus semicircularis in Ranid frogs. Anat Res. 1983;205:215A. [Google Scholar]
- Will U, Fritzch B. The eighth nerve of amphibians: peripheral and central distribution. In: Fritzch B, Ryan MJ, Wilczynski W, Walkowiak W, Fritzch B, Ryan MJ, Wilczynski W, Walkowiak W, Fritzch B, Ryan MJ, Wilczynski W, Walkowiak W, editors. The evolution of the amphibian auditory system. New York: Wiley; 1988. pp. 159–183. [Google Scholar]
- Will U, Luhede G, Görner P. The area octavo-lateralis in Xenopus laevis. I. The primary afferent projections. Cell Tissue Res. 1985a;239:147–161. [Google Scholar]
- Will U, Luhede G, Görner P. The area octavo-lateralis in Xenopus laevis II. Second order projections and cytoarchitecture. Cell Tissue Res. 1985b;239:163–175. [Google Scholar]
- Yovanof S, Feng A. Effects of estradiol on auditory evoked responses from the frog’s auditory midbrain. Neurosci Lett. 1983;36:291–297. doi: 10.1016/0304-3940(83)90015-0. [DOI] [PubMed] [Google Scholar]
- Zittlau KE, Claas B, Munz H. Horseradish peroxidase study of tectal afferents in Xenopus laevis with special emphasis on their relationship to the lateral-line system. Brain Behav Evol. 1988;32:208–219. doi: 10.1159/000116548. [DOI] [PubMed] [Google Scholar]
- Zittlau KE, Claas B, Munz H, Gorner P. Multisensory interaction in the torus semicircularis of the clawed toad Xenopus laevis. Neurosci Lett. 1985;60:77–81. doi: 10.1016/0304-3940(85)90384-2. [DOI] [PubMed] [Google Scholar]