Abstract
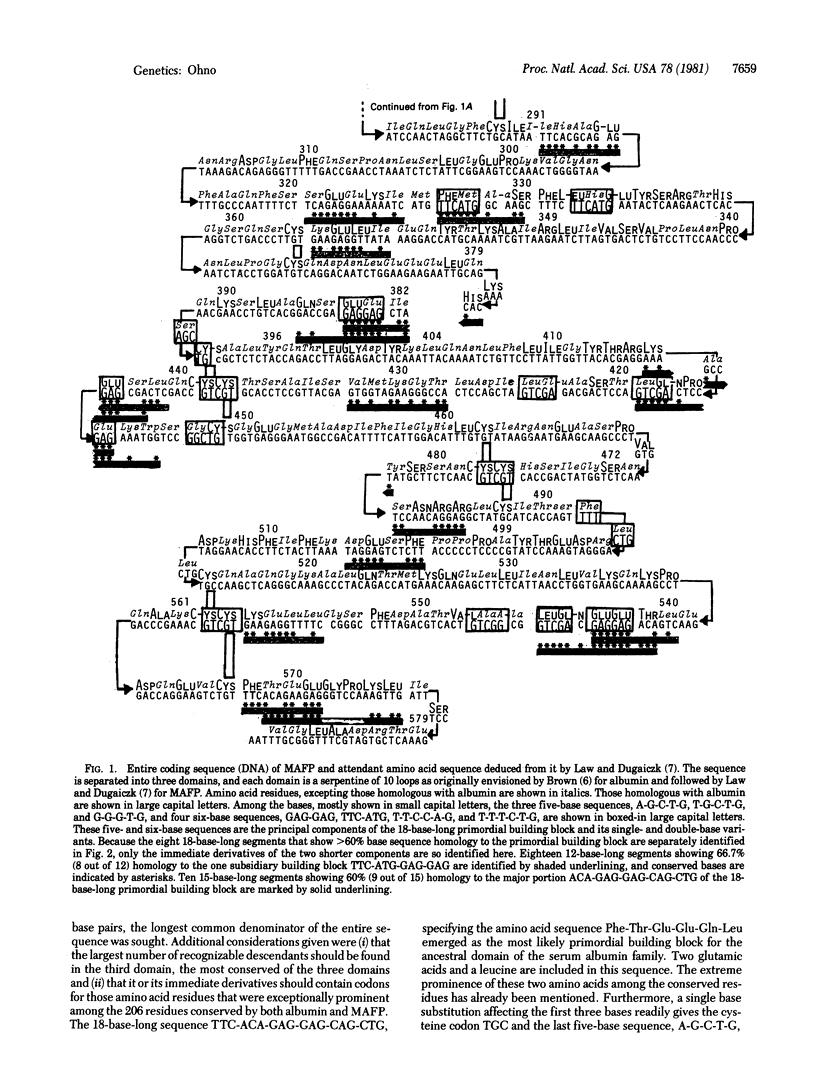
The characteristic three-domain structure has been conserved throughout mammalian evolution by serum albumin and its fetal counterpart, alpha-fetoprotein. Thus, one still detects 35.2% amino acid sequence homology between bovine serum albumin and murine alpha-fetoprotein. Yet, natural selection cannot be invoked as the major factor responsible for the observed conservation of these sequences, for the simple reason of their dispensability. Inherited analbuminemia is apparently a harmless trait in man and the rat. The conservation appears inherent in their repetitious origin. Each protein is made of triplicate copies of the ancestral domain. Furthermore, analysis of the published sequence data suggests that the original coding sequence for the ancestral domain arose as repeats of the 18-base-long primordial building block sequence TTC-ACA-GAG-GAG-CAG-CTG specifying Phe-Thr-Glu-Glu-Gln-Leu and its shorter subsidiary TTC-ATG-GAG-GAG specifying Phe-Met-Glu-Glu. Consequently, the homology between bovine serum albumin and alpha-fetoprotein is mostly confined to small segments still specified by recognizable descendants of these building block sequences. The point to be made here is that evolutionary conservation of coding sequences can be an inherent property; natural selection need not be invoked.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Attardi B., Ruoslahti E. Foetoneonatal oestradiol-binding protein in mouse brain cytosol is alpha foetoprotein. Nature. 1976 Oct 21;263(5579):685–687. doi: 10.1038/263685a0. [DOI] [PubMed] [Google Scholar]
- Boman H., Hermodson M., Hammond C. A., Motulsky A. G. Analbuminemia in an American Indian girl. Clin Genet. 1976 May;9(5):513–526. doi: 10.1111/j.1399-0004.1976.tb01606.x. [DOI] [PubMed] [Google Scholar]
- Brown J. R. Structural origins of mammalian albumin. Fed Proc. 1976 Aug;35(10):2141–2144. [PubMed] [Google Scholar]
- Esumi H., Okui M., Sato S., Sugimura T., Nagase S. Absence of albumin mRNA in the liver of analbuminemic rats. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3215–3219. doi: 10.1073/pnas.77.6.3215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris S. D., Whitt G. S. Loss of duplicate gene expression after polyploidisation. Nature. 1977 Jan 20;265(5591):258–260. doi: 10.1038/265258a0. [DOI] [PubMed] [Google Scholar]
- Law S. W., Dugaiczyk A. Homology between the primary structure of alpha-fetoprotein, deduced from a complete cDNA sequence, and serum albumin. Nature. 1981 May 21;291(5812):201–205. doi: 10.1038/291201a0. [DOI] [PubMed] [Google Scholar]
- Nagase S., Shimamune K., Shumiya S. Albumin-deficient rat mutant. Science. 1979 Aug 10;205(4406):590–591. doi: 10.1126/science.451621. [DOI] [PubMed] [Google Scholar]
- Nei M., Li W. H. Mathematical model for studying genetic variation in terms of restriction endonucleases. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5269–5273. doi: 10.1073/pnas.76.10.5269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno S. (AGCTG) (AGCTG) (AGCTG) (GGGTG) as the primordial sequence of intergenic spacers: the role in immunoglobulin class switch. Differentiation. 1981;18(2):65–74. doi: 10.1111/j.1432-0436.1981.tb01106.x. [DOI] [PubMed] [Google Scholar]
- Ohta T., Kimura M. Functional organization of genetic material as a product of molecular evolution. Nature. 1971 Sep 10;233(5315):118–119. doi: 10.1038/233118a0. [DOI] [PubMed] [Google Scholar]
- Plapinger L., McEwen B. Immunochemical comparison of estradiol-binding molecules in perinatal rat brain cytosol and serum. Steroids. 1975 Aug;26(2):255–265. doi: 10.1016/s0039-128x(75)80027-4. [DOI] [PubMed] [Google Scholar]
- Sarich V. M., Wilson A. C. Immunological time scale for hominid evolution. Science. 1967 Dec 1;158(3805):1200–1203. doi: 10.1126/science.158.3805.1200. [DOI] [PubMed] [Google Scholar]
- Wallace D. G., Maxson L. R., Wilson A. C. Albumin evolution in frogs: a test of the evolutionary clock hypothesis. Proc Natl Acad Sci U S A. 1971 Dec;68(12):3127–3129. doi: 10.1073/pnas.68.12.3127. [DOI] [PMC free article] [PubMed] [Google Scholar]



