Abstract
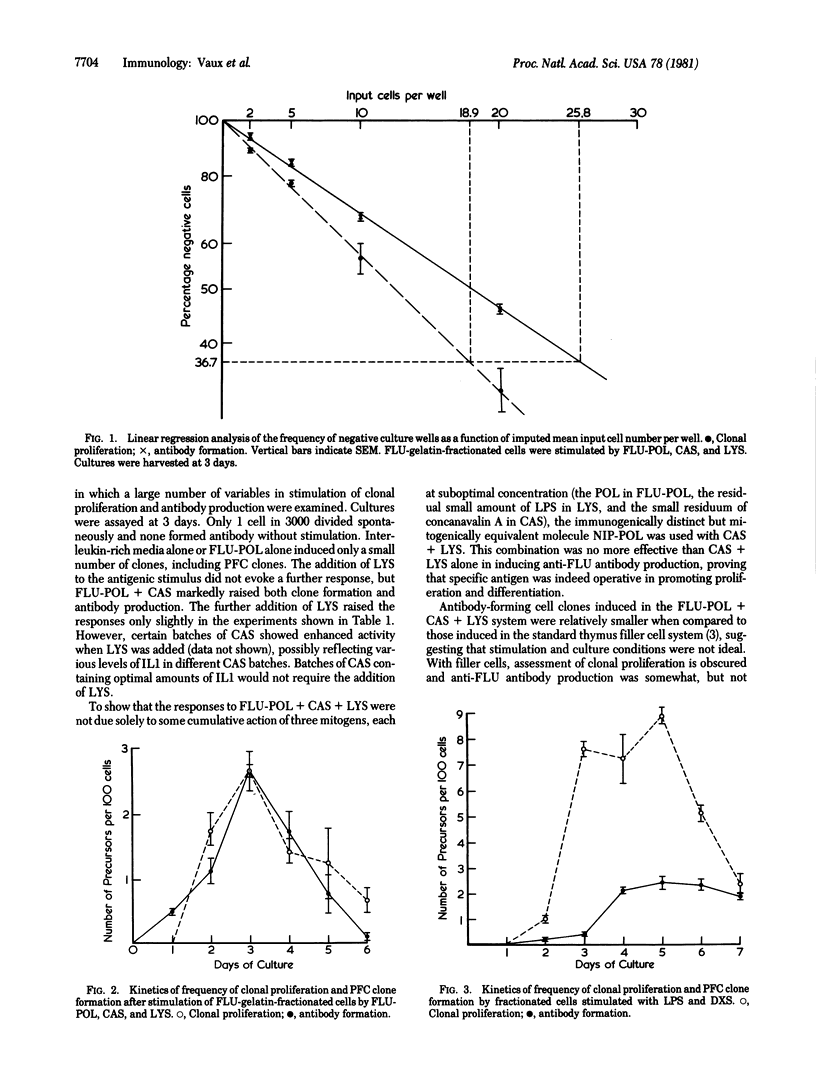
CBA mouse spleen cells were subjected to hapten affinity fractionation on thin layers of fluorescein (FLU)-gelatin. This procedure yields 97% B cells with varying FLU-binding avidities. One to 30 cells were placed in 10-microliter microcultures without any filler or accessory cells. the T-independent antigen polymerized flagellin coupled to FLU (FLU-POL) was ineffective in stimulating these cells to clonal proliferation or antibody production when used alone. Unpurified preparations rich in interleukins also failed to stimulate the cells. When specific antigen, but not in irrelevant hapten-POL, was combined with the interleukins, clonal proliferation was stimulated and most clones produced anti-FLU antibody-forming cells. The frequency of antibody-forming clones was only slightly lower than that in a system using antigen plus filler cells. In the absence of added interleukins, the mitogens Escherichia coli lipopolysaccharide plus dextran sulfate induced equivalent antibody production. However, a higher frequency of clonal proliferation was noted. Added interleukins did not aid these mitogen-driven responses. Such an antigen-dependent cloning system, free of filler and accessory cells, should permit more precise analysis of the respective roles of antigens and interleukins in the physiology of antibody-forming clone formation.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson J., Coutinho A., Melchers F., Watanabe T. Growth and maturation of single clones of normal murine T and B lymphocytes in vitro. Cold Spring Harb Symp Quant Biol. 1977;41(Pt 1):227–236. doi: 10.1101/sqb.1977.041.01.028. [DOI] [PubMed] [Google Scholar]
- Claësson M. H., Layton J. E., Luckenbach G. A. Specific antibody-forming B-lymphocyte colonies. I. Distribution and nature of SRBC antibody-forming B-lymphocyte colonies in mouse lyphomyeloid organs. Immunology. 1978 Aug;35(2):397–406. [PMC free article] [PubMed] [Google Scholar]
- Dutton R. W. Separate signals for the initiation of proliferation and differentiation in the b cell response to antigen. Transplant Rev. 1975;23:66–77. doi: 10.1111/j.1600-065x.1975.tb00149.x. [DOI] [PubMed] [Google Scholar]
- Gery I., Waksman B. H. Potentiation of the T-lymphocyte response to mitogens. II. The cellular source of potentiating mediator(s). J Exp Med. 1972 Jul 1;136(1):143–155. doi: 10.1084/jem.136.1.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goding J. W. Conjugation of antibodies with fluorochromes: modifications to the standard methods. J Immunol Methods. 1976;13(3-4):215–226. doi: 10.1016/0022-1759(76)90068-5. [DOI] [PubMed] [Google Scholar]
- Haas W., Layton J. E. Separation of antigen-specific lymphocytes. I. Enrichment of antigen-binding cells. J Exp Med. 1975 May 1;141(5):1004–1014. doi: 10.1084/jem.141.5.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann M. K. Macrophages and T cells control distinct phases of B cell differentiation in the humoral immune response in vitro. J Immunol. 1980 Nov;125(5):2076–2081. [PubMed] [Google Scholar]
- Kettman J., Wetzel M. Antibody synthesis in vitro, a marker of B cell differentiation. J Immunol Methods. 1980;39(3):203–222. doi: 10.1016/0022-1759(80)90056-3. [DOI] [PubMed] [Google Scholar]
- Klinman N. R., Press J. L. The B cell specificity repertoire: its relationship to definable subpopulations. Transplant Rev. 1975;24:41–83. doi: 10.1111/j.1600-065x.1975.tb00165.x. [DOI] [PubMed] [Google Scholar]
- Kurland J. I., Kincade P. W., Moore M. A. Regulation of B-lymphocyte clonal proliferation by stimulatory and inhibitory macrophage-derived factors. J Exp Med. 1977 Nov 1;146(5):1420–1435. doi: 10.1084/jem.146.5.1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson E. L. Mechanism of T cell activation. II. Antigen- and lectin-dependent acquisition of responsiveness to TCGF is a nonmitogenic, active response of resting T cells. J Immunol. 1981 Apr;126(4):1323–1326. [PubMed] [Google Scholar]
- Lefkovits I. Induction of antibody-forming cell clones in microcultures. Eur J Immunol. 1972 Aug;2(4):360–366. doi: 10.1002/eji.1830020412. [DOI] [PubMed] [Google Scholar]
- Marbrook J., Haskill J. S. The in vitro response to sheep erythrocytes by mouse spleen cells: segregation of distinct events leading to antibody formation. Cell Immunol. 1974 Jul;13(1):12–21. doi: 10.1016/0008-8749(74)90222-6. [DOI] [PubMed] [Google Scholar]
- Metcalf D., Nossal G. J., Warner N. L., Miller J. F., Mandel T. E., Layton J. E., Gutman G. A. Growth of B-lymphocyte colonies in vitro. J Exp Med. 1975 Dec 1;142(6):1534–1549. doi: 10.1084/jem.142.6.1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nossal G. J., Pike B. L., Battye F. L. Mechanisms of clonal abortion tolerogenesis. II. Clonal behaviour of immature B cells following exposure to anti-mu chain antibody. Immunology. 1979 May;37(1):203–215. [PMC free article] [PubMed] [Google Scholar]
- Nossal G. J., Pike B. L., Battye F. L. Sequential use of hapten-gelatin fractionation and fluorescence-activated cell sorting in the enrichment of hapten-specific B llymphocytes. Eur J Immunol. 1978 Mar;8(3):151–157. doi: 10.1002/eji.1830080302. [DOI] [PubMed] [Google Scholar]
- Nossal G. J., Pike B. L. Functional clonal deletion in immunological tolerance to major histocompatibility complex antigens. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3844–3847. doi: 10.1073/pnas.78.6.3844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nossal G. J., Pike B. L. Improved procedure for the fraction and in vitro stimulation of hapten-specific B lymphocytes. J Immunol. 1978 Jan;120(1):145–150. [PubMed] [Google Scholar]
- Nossal G. J., Pike B. L. Single cell studies on the antibody-forming potential of fractionated, hapten-specific B lymphocytes. Immunology. 1976 Feb;30(2):189–202. [PMC free article] [PubMed] [Google Scholar]
- Pike B. L. A microculture method for the generation of primary immune responses in vitro. J Immunol Methods. 1975 Nov;9(1):85–104. doi: 10.1016/0022-1759(75)90039-3. [DOI] [PubMed] [Google Scholar]
- Pike B. L., Nossal G. J. Requirement for persistent extracellular antigen in cultures of antigen-binding B lymphocytes. J Exp Med. 1976 Aug 1;144(2):568–571. doi: 10.1084/jem.144.2.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillai P. S., Scott D. W. Hapten-specific murine colony-forming B cells: in vitro response of colonies to fluoresceinated thymus independent antigens. J Immunol. 1981 May;126(5):1883–1886. [PubMed] [Google Scholar]
- Scher I., Steinberg A. D., Berning A. K., Paul W. E. X-linked B-lymphocyte immune defect in CBA/N mice. II. Studies of the mechanisms underlying the immune defect. J Exp Med. 1975 Sep 1;142(3):637–650. doi: 10.1084/jem.142.3.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schimpl A., Wecker E. A third signal in B cell activation given by TRF. Transplant Rev. 1975;23:176–188. doi: 10.1111/j.1600-065x.1975.tb00157.x. [DOI] [PubMed] [Google Scholar]
- Watson J., Aarden L. A., Shaw J., Paetkau V. Molecular and quantitative analysis of helper T cell-replacing factors on the induction of antigen-sensitive B and T lymphocytes. J Immunol. 1979 May;122(5):1633–1638. [PubMed] [Google Scholar]
- Watson J., Gillis S., Marbrook J., Mochizuki D., Smith K. A. Biochemical and biological characterization of lymphocyte regulatory molecules. I. Purification of a class of murine lymphokines. J Exp Med. 1979 Oct 1;150(4):849–861. doi: 10.1084/jem.150.4.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetzel G. D., Kettman J. R. Activation of murine B cells. II. Dextran sulfate removes the requirement for cellular interaction during lipopolysaccharide-induced mitogenesis. Cell Immunol. 1981 Jun;61(1):176–189. doi: 10.1016/0008-8749(81)90364-6. [DOI] [PubMed] [Google Scholar]
- Wetzel G. D., Kettman J. R. Activation of murine B lymphocytes. III. Stimulation of B lymphocyte clonal growth with lipopolysaccharide and dextran sulfate. J Immunol. 1981 Feb;126(2):723–728. [PubMed] [Google Scholar]



