Abstract
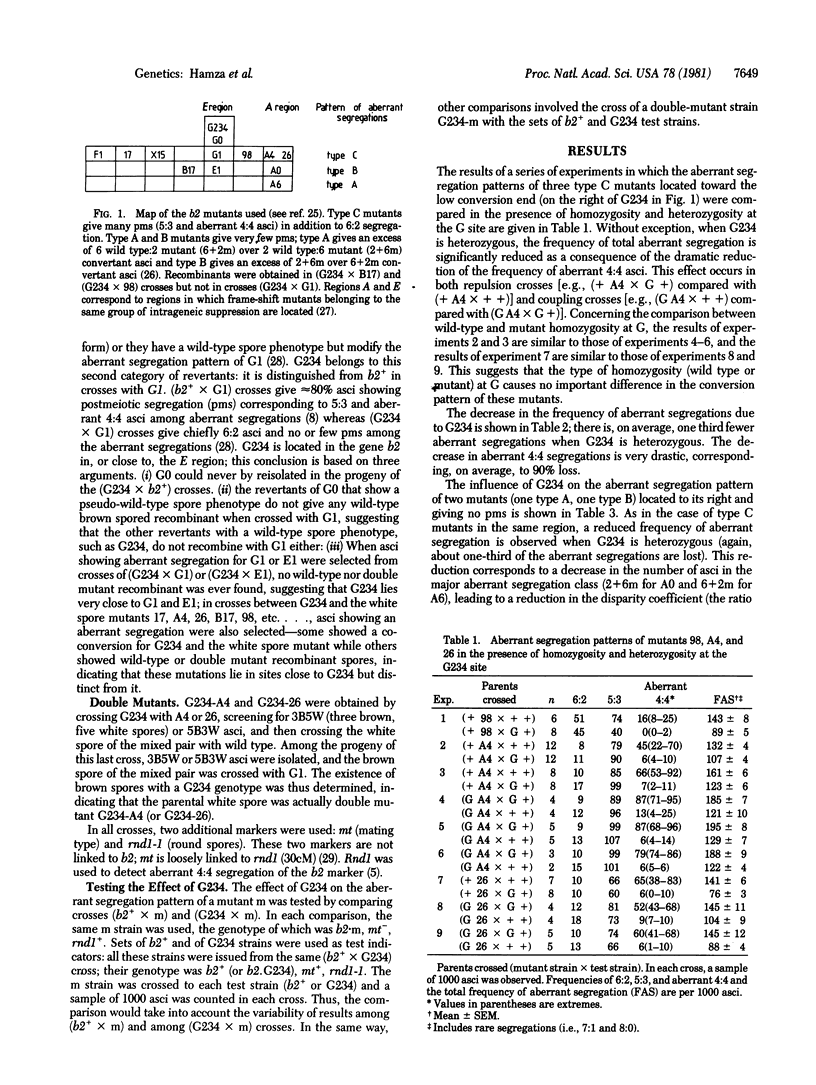
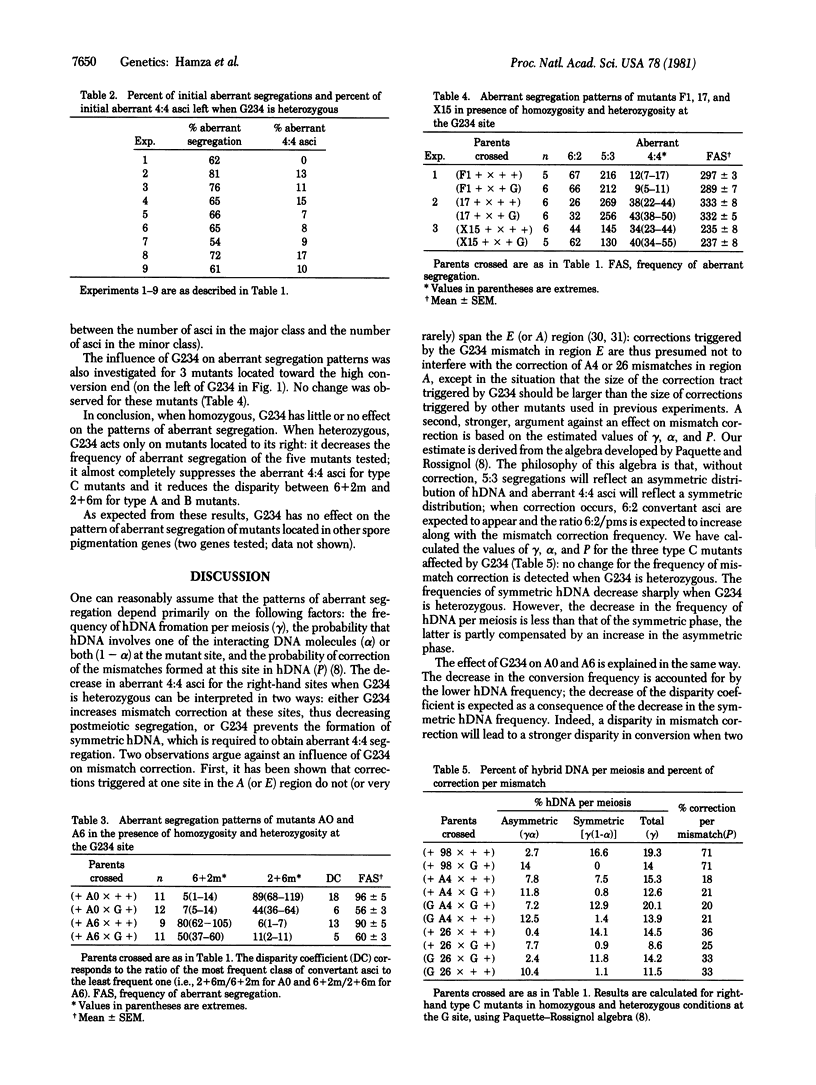
G234 is a silent mutation located in the middle of gene b2, which controls spore pigmentation in Ascobolus immersus. Its effect on the aberrant segregation patterns of while spore mutants located in the same gene was investigated. When heterozygous, G234 decreases the frequency of aberrant segregations of the mutants located on its right, toward the low conversion end. It almost completely suppresses the aberrant 4:4 asci for mutants giving postmeiotic segregation and decreases the disparity between the 6 wild-type:2 mutant and 2 wild-type:6 mutant aberrant asci for mutants giving only these types of convertant asci. These effects are polar; G234 does not change the aberrant segregation pattern of the mutants located on its left, toward the high conversion end. This behavior suggests that G234 blocks the migration of the symmetric phase of hybrid DNA that diffuses from the high conversion end but does not prevent the formation of asymmetrical hybrid DNA. Taking into account previous observations, we conclude that the high conversion end corresponds to a region of asymmetric initiation of recombination rather than to a region of preferential ending of recombination. The asymmetric hybrid DNA first formed is further changed into a symmetric phase that extends via branch migration toward the low conversion end.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Catcheside D. G. Fungal genetics. Annu Rev Genet. 1974;8:279–300. doi: 10.1146/annurev.ge.08.120174.001431. [DOI] [PubMed] [Google Scholar]
- Chattoraj D. K., Crasemann J. M., Dower N., Faulds D., Faulds P., Malone R. E., Stahl F. W., Stahl M. M. Chi. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 2):1063–1066. doi: 10.1101/sqb.1979.043.01.116. [DOI] [PubMed] [Google Scholar]
- DasGupta C., Shibata T., Cunningham R. P., Radding C. M. The topology of homologous pairing promoted by RecA protein. Cell. 1980 Nov;22(2 Pt 2):437–446. doi: 10.1016/0092-8674(80)90354-2. [DOI] [PubMed] [Google Scholar]
- Fogel S., Mortimer R., Lusnak K., Tavares F. Meiotic gene conversion: a signal of the basic recombination event in yeast. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 2):1325–1341. doi: 10.1101/sqb.1979.043.01.152. [DOI] [PubMed] [Google Scholar]
- Girard J., Rossignol J. L. The suppression of gene conversion and intragenic crossing over in Ascobolus immersus: evidence for modifiers acting in the heterozygous state. Genetics. 1974 Feb;76(2):221–243. doi: 10.1093/genetics/76.2.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitani Y., Olive L. S. Genetics of Sordaria fimicola. VI. Gene conversion at the g locus in mutant X wild type crosses. Genetics. 1967 Dec;57(4):767–782. doi: 10.1093/genetics/57.4.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leblon G., Paquette N. Intragenic suppression at the b2 locus in Ascobolus immersus. I. Identification of three distinct groups of suppression. Genetics. 1978 Nov;90(3):475–488. doi: 10.1093/genetics/90.3.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leblon G., Rossignol J. L. Mechanism of gene conversion in Ascobolus immersus. 3. The interaction of heteroallelas in the conversion process. Mol Gen Genet. 1973 Apr 12;122(2):165–182. doi: 10.1007/BF00435189. [DOI] [PubMed] [Google Scholar]
- Mekki-Berrada A., Rossignol J. L., Paquette N. Haute fréquence de réversion d'un mutant spontané chez Ascobolus immersus. C R Acad Sci Hebd Seances Acad Sci D. 1976 Oct 11;283(8):971–974. [PubMed] [Google Scholar]
- Meselson M. S., Radding C. M. A general model for genetic recombination. Proc Natl Acad Sci U S A. 1975 Jan;72(1):358–361. doi: 10.1073/pnas.72.1.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter H., Dressler D. On the mechanism of genetic recombination: electron microscopic observation of recombination intermediates. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3000–3004. doi: 10.1073/pnas.73.9.3000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RIZET G., ENGELMANN N., LEFORT C., LISSOUBA P., MOUSSEAU J. [On an Ascomycete of interest for the study of certain aspects of the problem of gene structure]. C R Hebd Seances Acad Sci. 1960 Mar 14;250:2050–2052. [PubMed] [Google Scholar]
- Rossignol J. L., Paquette N. Disparity of gene conversion in frameshift mutants located in locus b2 of Ascobolus immersus. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2871–2875. doi: 10.1073/pnas.76.6.2871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossignol J. L., Paquette N., Nicolas A. Aberrant 4:4 asci, disparity in the direction of conversion, and frequencies of conversion in Ascobolus immersus. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 2):1343–1352. doi: 10.1101/sqb.1979.043.01.153. [DOI] [PubMed] [Google Scholar]
- Sobell H. M. Molecular mechanism for genetic recombination. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2483–2487. doi: 10.1073/pnas.69.9.2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadler D. R., Towe A. M. Evidence for meiotic recombination in Ascobolus involving only one member of a tetrad. Genetics. 1971 Jul;68(3):401–413. doi: 10.1093/genetics/68.3.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson B. J., Camien M. N., Warner R. C. Kinetics of branch migration in double-stranded DNA. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2299–2303. doi: 10.1073/pnas.73.7.2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner R. E., Jr, Radman M. A mechanism for initiation of genetic recombination. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3619–3622. doi: 10.1073/pnas.72.9.3619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolgemuth D. J., Hsu M. T. Visualization of genetic recombination intermediates of human adenovirus type 2 DNA from infected HeLa cells. Nature. 1980 Sep 11;287(5778):168–171. doi: 10.1038/287168a0. [DOI] [PubMed] [Google Scholar]
- YU-SUN C. C. BIOCHEMICAL AND MORPHOLOGICAL MUTANTS OF ASCOBOLUS IMMERSUS. Genetics. 1964 Nov;50:987–998. doi: 10.1093/genetics/50.5.987. [DOI] [PMC free article] [PubMed] [Google Scholar]


