Abstract
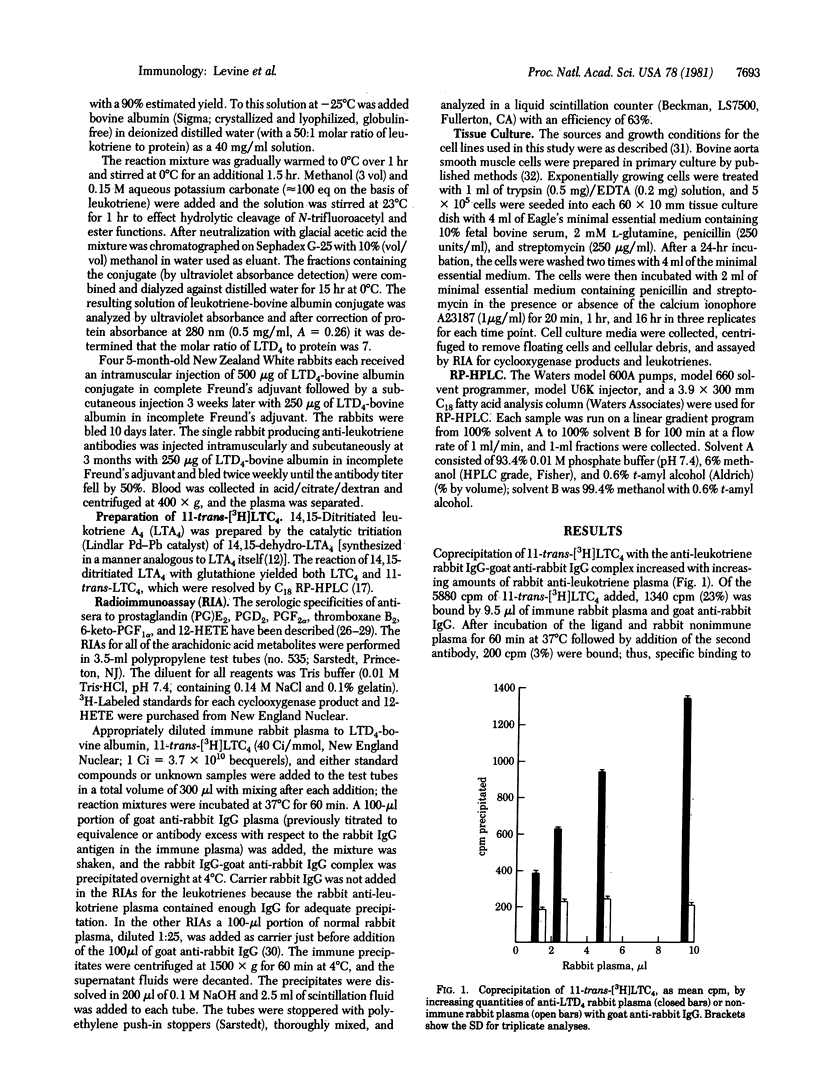
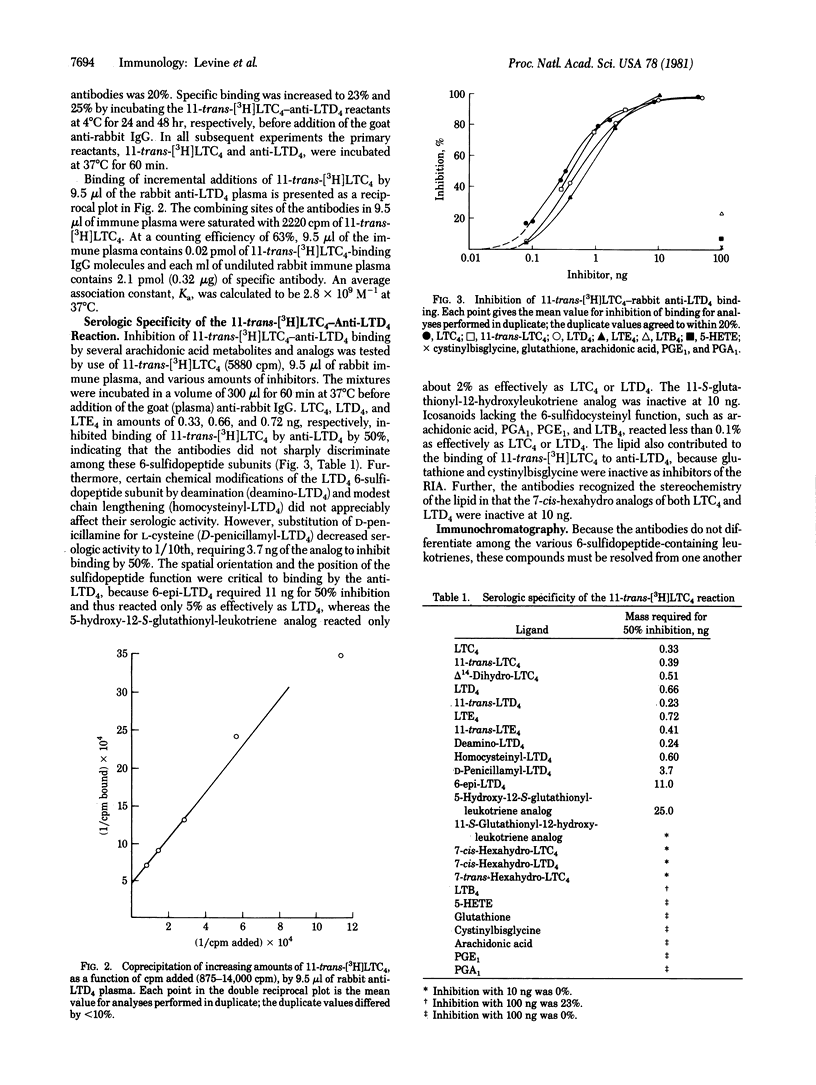
A rabbit immunized with a conjugate of leukotriene D4 (LTD4) and bovine albumin via the icosanoid carboxyl produced antibodies with comparable affinities for leukotrienes C4, D4, and E4 (LTC4, LTD4, and LTE4) and their 11-trans stereoisomers. The antibodies bound 3H-labeled 11-trans-LTC4 and 11-trans-LTC4 with the same average association constant (Ka) of 2.8 x 10(9) M-1 at 37 degrees C and were present at a concentration of 0.32 microgram/ml of the immune rabbit plasma. When 9.5 microliter of anti-LTD4 and 108 pmol of 11-trans-[3H]LTC4 (40 Ci/mmol) were incubated in a volume of 300 microliter with LTC4, LTD4, LTE4, or their 11-trans stereoisomers, 50% inhibition of 11-trans-[3H]LTC4 binding was achieved at levels varying between 0.3 and 0.7 ng. As assessed with synthetic analogs of the natural leukotrienes, the antibodies recognized neither those changes within the 6-sulfidopeptide unit of LTD4 produced by deamination or modest peptide lengthening nor the specific stereochemistry of the delta 14-cis double bond. However, the antibodies did recognize the triene lipid domain and the position and spatial orientation of the glutathione or cysteinylglycine function. Binding of 11-trans-[3H]LTC4 by anti-LTD4 was not inhibited by glutathione, cystinylbisglycine, arachidonic acid, or 5-hydroxy-6,8,11,14-icosatetraenoic acid, and leukotriene B4 (LTB4) was about 1/1000th as active as LTC4, LTD4, or LTE4. Mouse lymphoma (WEHI-5) and rat basophil leukemia (RBL-1) cells, when stimulated with calcium ionophore A23187, each produced immunoreactive leukotrienes; and LTC4, LTD4, and LTE4 from RBL-1 cells were individually quantitated by radioimmunoassay after resolution by high-performance liquid chromatography.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alam I., Ohuchi K., Levine L. Determination of cyclooxygenase products and prostaglandin metabolites using high-pressure liquid chromatography and radioimmunoassay. Anal Biochem. 1979 Mar;93(2):339–345. doi: 10.1016/s0003-2697(79)80160-8. [DOI] [PubMed] [Google Scholar]
- BROCKLEHURST W. E. Occurrence of an unidentified substance during anaphylactic shock in cavy lung. J Physiol. 1953 Apr 28;120(1-2):16P–17P. [PubMed] [Google Scholar]
- BROCKLEHURST W. E. Slow reacting substance and related compounds. Prog Allergy. 1962;6:539–558. [PubMed] [Google Scholar]
- Bach M. K., Brashler J. R., Gorman R. R. On the structure of slow reacting substance of anaphylaxis: evidence of biosynthesis from arachidonic acid. Prostaglandins. 1977 Jul;14(1):21–38. doi: 10.1016/0090-6980(77)90154-x. [DOI] [PubMed] [Google Scholar]
- Coughlin S. R., Moskowitz M. A., Zetter B. R., Antoniades H. N., Levine L. Platelet-dependent stimulation of prostacyclin synthesis by platelet-derived growth factor. Nature. 1980 Dec 11;288(5791):600–602. doi: 10.1038/288600a0. [DOI] [PubMed] [Google Scholar]
- Drazen J. M., Lewis R. A., Austen K. F., Toda M., Brion F., Marfat A., Corey E. J. Contractile activities of structural analogs of leukotrienes C and D: necessity of a hydrophobic region. Proc Natl Acad Sci U S A. 1981 May;78(5):3195–3198. doi: 10.1073/pnas.78.5.3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaudet R. J., Alam I., Levine L. Accumulation of cyclooxygenase products of arachidonic acid metabolism in gerbil brain during reperfusion after bilateral common carotid artery occlusion. J Neurochem. 1980 Sep;35(3):653–658. doi: 10.1111/j.1471-4159.1980.tb03704.x. [DOI] [PubMed] [Google Scholar]
- Gershman H., Powers E., Levine L., Van Vunakis H. Radioimmunoassay of prostaglandins, angiotensin, digoxin, morphine and adenosine-3',5'-cyclic-monophosphate with nitrocellulose membranes. Prostaglandins. 1972 May;1(5):407–423. doi: 10.1016/0090-6980(72)90055-x. [DOI] [PubMed] [Google Scholar]
- Jakschik B. A., Falkenhein S., Parker C. W. Precursor role of arachidonic acid in release of slow reacting substance from rat basophilic leukemia cells. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4577–4581. doi: 10.1073/pnas.74.10.4577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine L., Alam I., Gjika H., Carty T. J., Goetzl E. J. The development of a radioimmunoassay for 12-L-hydroxyeicosatetraenoic acid. Prostaglandins. 1980 Nov;20(5):923–934. doi: 10.1016/0090-6980(80)90142-2. [DOI] [PubMed] [Google Scholar]
- Levine L., Gjtierrez Cernosek R. M., Van Vunakis H. Specificities of prostaglandins B 1 , F 1 , and F 2 antigen-antibody reactions. J Biol Chem. 1971 Nov 25;246(22):6782–6785. [PubMed] [Google Scholar]
- Lewis R. A., Austen K. F., Drazen J. M., Clark D. A., Marfat A., Corey E. J. Slow reacting substances of anaphylaxis: identification of leukotrienes C-1 and D from human and rat sources. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3710–3714. doi: 10.1073/pnas.77.6.3710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis R. A., Drazen J. M., Austen K. F., Clark D. A., Corey E. J. Identification of the C(6)-S-conjugate of leukotriene A with cysteine as a naturally occurring slow reacting substance of anaphylaxis (SRS-A). Importance of the 11-cis-geometry for biological activity. Biochem Biophys Res Commun. 1980 Sep 16;96(1):271–277. doi: 10.1016/0006-291x(80)91210-3. [DOI] [PubMed] [Google Scholar]
- Lewis R. A., Drazen J. M., Austen K. F., Toda M., Brion F., Marfat A., Corey E. J. Contractile activities of structural analogs of leukotrienes C and D: role of the polar substituents. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4579–4583. doi: 10.1073/pnas.78.7.4579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris H. R., Taylor G. W., Piper P. J., Tippins J. R. Structure of slow-reacting substance of anaphylaxis from guinea-pig lung. Nature. 1980 May 8;285(5760):104–106. doi: 10.1038/285104a0. [DOI] [PubMed] [Google Scholar]
- Murphy R. C., Hammarström S., Samuelsson B. Leukotriene C: a slow-reacting substance from murine mastocytoma cells. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4275–4279. doi: 10.1073/pnas.76.9.4275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orange R. P., Austen K. F. Slow reacting substance of anaphylaxis. Adv Immunol. 1969;10:105–144. doi: 10.1016/s0065-2776(08)60416-2. [DOI] [PubMed] [Google Scholar]
- Orange R. P., Chang P. L. The effect of thiols on immunologic release of slow reacting substance of anaphylaxis. I. Human lung. J Immunol. 1975 Oct;115(4):1072–1077. [PubMed] [Google Scholar]
- Orange R. P., Moore E. G. The effect of thiols on the immunologic release of slow reacting substance of anaphylaxis. II. Other in vitro and in vivo models. J Immunol. 1976 Feb;116(2):392–397. [PubMed] [Google Scholar]
- Orange R. P., Murphy R. C., Austen K. F. Inactivation of slow reacting substance of anaphylaxins (SRS-A) by arylsulfatases. J Immunol. 1974 Jul;113(1):316–322. [PubMed] [Google Scholar]
- Orange R. P., Murphy R. C., Karnovsky M. L., Austen K. F. The physicochemical characteristics and purification of slow-reacting substance of anaphylaxis. J Immunol. 1973 Mar;110(3):760–770. [PubMed] [Google Scholar]
- Orning L., Hammarström S., Samuelsson B. Leukotriene D: a slow reacting substance from rat basophilic leukemia cells. Proc Natl Acad Sci U S A. 1980 Apr;77(4):2014–2017. doi: 10.1073/pnas.77.4.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigas A., Levine L. Effect of propranolol on arachidonic acid metabolism by cells in culture. Prostaglandins Med. 1981 Sep;7(3):217–228. doi: 10.1016/0161-4630(81)90101-4. [DOI] [PubMed] [Google Scholar]



