Abstract
The mitogen-activated protein kinase kinase kinase (MAPKKK) Constitutive Triple-Response1 (CTR1) plays a key role in mediating ethylene receptor signaling via its N-terminal interaction with the ethylene receptor C-terminal histidine kinase (HK) domain. Loss-of-function mutations of CTR1 prevent ethylene receptor signaling, and corresponding ctr1 mutants show a constitutive ethylene response phenotype. We recently reported in Plant Physiology that expression of the truncated ethylene receptor Ethylene Response1 (ETR1) isoforms etr11-349 and dominant ethylene-insensitive etr1-11-349, lacking the C-terminal HK and receiver domains, both suppressed the ctr1 mutant phenotype. Therefore, the ETR1 N terminus is capable of receptor signaling independent of CTR1. The constitutive ethylene response phenotype is stronger for ctr1-1 than ctr1-1 lines expressing the etr11-349 transgene, so N-terminal signaling by the full-length but not truncated ETR1 is inhibited by ctr1-1. We address possible modulations of ETR1 N-terminal signaling with docking of CTR1 on the ETR1 HK domain.
Keywords: ethylene, ethylene receptor, ETR1, CTR1, ETR1 N-terminal signaling
The gaseous plant hormone ethylene is perceived by an ethylene receptor family of five members in Arabidopsis and regulates many aspects of biological processes.1-6 The ethylene receptors structurally resemble prokaryotic two-component histidine kinase (HK) modules, which perceive and transduce external signals to trigger an array of responses. Acting directly downstream of the ethylene receptors is the mitogen-activated protein kinase kinase kinase (MAPKKK) Constitutive Triple-Response1 (CTR1) protein, which mediates the receptor signal by docking its N portion to the receptor C-terminal HK domain.7,8 Mutations to abolish HK activity or delete the HK and receiver domains do not prevent ETR1 receptor signaling, and the biochemical nature of the ethylene receptor signal is currently unknown.9-12
Members of the ethylene receptor family act cooperatively as clusters.13,14 The ETR1 C terminus is the CTR1-docking site for the receptor signal output; receptor signaling by truncated, C-terminus–lacking ETR1 isoforms is conceivably mediated by CTR1 via cooperation with other full-length ethylene receptors or by interaction with a third component.10,15 Alternatively, the truncated ETR1 isoforms may mediate the receptor signal independent of CTR1.10 The latter scenario is supported by our recent finding that expression of the transgenes etr11–349 and dominant ethylene-insensitive etr1–11–349, which encode the ETR1 isoforms lacking the HK and receiver domains, each greatly suppressed the ctr1–1 and ctr1–2 mutant phenotype.12 Excess CTR1 N-terminal CTR17–560 prevents the receptor signaling, possibly by titrating out available ethylene receptors, and CTR17–560 overexpressor shows a constitutive ethylene response phenotype.8,12 Cooperative ethylene receptor signaling of the truncated etr11–349 and etr1–11–349 isoforms with other family members was inferred from transformation studies: expression of etr11–349 or etr1–11–349 suppressed the constitutive ethylene response phenotype of CTR17–560 overexpressor and restored ethylene insensitivity conferred by a dominant ethylene-insensitive receptor in the ctr1–1 background.12
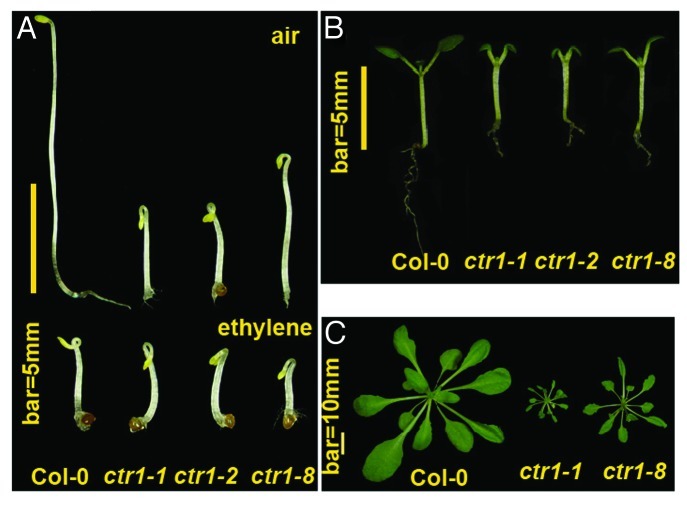
Of note, the ctr1–1 and ctr1–2 mutants show a constitutive ethylene response phenotype throughout development, so the ctr1–1 and ctr1–2 isoforms prevent full-length ETR1 N-terminal signaling. Both etr11–349 and etr1–11–349 do not have the CTR1 docking site, and probably their receptor signaling is not prevented by these ctr1 isoforms. The hypothesis that the full-length ETR1 can mediate N-terminal signaling without CTR1 docking can be addressed in a mutation background with lack of CTR1 or the ETR1–CTR1 association. The ethylene receptors are associated with the endoplasmic reticulum, and the ethylene receptor–CTR1 interaction brings CTR1 to the membrane fraction.16 The ctr1–8 mutation results from the G354E substitution, which greatly prevents the ETR1–CTR1 interaction, and the ctr1–8 protein predominantly localizes to the soluble but not membrane fraction. ctr1–8 could be a strong allele because ctr1–8 protein cannot act directly with ethylene receptors. Interestingly, the ctr1–8 mutant shows a relatively mild constitutive ethylene response phenotype throughout development (Fig. 1). The mild phenotype of ctr1–8 as compared with ctr1–1 and ctr1–2 could imply that ETR1 receptor signaling is mediated without ctr1–8 docking to the ETR1 HK domain. Therefore, ETR1 can still mediate the N-terminal signaling without the association of CTR1 and the HK domain, and the GAF domain is likely responsible for ETR1 N-terminal signal output (Fig. 2A and 2B). A CTR1 locus-deficient null mutant needs to be identified to support this hypothesis. A very small amount of ctr1–8 may still associate with ETR1 to mediate the receptor signaling via the HK domain; however, we do not know whether the ctr1–8 isoform can mediate the receptor signaling.
Figure 1. The ctr1–8 mutant shows a mild constitutive ethylene response phenotype. The constitutive ethylene response phenotypes are shown for wild type (Col-0), ctr1–1, ctr1–2, and ctr1–8. a. Etiolated seedlings of air-grown ctr1–8 show weaker hypocotyl growth inhibition than ctr1–1 and ctr1–2. Ethylene treatment inhibits the seedling hypocotyl elongation of each genotype to a similar level. b. Light-grown seedlings show stronger growth inhibition in cotyledons and roots for ctr1–1 and ctr1–2 than ctr1–8. c. At the adult stage, ctr1–8 produces a much larger rosette than ctr1–1 and smaller rosette than the wild-type plant.
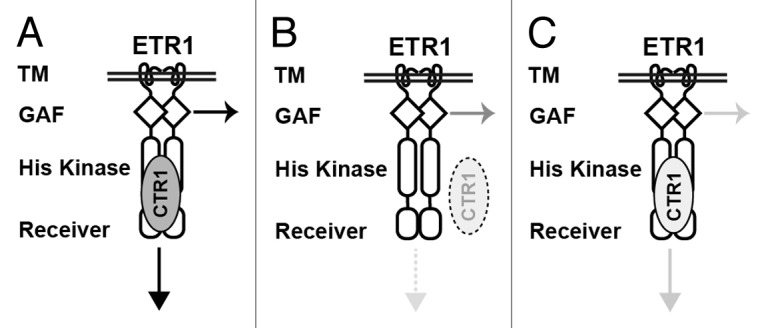
Figure 2. A model for possible modulation of the ETR1 receptor signaling by CTR1. (A) The docking of CTR1 to the ETR1 receptor facilitates receptor signaling by the N terminus and histidine kinase (HK) domain. (B) Without the docking of CTR1 to the ETR1 HK domain, ETR1 receptor signaling is partly mediated by the N terminus but not HK domain. (C) When associated with CTR1 of reduced kinase activity, the ETR1 receptor signaling mediated by N and C termini is attenuated. Arrows indicate ethylene receptor signaling and shading indicate different levels of strength in receptor signal output (arrows) or CTR1 kinase activity (ovals). Dotted lines indicate the absence of receptor signaling (arrow) or ETR1–CTR1 association (oval). TM, transmembrane domain.
The association of CTR1 kinase activity and strength of the receptor signaling is consistent with the difference in degree of the constitutive ethylene response phenotype between ctr1–1 and ctr1btk. ctr1btk has higher kinase activity than ctr1–1, and the ctr1btk mutant has a weaker constitutive ethylene response phenotype than does ctr1–1 17. Conceivably, signaling by the ETR1 N terminus, as well as C terminus, can be modulated by alterations in the CTR1 kinase activity: the docking of the ETR1 HK domain with CTR1 with high kinase activity may facilitate strong ETR1 receptor signaling. In contrast, when ETR1 associates with CTR1 with weak kinase activity, ETR1 receptor signaling could be weak (Fig. 2A and 2C). CTR1 phosphorylation status could be modulated by a phosphatase or by an ETR1 conformation that is changed on ethylene perception. A recent study showed that HG2 mutations of ETR1 (H353Q and G545A G547A) facilitate ETR1 receptor signaling18; we consider the possibility that the ETR1[HG2] isoform could affect CTR1 phosphorylation and alter receptor signaling strength.
Without knowledge of the biochemical nature of the ethylene receptor signal, we face challenges in revealing the underlying mechanism of the ethylene receptor signal output mediated by the ETR1–CTR1 interaction. The current model for ethylene receptor signaling is proposed primarily from genetic studies. Biochemical evidence is lacking for the model that CTR1 mediates ETR1 receptor signaling via the HK domain. CTR1 may be an activator promoting the ETR1 receptor signaling. Or, ETR1 receptor signaling may be mediated by both the GAF and HK domains by different mechanisms. Biochemical and genetic evidence supports that ethylene receptor signaling strength is associated with CTR1 kinase activity.17 Understanding the regulation of CTR1 kinase activity may advance our knowledge of modulation of ETR1 receptor signaling.
Acknowledgments
This work was supported in part by the National Natural Sciences Foundation of China (31123006, 31070249, and 30770199).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/21545
References
- 1.Guzmán P, Ecker JR. Exploiting the triple response of Arabidopsis to identify ethylene-related mutants. Plant Cell. 1990;2:513–23. doi: 10.1105/tpc.2.6.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahlfors R, Macioszek V, Rudd J, Brosché M, Schlichting R, Scheel D, et al. Stress hormone-independent activation and nuclear translocation of mitogen-activated protein kinases in Arabidopsis thaliana during ozone exposure. Plant J. 2004;40:512–22. doi: 10.1111/j.1365-313X.2004.02229.x. [DOI] [PubMed] [Google Scholar]
- 3.Berrocal-Lobo M, Molina A, Solano R. Constitutive expression of ETHYLENE-RESPONSE-FACTOR1 in Arabidopsis confers resistance to several necrotrophic fungi. Plant J. 2002;29:23–32. doi: 10.1046/j.1365-313x.2002.01191.x. [DOI] [PubMed] [Google Scholar]
- 4.Hua J, Meyerowitz EM. Ethylene responses are negatively regulated by a receptor gene family in Arabidopsis thaliana. Cell. 1998;94:261–71. doi: 10.1016/S0092-8674(00)81425-7. [DOI] [PubMed] [Google Scholar]
- 5.Zhang W, Zhou X, Wen C-K. Modulation of ethylene responses by OsRTH1 overexpression reveals the biological significance of ethylene in rice seedling growth and development. J Exp Bot. 2012;63:4151–64. doi: 10.1093/jxb/ers098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xu C, Gao X, Sun X, Wen C-K. The Basal Level Ethylene Response is Important to the Wall and Endomembrane Structure in the Hypocotyl Cells of Etiolated Arabidopsis Seedlings. J Integr Plant Biol. 2012;54:434–55. doi: 10.1111/j.1744-7909.2012.01130.x. [DOI] [PubMed] [Google Scholar]
- 7.Clark KL, Larsen PB, Wang X, Chang C. Association of the Arabidopsis CTR1 Raf-like kinase with the ETR1 and ERS ethylene receptors. Proc Natl Acad Sci U S A. 1998;95:5401–6. doi: 10.1073/pnas.95.9.5401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang Y, Li H, Hutchison CE, Laskey J, Kieber JJ. Biochemical and functional analysis of CTR1, a protein kinase that negatively regulates ethylene signaling in Arabidopsis. Plant J. 2003;33:221–33. doi: 10.1046/j.1365-313X.2003.01620.x. [DOI] [PubMed] [Google Scholar]
- 9.Qu X, Hall BP, Gao Z, Schaller GE. A strong constitutive ethylene-response phenotype conferred on Arabidopsis plants containing null mutations in the ethylene receptors ETR1 and ERS1. BMC Plant Biol. 2007;7:3. doi: 10.1186/1471-2229-7-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xie F, Liu Q, Wen C-K. Receptor signal output mediated by the ETR1 N terminus is primarily subfamily I receptor dependent. Plant Physiol. 2006;142:492–508. doi: 10.1104/pp.106.082628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang W, Hall AE, O’Malley R, Bleecker AB. Canonical histidine kinase activity of the transmitter domain of the ETR1 ethylene receptor from Arabidopsis is not required for signal transmission. Proc Natl Acad Sci U S A. 2003;100:352–7. doi: 10.1073/pnas.0237085100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qiu L, Xie F, Yu J, Wen C-K. Arabidopsis RTE1 Is Essential to Ethylene Receptor ETR1 Amino-Terminal Signaling Independent of CTR1. Plant Physiol. 2012;159:1263–76. doi: 10.1104/pp.112.193979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gao Z, Wen C-K, Binder BM, Chen Y-F, Chang J, Chiang Y-H, et al. Heteromeric interactions among ethylene receptors mediate signaling in Arabidopsis. J Biol Chem. 2008;283:23801–10. doi: 10.1074/jbc.M800641200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gao Z, Schaller GE. The role of receptor interactions in regulating ethylene signal transduction. Plant Signal Behav. 2009;4:1152–3. doi: 10.4161/psb.4.12.9943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Qu X, Schaller GE. Requirement of the histidine kinase domain for signal transduction by the ethylene receptor ETR1. Plant Physiol. 2004;136:2961–70. doi: 10.1104/pp.104.047126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gao Z, Chen YF, Randlett MD, Zhao XC, Findell JL, Kieber JJ, et al. Localization of the Raf-like kinase CTR1 to the endoplasmic reticulum of Arabidopsis through participation in ethylene receptor signaling complexes. J Biol Chem. 2003;278:34725–32. doi: 10.1074/jbc.M305548200. [DOI] [PubMed] [Google Scholar]
- 17.Ikeda Y, Men S, Fischer U, Stepanova AN, Alonso JM, Ljung K, et al. Local auxin biosynthesis modulates gradient-directed planar polarity in Arabidopsis. Nat Cell Biol. 2009;11:731–8. doi: 10.1038/ncb1879. [DOI] [PubMed] [Google Scholar]
- 18.Hall BP, Shakeel SN, Amir M, Ul Haq N, Qu X, Schaller GE. Histidine kinase activity of the ethylene receptor ETR1 facilitates the ethylene response in Arabidopsis. Plant Physiol. 2012;159:682–95. doi: 10.1104/pp.112.196790. [DOI] [PMC free article] [PubMed] [Google Scholar]