Abstract
Tissues expressing mRNAs of three cold-induced genes, blt101, blt14, and blt4.9, and a control gene, elongation factor 1α, were identified in the crown and immature leaves of cultivated barley (Hordeum vulgare L. cv Igri). Hardiness and tissue damage were assessed. blt101 and blt4.9 mRNAs were not detected in control plants; blt14 was expressed in control plants but only in the inner layers of the crown cortex. blt101 was expressed in many tissues of cold-acclimated plants but most strongly in the vascular-transition zone of the crown; blt14 was expressed only in the inner layers of the cortex and in cell layers partly surrounding vascular bundles in the vascular-transition zone; expression of blt4.9, which codes for a nonspecific lipid-transfer protein, was confined to the epidermis of the leaf and to the epidermis of the older parts of the crown. None of the cold-induced genes was expressed in the tunica, although the control gene was most strongly expressed there. Thus, the molecular aspects of acclimation differed markedly between tissues. Damage in the vascular-transition zone of the crown correlated closely with plant survival. Therefore, the strong expression of blt101 and blt14 in this zone may indicate a direct role in freezing tolerance of the crown.
Freezing is one of the most widespread environmental stresses. Plants able to overwinter in temperate and colder regions acclimate, i.e. they respond to low temperatures by increasing the frost intensity that they can survive. The expression of numerous genes is greatly increased during acclimation, but the biochemical and physiological functions of many of them are unknown.
In nature freezing can be accompanied by other winter stresses, including water-logging and ice encasement, which cause anaerobic stress, and wind and direct sunlight, which, if the soil is frozen, can cause shoot dehydration. Plants adapted to these environments will acclimate to tolerate these stresses as well as the direct effects of freezing. In addition, growth and primary metabolism acclimate to cold (Guy, 1990); this allows the plant to compensate slightly for the adverse effect of cold on the growth rate and helps to provide the metabolic support essential for acclimation to frost and other winter stresses. It is possible that genes expressed during acclimation have a function related to any of these adaptations to winter.
Identifying the tissues in which cold-induced genes are expressed would be an important step toward understanding their possible functions, since this will eliminate some functional associations from consideration and will suggest others. This is best done in a heterogeneous organ in which the different parts are functionally distinct and some parts are likely to be more frost sensitive than others. The cereal crown is particularly appropriate. It includes the shoot apex, leaf and tiller initials, bases of the unemerged leaves and of still-growing leaf sheaths, and the subtending stem, including root initials and newly emerging roots. Within the crown, the following tissues can be identified: vascular bundles that develop in the newly forming tissues, servicing the older organs and forming the vascular-transition zone; the epidermis and cortex; stem tissue surrounding vascular bundles in the transition zone; the tunica, comprising several cell layers at the apex; and the pith meristem and subapical meristems. These parts differed in sensitivity to frost when tested in other Gramineae (Tanino and McKersie, 1985; Shibata and Shimada, 1986). In addition, the crown is the part of the barley (Hordeum vulgare) plant in which survival has the most impact on survival of the plant as a whole (Olien, 1967). Although most of our experiments focused on the crown, expression in leaves was also examined for comparison.
We cloned and characterized a number of cold-induced genes expressed in the crown of the barley cv Igri. blt4.9 and closely related genes form a small multigene family with members coding for nsLTPs (Dunn et al., 1991; White et al., 1994). In barley some nsLTPs are expressed in response to drought and some in response to cold (Molina and García-Olmedo, 1993; White et al., 1994). blt14 is also a member of a small multigene family but has no similarity to any sequence of known function; expression of all of the known members of this multigene family is strongly up-regulated in response to cold (Dunn et al., 1990; Phillips et al., 1997); other stresses, including drought, had a lesser effect on expression (Pearce et al., 1996). blt101 is one of two closely related genes in barley that have no similarity to any sequence of known function (Goddard et al., 1993); its expression is strongly up-regulated in response to cold but not in response to drought (Pearce et al., 1996). These genes, or closely related sequences, are all expressed in response to cold in the mature shoot tissues, and two (blt14 and blt101) are also expressed in the roots of cold-grown barley (Dunn et al., 1990; Goddard et al., 1993). The mechanisms by which their mRNA levels are controlled differ: the cold up-regulation of blt4.9 and blt101 mRNAs is transcriptionally controlled, whereas the cold up-regulation of blt14 is posttranscriptionally controlled (Dunn et al., 1994). Overall, the three genes, although all cold up-regulated, are representative of different expression patterns and mechanisms. A control gene coding for EF-1α (blt63), which has high sequence similarity to other EF-1α genes (Dunn et al., 1993), was also included in the experiments.
The objectives of the experiments were (a) to use in situ hybridization of mRNA to determine in which tissues of the cold-acclimating barley crown meristem and developing leaves the three cold up-regulated genes (blt101, blt14, and blt4.9) are expressed, (b) to compare these expression patterns with the frost susceptibility of the tissues, and (c) to use this as a basis for drawing conclusions about possible functional relationships of the genes.
MATERIALS AND METHODS
Plant Material and Growth Environments
Seeds of cultivated barley (Hordeum vulgare L. cv Igri) were sown in John Innes no. 2 potting compost (12 seeds/10-cm pot) and germinated in a controlled-environment room set at 20/15°C (day/night), a 10-h photoperiod, and 175 μmol m−2 s−1 photon flux density (400–700 nm). This was designated the control environment. When the third leaf had emerged in 50 to 75% of plants (about 14 d after sowing), plants for cold acclimation were transferred to a controlled environment set at 6/2°C (day/night) and with the other environmental parameters the same as the control; control plants remained in the 20/15°C environment. Both control and cold-grown plants were analyzed when the fourth leaf was emerging in about 50% of the plants, by which time frost tolerance in the cold-acclimated plants was approaching the maximum for this cultivar and environment (Pearce et al., 1996).
Harvesting and Fixation of the Plant Samples and Processing to Prepare Sections for Hybridization
The plants were washed free of soil, dabbed dry, and the outer, fully emerged leaf blades and sheaths were stripped off. Leaf material 5 mm above the base and emerged roots were cut off and discarded, and the resultant plant piece was transferred to fixative on ice while further samples were collected. Leaf blades almost emerging or just emerged from enclosing sheaths were also collected and fixed.
To protect the samples against exogenous RNase during the procedure, solutions were treated with diethyl pyrocarbonate and autoclaved, and glassware and metal items were baked. The method for fixation and processing for hybridization was modified from Gurr et al. (1992) as follows (all percentages are weight per volume): Plant pieces were fixed in 4% formaldehyde using vacuum infiltration for 20 min at room temperature and then kept in the fixative overnight at 4°C. They were then dehydrated and transferred via Histoclear (National Diagnostics, Hull, UK) to wax and vacuum embedded. Eight-micrometer sections were cut and floated on sterile distilled water heated to 50°C and then transferred onto poly l-Lys-coated slides. The sections were rehydrated and transferred sequentially to PBS (0.13 m NaCl, 0.007 m Na2HPO4, and 0.003 m NaH2PO4) for 2 min, to a 125-μg/mL solution of a protease from Streptomyces griseus (Sigma) in buffer (0.05 m Tris-HCl and 0.005 m EDTA, pH 7.5) for 10 min, to Gly (0.2% in PBS), and then to PBS, refixed in 4% paraformaldehyde in PBS for 10 min, washed in PBS, transferred to 0.85% NaCl, and dehydrated. Some sections were treated with RNase type 1-A (Sigma; 20 μg/μL in 0.5 m NaCl, 10 mm Tris-HCl, pH 7.5, and 1 mm EDTA, at 37°C for 30 min) immediately after the Gly step, after which they were treated in the same way as other sections.
Preparation of Riboprobes, in Situ Hybridization, and Probe Detection
Gene sequences were subcloned into pGEM-5Zf (Promega). The SP6 and T7 RNA polymerase promoters on complementary strands were used to promote transcription of either the sense or antisense RNA strand. Digoxigenin-labeled RNA was synthesized using a kit (Boehringer Mannheim). Before use the riboprobe was heated for 2 min at 80°C and then cooled on ice and centrifuged. The supernatant was added to hybridization buffer (final composition: 1 m NaCl, 0.033 m Tris-HCl, 0.033 m NaH2PO4, 16.7 mm EDTA, pH 6.8, 30% formamide, 3.3 mg/mL tRNA, and 3.3× Denhardt's solution; all solutions used in preparation were treated with diethyl pyrocarbonate) to make the hybridization mixture. The probe was used in the concentration range of 0.1 to 0.3 ng μL−1 kb−1.
Hybridization was carried out overnight at 50°C and the samples were washed with shaking in sequence as follows: first with 2× SSC buffer, 50% formamide for 30 min at 50°C; then twice with 2× SSC buffer for 1.5 h at 50°C; twice with 0.5 m NaCl, 10 mm Tris-HCl, pH 7.5, and 1 mm EDTA (NTE buffer) for 5 min each at 37°C; once with RNase A (20 μg/mL RNase A in NTE buffer) for 30 min at 37°C; twice with NTE buffer for 5 min at 37°C; once with 2× SSC buffer for 1 h at 50°C; and, finally, once with PBS for 2 min.
A digoxigenin-detection kit (Boehringer Mannheim) was used to detect the probe. When development had taken place, the sections were washed in sterile distilled water, dehydrated, and mounted in Canada balsam (Sigma). The result was recorded on tungsten-balanced transparency film (RTP, ASA 64, Fuji, Tokyo, Japan).
Tests of Plant and Tissue Tolerance of Freezing Stress
To test frost survival directly by a regrowth test after freezing, plants were trimmed to remove roots and leaf blades, placed in tubes, immersed in an alcohol bath with ice added to initiate freezing, exposed to subfreezing temperatures, thawed, planted in John Innes no. 2 potting compost, and returned to the control environment, as described previously (Pearce et al., 1996). Survival was judged after 7 and 28 d by continued regrowth above 3 mm.
For the tests of tissue damage, plants were exposed to frost, thawed, and planted in compost in the same way as for the regrowth test described above. After 1 d in the control environment the tillers were removed from soil and washed, and the bases were bisected. These were then immersed in 0.5% TTC in 50 mm Hepes, pH 7.4, at room temperature in the dark for 1.5 h, and then rinsed in water (Tanino and McKersie, 1985). With this test, formazan, the reduction product of TTC, stains red in living tissue, whereas nonliving tissue remains white. Staining of different parts exposed at the cut surface was assessed microscopically using a low-power binocular microscope to identify which regions were alive and which were dead (figures in Tanino and McKersie, 1985, illustrate the appearance), and the appearance was photographed (Kodak Gold 200 ASA color-negative film). The regions documented were leaf sheaths surrounding apex and stem, the apex, the inner part of the subapical region, the inner part of the base, and the cortex.
Statistical tests of differences between the results for tiller survival (by the regrowth test) and tissue survival (by the TTC test) were determined by ascribing confidence intervals to the individual percent-survival values, taking account of the sample sizes (table 41 in Pearson and Hartley, 1956) and using correlation analysis.
RESULTS
Controls for the in Situ Hybridization Methodology
Treatment of sections with RNase before hybridization, exclusion of the probe from the normal procedure, and the use of the sense probe gave no signal. Figure 1, A and B, substantiates the last point for the crown tip and the vascular-transition zone for two sense sequences, blt63 and blt101; blt14 and blt4.9 sense sequences were also tested and also gave no signal either with tissue from control (20/15°C) or from cold-grown (6/2°C) plants. These tests showed that the positive signals obtained with the sections given the normal procedure and using the antisense sequences were attributable to mRNA rather than to nonspecific binding or endogenous phosphatase activity.
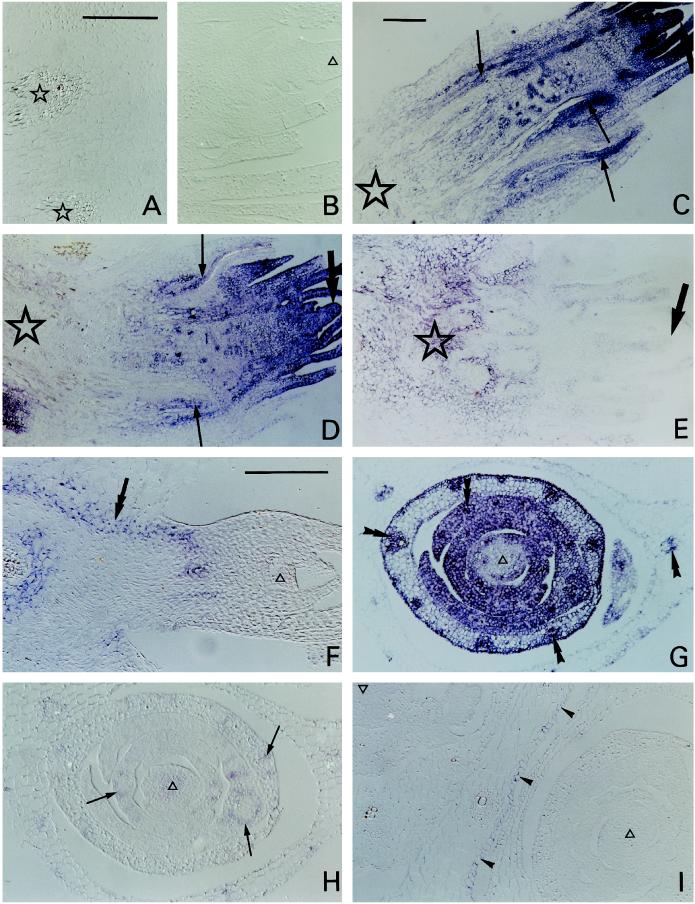
Figure 1.
Sections of barley crown hybridized with sense (A and B) or antisense (C–I) digoxigenin-labeled sequences. A blue-purple color indicates a positive signal. A and B, Longitudinal sections of the basal (A) and apical (B) region of the crown from cold-grown plants, hybridized with sense blt101 (A) or sense blt63 (B). Stars, Vascular bundles; ▵, tissue within the apical tip. C to E, Longitudinal sections of crowns from control (C) or cold-grown (D and E) plants hybridized with antisense blt63 (C and D) or antisense blt101 (E). Stars, Base; thick arrows, apex; thin arrows, stain (blt63) around developing vascular tissue. F, Longitudinal section of a subsidiary apex in the crown of a cold-grown plant hybridized with antisense blt14. Double arrow, Stain in the inner region of the cortex. G to I, Transverse sections of the apical regions of crowns from cold-grown plants hybridized with antisense blt63 (G), blt101 (H), or blt4.9 (I). ▵, Tissue within the apical tip; double arrowheads, stain (blt63) within vascular bundles in leaf initials; thin arrows, stain (blt101) around vascular bundles in leaf initials; arrowheads, stain (blt4.9) in one epidermis. C and G, Bright-field microscopy; A, B, D–F, H, and I, Interference contrast microscopy. Bars in A, C, and F = 200 μm (A and B are same scale; C to E are same scale; and F to I are same scale).
The blt63 antisense probe (Fig. 1, C, D, and G) hybridized with every living cell, although with varying intensity, and thus was used as a positive control for the method. This positive signal showed that the absence of a signal from any section or from any particular tissue in the section, when probed with the antisense strand of one of the other genes, was due to the absence of sufficient corresponding mRNA to generate a signal and was not an artifact (such as from local impenetrability). The blt63 probe was also used to indicate areas of probable active protein synthesis and thus the areas that were metabolically most active.
Localization of Expression in Crowns
The pattern of blt63 signal intensity was the same for both cold-acclimated and control plants: the signal was strongest at the apex and weakest at the base of the crown (Fig. 1, C and D). In contrast to this pattern, the blt101 signal in the cold-acclimated plants was weakest at the apex and strongest at the base (Fig. 1E). In the apex the blt63 signal was strongest in the outermost cell layers of the shoot meristem tip and weaker in the pith (Fig. 1, C and D). Again, in contrast to this, there was no blt101 signal from the peripheral cell layers at the apex, but there was a distinct but weak signal from the adjacent core of the apex (Fig. 1E). The contrast was even greater with blt14, which gave no signal from any part of the apex from cold-acclimated plants but gave a strong signal from the inner layers of the cortex and other tissues lower in the crown (Fig. 1F).
The contrasting patterns of expression at the apex of cold-acclimated plants are shown in greater detail by transverse sections (Fig. 1, G–I; control plants gave no signal for blt101, blt14, or blt4.9 [not shown]). There was a strong blt63 signal from the youngest leaf initials and from vascular bundles in younger and older organs, a weaker signal from other tissues in the more developmentally advanced leaf tissue, and, again, a weaker signal from the core of the apex (Fig. 1G). In contrast, there was a detectable but weak blt101 signal only from the core of the apex and from the cells immediately surrounding but not in the vascular bundles in the developing leaves (Fig. 1H). In contrast to both, blt4.9 gave a signal only from the epidermis of the oldest leaf in the section shown, subtending the main apex, but not from the surface cell layers of any of the younger organs (Fig. 1I); there was no blt4.9 signal from the corresponding epidermis in control plants (not shown). Only the abaxial epidermis gave a signal, illustrating a remarkable difference in response within a single organ between the two surfaces; however, in older leaves the adaxial as well as the abaxial epidermis gave a signal (not shown).
These comparisons make it clear that, whereas blt63 gave signals from all living cell types and tissues, blt101, blt14, and blt4.9 did not.
There were also many detailed differences in patterns of expression in the basal regions of the crown. Control plants gave no blt101 or blt4.9 signals (Fig. 2, A and B); however, the inner region of the cortex (but no other tissue) did give a blt14 signal (Fig. 2C). The inner region of the crown cortex from cold-grown plants gave signals for blt101 and blt14 as well as for blt63 (Fig. 2, D–F). Cell layers immediately surrounding but not in the vascular bundles in this basal region gave strong blt101 and blt14 signals (Fig. 2, D and E). A detailed comparison between the patterns of expression of the blt63 and blt101 sequences revealed clear differences: an arc of tissue immediately surrounding part of the vascular bundles gave a strong blt101 signal but very faint blt63 signal, whereas cell layers between these gave no blt101 signal but did give a clear blt63 signal (Fig. 2, D and F). Again, unlike the other sequences, a blt4.9 signal was given only by the epidermis (Fig. 2G).
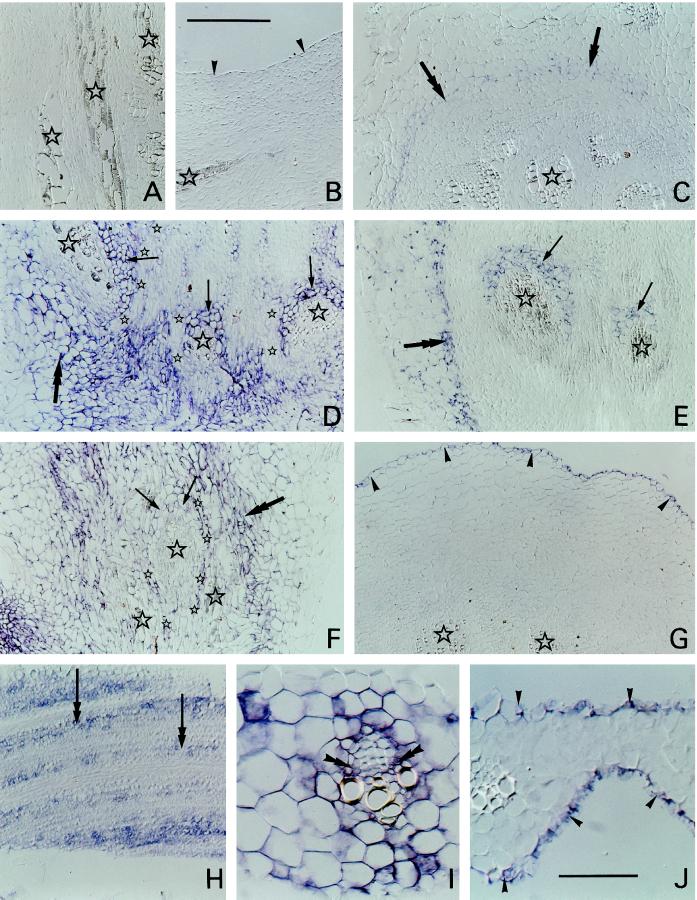
Figure 2.
Sections of barley crown and leaves hybridized with antisense digoxigenin-labeled sequences. A blue-purple color indicates a positive signal. A and B, Longitudinals sections from the basal region of crowns from control plants hybridized with antisense blt101 (A) or blt4.9 (B). Stars, Vascular bundles; arrowheads, epidermis. C, Transverse section from the basal region of a control crown hybridized with antisense blt14. Stars, Vascular bundles; double arrows, stain in the inner region of the cortex. D to F, Longitudinal sections from the basal regions of crowns from cold-grown plants hybridized with antisense blt101 (D), blt14 (E), or blt63 (F). Large stars, Vascular bundles; double arrows, stain in the cortex; single arrows, tissue around part of each vascular bundle either stained (blt101 and blt14) or not stained (blt63); small stars, tissue layers between vascular bundles either not stained (blt101) or stained (blt63). G, Transverse section from the basal region of a crown from a cold-grown plant hybridized with antisense BLT4.9. Stars, Vascular bundles; arrowheads, stain in the epidermis. H, Glancing longitudinal section of a developing leaf initial from a cold-grown plant hybridized with antisense blt14. Double arrows, Stain between developing vascular bundles. I and J, Transverse section of emerging leaf blades from cold-grown plants hybridized with blt101 (I) or blt4.9 (J). Double arrowheads, Stain (blt101) in cells within a vascular bundle; single arrowheads, stain (blt4.9) in epidermis. All sections viewed using interference contrast microscopy. Bar in B = 200 μm (A–H are the same scale); bar in J = 50 μm (I and J are the same scale).
Localization of Expression in Leaves
In the older leaf initials from cold-acclimated plants there was a blt14 signal from cells between but not in the vascular bundles (Fig. 2H). However, cells throughout the emerging leaf blades and surrounding sheaths of cold-acclimated plants probed with blt101 gave a signal, including cells within the vascular bundles (Fig. 2I). The absence of signal from some cells could reflect the apparent absence of cytoplasm in the section of that cell, and, similarly, variation in signal between individual cells could at least partly reflect variations in the amount and local thickness of cytoplasm remaining after sectioning had removed part. As in the crown, only the epidermal cells gave a blt4.9 signal (Fig. 2J). No blt14, blt101, or blt4.9 signals were detectable in leaf sections from control plants (not shown).
Hardiness of Crown Parts
After the TTC test, controls were stained red throughout, indicating that all tissues were alive. Specimens exposed to freezing temperatures were generally less intensely red after exposure to progressively lower temperatures (not shown). The parts that were colorless at the warmest damaging freezing temperatures were the apex and central area of the base, indicating that these areas were the parts that were the most sensitive to freezing temperatures. At lower temperatures other tissues were also colorless and, therefore, dead.
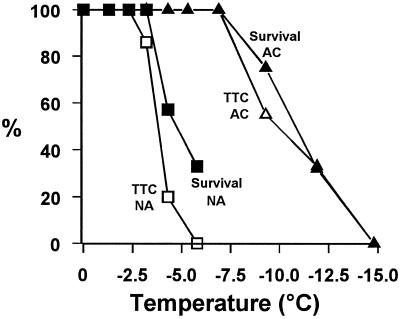
The results of the TTC test for tissue damage in the central area of the base, together with the results of the regrowth test of tiller frost survival for control and acclimated material, are summarized in Figure 3. The relationship between survival and damage to the central area of the base of the crown was similar in both nonacclimated and acclimated plants. Putting the results for control and acclimated material together and including one each, only, of the extreme values (100% survival with 100% stained red and 0% survival with 0% stained red) indicated that there was a correlation between tiller survival and survival of the central area of the base (P < 0.001 for the null hypothesis; correlation coefficient = 0.88; n = 7).
Figure 3.
Tiller survival and tissue damage in the vascular-transition zone of barley following exposure to frost. Young barley cv Igri plants were acclimated (ACC) or not acclimated (NA) to cold. Survival, Percentage of tillers regrowing after exposure to freezing temperatures (n = 12 for each point). TTC, Percentage of center bases of main axes alive after testing for reduction of TTC (n = 11–12 for acclimated plants and 7–10 for nonacclimated plants for each point). Survival and TTC reduction were tested on tillers grown at the same time in the same environments and then exposed at the same time to the same freezing stresses.
DISCUSSION
The results showed very distinct patterns of expression of the cold-inducible genes at the mRNA level in the cold-acclimated barley crown. Differences in signal intensity between tissues noted in Results for any of the specific mRNAs probed for do not directly indicate differences in specific mRNA content per cell or per unit of cytoplasm, because the cells differ in size and in cytoplasmic content between tissues. Instead, they indicate differences per unit volume of section and hence per unit volume of tissue.
Tissues having high expression of the EF-1α mRNA sequence (blt63) were interpreted as indicating areas with a high capacity for protein synthesis and, presumably, high levels of metabolism and total mRNA. blt101 and blt14 were more strongly expressed toward the lower part of the crown, whereas blt63 was more strongly expressed toward the upper part; expression of blt4.9 was confined to the epidermis. Thus, viewed broadly, tissues strongly expressing the cold-induced genes did not correlate with tissues having high metabolic activity. Detailed comparisons confirmed this: (a) none of the three cold-induced genes was detectably expressed in the tunica, although blt63 was most strongly expressed there; (b) the distribution of expression of blt101, and partly of blt14, in the tissue layers around and between the vascular bundles in the vascular-transition zone was the reverse of the distribution of expression of blt63 in this region. This indicates that the cold-induced genes studied here do not have a role in general maintenance of metabolism under stress conditions and, hence, that associations with more specific aspects of acclimation occurring at the tissue level might be detectable. Although metabolic responses are an important component of the response to cold, the results clearly indicate that acclimation-related processes and not a high level of total metabolism is the major activity in those tissues in which these cold-induced genes are most strongly expressed.
blt4.9
Some isoforms of nsLTPs are expressed in shoots of nonstressed plants, and these control isoforms and the genes that code for them have been extensively studied (Kader, 1996). The mRNA occurs in the epidermis and the protein is localized extracellularly, in the cuticle and wax layers (Sterk et al., 1991; Clarke and Bohnert, 1993; Thoma et al., 1993; Pyee and Kolattukudy, 1995). Like all other plant nsLTP genes studied so far (Bernhard and Somerville, 1989), the coding region of blt4.9 includes a leader sequence, consistent with an extracellular location for the protein (White et al., 1994). In several members of the Poacea tested the total expression of nsLTP mRNAs is much higher in plants grown in environments imposing cold or dehydrative stresses than in nonstressful environments (H. vulgare: White et al., 1994; Hordeum murinum: Rixon et al., 1994; Lophopyrum elongatum, and Agropyron desertorum: Tabaei Aghdaei, 1997). Clearly, the question arises of whether the stress-associated isoforms have the same function(s) as those suggested for the nonstress-associated isoforms; if they do, a similar location for expression would be predicted. Consistent with this, our results confirm that the site of expression of the cold-inducible nsLTP genes in barley is the epidermis.
The functions suggested for the nonstress-associated isoforms of shoot nsLTPs include roles in cuticle formation (Sterk et al, 1991; Meijer et al., 1993) and in resistance to pathogens (Molina et al., 1993). These functions, if correct, may indicate a role for stronger expression under stress conditions. In the case of cold acclimation, the plant can be regarded more accurately as acclimating to winter stress. This can include conditions in which water uptake from the soil is limited because of soil freezing or adverse effects of cold on root-uptake processes, combining with conditions in which the sun, warming the leaf, or high winds cause rapid depletion of shoot moisture. Under these conditions, cuticular properties may contribute to limiting moisture loss from the shoot, helping reduce windburn and sunburn.
If the role of nsLTPs is to help reduce stress-induced shoot water loss, then one would expect this function to be applied to all parts of the leaf surface; therefore, all epidermal cells should show up-regulation of stress-induced nsLTP mRNA levels. Consistent with this, blt4.9 mRNA was strongly expressed in all epidermal cells of emerging leaves of the cold-acclimated barley.
Experiments with maize and barley have also suggested expression in vascular tissues (Sossountzov et al., 1991; Molina and García-Olmedo, 1993). All of the studies designed to test the location of expression have pointed to the epidermis, whereas only some studies have identified expression in vascular tissue. Thus, it is not clear whether this reflects species or cultivar differences or whether, as indicated by Sossountzov et al. (1991), vascular tissue is a site in which only some isoforms are expressed. However, it is clear from the in situ results presented here that, at least in the barley cv Igri, the cold-inducible nsLTP mRNAs detected with the blt4.9 probe are present only in the epidermis.
Expression of nsLTP genes in the crown may have a role similar to their expression in the leaf. Snow molds make up a group of pathogens attacking the crown of overwintering cereals and grasses (Gaudet, 1994); therefore, it is possible that genes contributing to pathogen resistance would be expressed in the crown. On the other hand, extraorgan ice can dehydrate cereal crown cells (Pearce and Willison, 1985a); therefore, a role in slowing cell dehydration by slowing water transport through the cuticle (to condense on the extraorgan ice) is also a possibility.
blt101 and blt14
The central region of the base of the stem in overwintering grasses and cereals is of particular interest in relation to their susceptibility to frost, because this region, which contains the vascular-transition zone, is relatively susceptible to freezing damage. Both the vascular-transition zone and the apical meristem are more susceptible than other parts of the crown to freezing damage. In wheat the vascular-transition zone is the more susceptible of the two (Tanino and McKersie, 1985), whereas in Dactylis glomerata the apex is slightly more susceptible (Shibata and Shimada, 1986). When barley cv Igri was acclimated in our experiments, the central basal region and shoot apex were of similar susceptibility to freezing damage, and all other parts of the crown were more tolerant.
blt14 and blt101 were strongly expressed in perivascular cell layers in the vascular-transition zone of the crown of cold-acclimated barley. They were also expressed in the inner regions of the cortex, including the basal part of the crown adjacent to the vascular-transition zone. Thus, it is possible that they have a role in acclimation in this region, which is relatively susceptible to freezing stress. The survival of this part correlates with the survival of the tiller; therefore, the demonstration here of genes expressed in this part could be a key to understanding frost survival of the whole plant. In wheat a late embryogenesis abundant-like protein coded for by the Wcs120 gene is also expressed in or near crown vascular tissue during cold acclimation (Houde et al., 1995).
blt14 and blt101 did not have identical patterns of expression. There were only two tissues in the crown in which blt14 was expressed, in the cortex (especially the inner region) and in an arc of tissue partly surrounding the vascular bundles in the vascular-transition zone. blt101, however, was also expressed more generally in the crown, including in the apical pith. Therefore, the two genes do not necessarily relate to the same aspects of low-temperature acclimation.
Both blt14 and blt101 (and Wsc120) are expressed in other tissues and organs apart from their expression in the base of the crown. It follows that the mechanisms of their actions are unlikely to be related to unique features of the vascular-transition zone. Presumably, the genes are strongly expressed in the vascular-transition zone because of its particular susceptibility to freezing damage. However, it is not clear why the vascular-transition zone has more susceptibility than most other parts.
Olien (1964) suggested that the crown of barley could be damaged by either direct ice effects, disrupting xylem elements and disrupting tissues within and surrounding the vascular bundles, or indirectly, by freeze-induced dehydration. Which of these predominated, he proposed, depended on the water content of the crown and the consequent difference in ice crystal size and location. Dehydration is probably the most common cause of freeze-induced cell death in plants and is not dependent on specific tissue structures or specific locations. In contrast, direct ice damage, if it occurs, can depend on specific features of the tissue or organ affected. The expression of blt14 in the crown is confined to the cortex and an arc of tissue surrounding the vascular bundles of the transition zone. In addition, it may code for an extracellular protein (Phillips et al., 1997). Therefore, a role involving direct interaction with ice, as other extracellular proteins are proposed to do (Griffiths and Antikienen, 1996), is possible. However, rather than directly interacting with the ice, it is equally possible that its role could be to influence tissue structure or cell-to-cell contact and thus to contribute to resistance to local tissue disruption by ice.
blt101 was expressed, although sometimes at much lower intensity, in most tissues examined. Therefore, it may have a general involvement in tolerance of the cellular dehydration caused by extracellular freezing. The protein it codes for has a leader sequence that may direct it to the secretary pathway, and the mature protein is predicted to be highly hydrophobic (Goddard et al., 1993); therefore, it could be located in the plasma membrane. Membranes, particularly the plasma membrane, are susceptible to damage from freezing-induced dehydration (Pearce and Willison, 1985b) and may well be the limiting factor in cell survival (Steponkus et al., 1990).
The outermost cell layers of the shoot apex did not express any of the cold-induced genes. These cell layers, unlike most other shoot cells, are unexpanded and lack large vacuoles, and consequently they may experience a lesser volumetric collapse than expanded cells when exposed to the same extracellular freezing stress. Hence, it is possible that the specific function of blt101 relates to tolerance of the stresses associated with considerable volumetric collapse.
CONCLUSIONS
Acclimation in the crown is not a single process equally applied in all cells but, rather, involves in different tissues the expression of different cold-inducible genes in different proportions to each other. Suggestions of function must explain this. The cold-induced nsLTP gene is expressed in the epidermis of the leaf and the older parts of the crown and hence has the same location of expression as nsLTPs expressed (at lower levels) in nonstressed plants of other species. Thus, the function of stress-related and nonstress-related expression of nsLTPs may be the same. The strong expression of blt101 and blt14 in tissues of the vascular-transition zone indicates direct roles in the frost tolerance of the crown and therefore of the plant.
Abbreviations:
- EF
elongation factor
- nsLTP
nonspecific lipid-transfer protein
- TTC
2,3,5-triphenyl tetrazolium chloride
Footnotes
The research was supported by the Biotechnological and Biological Sciences Research Council of the UK (grant no. 13/A000191).
LITERATURE CITED
- Bernhard WR, Somerville CR. Coidentity of putative amylase inhibitors from barley and finger millet with phospholipid transfer proteins inferred from amino acid sequence homology. Arch Biochem Biophys. 1989;269:695–697. doi: 10.1016/0003-9861(89)90154-9. [DOI] [PubMed] [Google Scholar]
- Clarke AM, Bohnert HJ. Epidermis-specific transcript. Nucleotide sequence of a full-length cDNA of EP112, encoding a putative lipid transfer protein. Plant Physiol. 1993;103:677–678. doi: 10.1104/pp.103.2.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn MA, Goddard NJ, Zhang L, Pearce RS, Hughes MA. Different control mechanisms mediate the low temperature response of genes in barley. Plant Mol Biol. 1994;24:879–888. doi: 10.1007/BF00014442. [DOI] [PubMed] [Google Scholar]
- Dunn MA, Hughes MA, Pearce RS, Jack PL. Molecular characterisation of a barley gene induced by cold treatment. J Exp Bot. 1990;41:1405–1413. [Google Scholar]
- Dunn MA, Hughes MA, Zhang L, Pearce RS, Quigley AS, Jack PL. Nucleotide sequence and molecular analysis of the low temperature induced cereal gene, BLT4. Mol Gen Genet. 1991;229:389–394. doi: 10.1007/BF00267460. [DOI] [PubMed] [Google Scholar]
- Dunn MA, Morris A, Jack PL, Hughes MA. A low-temperature-responsive translation elongation factor 1α from barley (Hordeum vulgare L.) Plant Mol Biol. 1993;23:221–225. doi: 10.1007/BF00021434. [DOI] [PubMed] [Google Scholar]
- Gaudet DA. Progress towards understanding interaction between cold-hardiness and snow mold resistance and development of resistant cultivars. Can J Plant Pathol. 1994;16:241–246. [Google Scholar]
- Goddard NJ, Dunn MA, Zhang L, White AJ, Jack PL, Hughes MA. Molecular analysis and spatial expression pattern of a low-temperature-specific barley gene, blt101. Plant Mol Biol. 1993;23:871–897. doi: 10.1007/BF00021541. [DOI] [PubMed] [Google Scholar]
- Griffiths M, Antikienen M. Extracellular ice formation in freezing-tolerant plants. Adv Low-Temp Biol. 1996;3:107–139. [Google Scholar]
- Gurr SJ, McPherson MJ, Bowles DJ. Molecular Plant Pathology—A Practical Approach, Vol 1. Oxford, UK: IRC Press; 1992. [Google Scholar]
- Guy CL. Cold acclimation and freezing stress tolerance: role of protein metabolism. Annu Rev Plant Physiol Plant Mol Biol. 1990;41:187–223. [Google Scholar]
- Houde M, Daniel C, Lachapelle M, Allard F, Laliberté S, Sarhan F. Immunolocalisation of freezing-tolerance-associated proteins in the cytoplasm and nucleoplasm of wheat crown tissues. Plant J. 1995;8:583–593. doi: 10.1046/j.1365-313x.1995.8040583.x. [DOI] [PubMed] [Google Scholar]
- Kader J-C. Lipid-transfer proteins in plants. Annu Rev Plant Physiol Plant Mol Biol. 1996;47:627–654. doi: 10.1146/annurev.arplant.47.1.627. [DOI] [PubMed] [Google Scholar]
- Meijer EA, de Vries SC, Sterk P, Gadella DWJ, Wirtz KWA, Hendriks T. Characterisation of the nonspecific lipid transfer protein—EP2 from carrot (Daucus carota L.) Mol Cell Biochem. 1993;123:159–166. doi: 10.1007/BF01076488. [DOI] [PubMed] [Google Scholar]
- Molina A, García-Olmedo F. Developmental and pathogen-induced expression of three barley genes encoding lipid transfer proteins. Plant J. 1993;4:983–991. doi: 10.1046/j.1365-313x.1993.04060983.x. [DOI] [PubMed] [Google Scholar]
- Molina A, Segura A, García-Olmedo F. Lipid transfer proteins (nsLTPs) from barley and maize leaves are potent inhibitors of bacterial and fungal plant pathogens. FEBS Lett. 1993;316:119–122. doi: 10.1016/0014-5793(93)81198-9. [DOI] [PubMed] [Google Scholar]
- Olien CR. Freezing processes in the crown of Hudson barley, Hordeum vulgare (L. emend. Lam.) Hudson. Crop Sci. 1964;4:91–95. [Google Scholar]
- Olien CR. Freezing stresses and survival. Annu Rev Plant Physiol. 1967;18:387–408. [Google Scholar]
- Pearce RS, Dunn MA, Rixon JE, Harrison P, Hughes MA. Expression of cold-induced genes and frost hardiness in the crown meristem of young barley (Hordeum vulgare L. cv. Igri) plants grown in different environments. Plant Cell Environ. 1996;12:275–290. [Google Scholar]
- Pearce RS, Willison JHM. Wheat tissues freeze-etched during exposure to extracellular freezing: distribution of ice. Planta. 1985a;163:295–303. doi: 10.1007/BF00395139. [DOI] [PubMed] [Google Scholar]
- Pearce RS, Willison JHM. A freeze-etch study of the effect of extracellular freezing on the cellular membranes of wheat. Planta. 1985b;163:304–316. doi: 10.1007/BF00395140. [DOI] [PubMed] [Google Scholar]
- Pearson ES, Hartley HO. Biometrika Tables for Statisticians, Vol 1. Cambridge, UK: Cambridge University Press; 1956. [Google Scholar]
- Phillips JR, Dunn MA, Hughes MA. mRNA stability and localization of the low-temperature-responsive barley gene family blt14. Plant Mol Biol. 1997;33:1013–1023. doi: 10.1023/a:1005717613224. [DOI] [PubMed] [Google Scholar]
- Pyee J, Kolattukudy PE. The gene for the major cuticular wax-associated protein and three homologous genes from broccoli (Brassica oleracea) and their expression patterns. Plant J. 1995;7:49–59. doi: 10.1046/j.1365-313x.1995.07010049.x. [DOI] [PubMed] [Google Scholar]
- Rixon JE, Pearce RS, Dunn MA, Hughes MA, Atherton KM, Cairns PM. Expression of low temperature induced genes in different cultivars and populations of Hordeum (supplement) J Exp Bot. 1994;45:21. [Google Scholar]
- Shibata S, Shimada T. Anatomical observation of the development of freezing injury in orchardgrass crown. J Jpn Grass Sci. 1986;32:197–204. [Google Scholar]
- Sossountzov L, Riz-Avila L, Vignols F, Jolliot A, Arondel V, Tchang F, Grosbois M, Guerbette F, Miginiac E, Delseny M and others. Spatial and temporal expression of a maize lipid transfer protein gene. Plant Cell. 1991;3:923–933. doi: 10.1105/tpc.3.9.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steponkus PL, Lynch DV, Uemura M. The influence of cold-acclimation on the lipid-composition and cryobehavior of the plasma-membrane of isolated rye protoplasts. Philos Trans R Soc Lond-Biol Sci. 1990;326:571–583. [Google Scholar]
- Sterk P, Booij H, Schellekens GA, Van Kammen A, de Vries SC. Cell-specific expression of the carrot EP2 lipid transfer protein gene. Plant Cell. 1991;3:907–921. doi: 10.1105/tpc.3.9.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabaei Aghdaei SR (1997) Studies of stress responses in Gramineae (Poaceae) using biotechnological methods. PhD thesis. University of Newcastle upon Tyne, UK
- Tanino KK, McKersie BD. Injury within the crown of winter wheat seedlings after freezing and icing stress. Can J Bot. 1985;63:432–436. [Google Scholar]
- Thoma S, Kaneko Y, Somerville C. A nonspecific lipid transfer protein from Arabidopsis is a cell wall protein. Plant J. 1993;3:427–436. doi: 10.1046/j.1365-313x.1993.t01-25-00999.x. [DOI] [PubMed] [Google Scholar]
- White AJ, Dunn MA, Brown K, Hughes MA. Comparative analysis of genomic sequence and expression of a lipid transfer protein gene family in winter barley. J Exp Bot. 1994;45:1885–1892. [Google Scholar]