Abstract
Elaboration of a complex leaves depends on the morphogenetic activity of a specific tissue at the leaf margin termed marginal-blastozon (MB). In tomato (Solanum lycopersicym), prolonged activity of the MB leads to the development of compound leaves. The activity of the MB is restricted by the TCP transcription factor LANCEOLATE (LA). Plants harboring the dominant LA mutant allele La-2 have simple leaves with a uniform blade. Conversely, leaves of pFIL > > miR319 are compound and grow indeterminately in their margins due to leaf overexpression of miR319, a negative regulator of LA and additional miR319-sensitive genes. We have recently shown that the auxin-response sensor DR5::VENUS marks and precedes leaflet initiation events in the MB. Mutations in ENTIRE (E), an auxin signal inhibitor from the Aux/IAA family, lead to the expansion of the DR5::VENUS signal to throughout the leaf-primordia margin, and to a simplified leaf phenotype. Here, we examined the interaction between auxin, E, and LA in tomato leaf development. In La-2 leaf primordia, the auxin signal is very weak and is diffused to throughout the leaf margin, suggesting that auxin acts within the developmental-context of MB activity, which is controlled by LA. e La-2 double mutants showed an enhanced simple leaf phenotype and e pFIL > > miR319 leaves initiated less leaflets than wild-type, but their margins showed continuous growth. These results suggest that E and auxin affect leaflet initiation within the context of the extended MB activity, but their influence on the extent of indeterminate growth of the leaf is minor.
Keywords: Auxin, CIN-TCP, LANCEOLATE, SlIAA9/ENTIRE, Tomato, compound leaf, leaf development
The diversity of leaf forms arises from flexible tuning of a common developmental program. Leaf development has been described by three successive and overlapping stages.1,2 At the initiation (I) stage, the leaves arise at the flanks of the shoot apical meristem (SAM). During primary morphogenesis (PM) the leaf expands laterally and acquires its principal shape. In the final stage of secondary morphogenesis (SM), the leaf grows substantially, mainly through cell expansion, and the functional tissues differentiate. The elaboration of compound leaves often depends on prolonged morphogenetic activity of a specific region at the leaf margin, termed marginal blastozone (MB). During PM, the MB is responsible for the organogenesis of structures such as leaflets and lobes.3 Studies of leaf development have identified several genes and hormones that promote the morphogenetic activity of the MB. For example, class I knotted like homeobox (KNOXI) genes are important for the maintenance of morphogenetic activity during early stages of compound-leaf development in many species.4-8 Dominant mutations or transgenic overexpression of KNOXI genes lead to increased leaf complexity. In tomato, the KNOXI gene TKN2 (also called LeT6) was proposed to promote the morphogenetic activity by inhibiting the transition between the PM to the SM. The plant hormone cytokinin (CK) was shown to act downstream of KNOXI proteins in prolonging the morphogenetic activity of the tomato leaf margin.9
Conversely, the tomato CIN-TCP transcription factor LANCEOLATE (LA), and additional miR319-regulated CIN-TCPs, were shown to restrict the duration of the MB activity by promoting differentiation.10-14 Precocious elevation of LA expression in the gain-of-function mutant La-2, in which the miR319-recognition site is mutated, results in early differentiation and simplified leaf form (Fig. 2C).14 Downregulation of LA-like genes by leaf-specific expression of miR319 leads to prolonged morphogenetic activity of the leaf margin and to partially indeterminate leaf growth.12-14 (Fig. 2D). The plant hormone gibberellin (GA) was shown to negatively regulate leaf complexity.15-19 Recently LA was shown to act in part by modulating GA levels.20
Figure 2. Interaction between e and genotypes with alterd LA activity. Shown are mature fifth leaves of the indicated genotypes. Scale bars: 1cm.
Auxin mediates the positioning of initiating lateral appendage such as leaflets and lobes via the establishment of auxin maxima in the initiation sites.21-26 In tomato, the Aux/IAA protein SlIAA9/ENTIRE (E) is a specific auxin-response inhibitor that affects fruit and leaf development.22,25,27-30 We and others have recently shown that in the leaf, E inhibits auxin response between initiating leaflets, which enables the establishment of distinct auxin maxima and leaflet separation.22,23,25 In loss-of-function e mutants, leaflets initiate but the mature leaf is simple as a result of expanded auxin response and ectopic blade outgrowth between leaflets (Fig. 2B).22,25,31
We have recently shown that E mRNA expression is restricted to the intercalary area between initiating leaflets and that E restricts auxin maxima to leaflet initiation sites.22 While these studies show that E and auxin are important in leaf patterning, it is still unclear whether they also affects the extent of morphogenetic potential of the leaf margin, or acts within the developmental window of morphogenetic potential to determine leaf initiation sites. To address this question, we tested the effect of the La-2 mutant on the distribution of the DR5::VENUS signal and examined the genetic interaction between e and genotypes with altered LA activity.
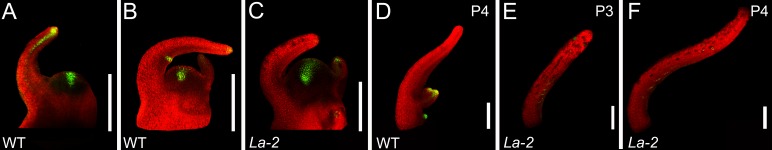
We have recently shown that the auxin response sensor DR5::VENUS marks and precedes the initiation of marginal appendages.22 In La-2 mutants, the DR5::VENUS signal appeared at the site of the incipient leaf primordia and in the prevascular tissue of young leaf primordia, similar to wild type (Fig. 1A-C). However, the auxin maxima at the leaflet-initiation sites that are typical to older wild-type leaf primordial (Fig. 1B, D) were lost, and a very weak signal appeared throughout the leaf margins (Fig. 1D-F). This is in accordance with the lack of leaflet initiation in this genotype. The loss of auxin maxima implies that a proper auxin gradient cannot be established in differentiated leaf-margins, and that the auxin maxima form within the developmental context defined by LA.
Figure 1. Leaf primordia of La-2 exhibit weak and diffused auxin signal throughout their margins. Confocal micrographs showing the spatial expression of the auxin signal reporter DR5::VENUS (green). Genotypes are indicated at the bottom left corner of each panel. (A-C) Longitundal view of the SAM and two young leaf primordia. (D-F) Longitundal view of leaf primordia. The developmental stage is indicated at the top right corner. Scale bars: 200µm.
e La-2/+ double mutants show an enhanced phenotype of a simple leaf with a single entire lamina (Fig. 2E), indicating that E and LA act through at least partially independent pathways. In e pFIL > > miR319 leaves, e is epistatic to pFIL > > miR319 with respect to the simple-leaf phenotype and the reduction in leaflet number, but the growth of the leaf margin was not affected by e and was indeterminate as in pFIL > > miR319 (Fig. 2F). This implies that LA-like and E affect different aspects of the compound-leaf development: Timing of LA-like activity defines the potential to elaborate marginal structures, but E is required for specification, localization and separation of these marginal structures. The initiation of leaflets in e may indicate that in young leaf primordia the MB is active despite the expanded auxin signal. Thus, it seems that the maintenance of a morphogenetic potential in the leaf margin might be partially mediated by the antagonistic activity of GA and CK, and that auxin maxima are involved in the positioning and separation of leaflets within the morphogenetic active tissue.
The previously reported genetic interaction between e and 35S::kn1 plants supports these observations. Transgenic tomato plants overexpressing the maize KNOXI gene Kn1 display a range of phenotypic abnormalities, including the formation of super-compound leaves featuring several orders of leaflet reiteration.5 In e 35S::kn1 leaves leaflet number was reduced relative to 35S::kn1, but the leaflet margin showed prolonged lamina growth.32 These results suggest that distinct programs affect the indeterminacy of the MB, the first allows the initiation of leaf marginal appendages at early stages of leaf development and the other enables the growth of the lamina at the leaf margin and the formation of late arising leaflets after leaf expansion. Thus, auxin and E appear to affect the specification of marginal outgrowths within a developmental window of morphogenetic potential.
Acknowledgments
This work was supported by grants from BARD (no. IS 04140–08C) and the Israeli Ministry of Agriculture (no. 837–0055–09) to NO. HB is funded in part by a Kaye Einstein Scholarship.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/21550
References
- 1.Dengler NG, Tsukaya H. Leaf morphogenesis in dicotyledons: current issues. Int J Plant Sci. 2001;162:459–64. doi: 10.1086/320145. [DOI] [Google Scholar]
- 2.Kaplan DR. Fundamental concepts of leaf morphology and morphogenesis: a contribution to the interpretation of molecular genetic mutants. Int J Plant Sci. 2001;162:465–74. doi: 10.1086/320135. [DOI] [Google Scholar]
- 3.Hagemann W, Gleissberg S. Organogenetic capacity of leaves: the significance of marginal blastozones in angiosperms. Plant Syst Evol. 1996;199:121–52. doi: 10.1007/BF00984901. [DOI] [Google Scholar]
- 4.Barth S, Geier T, Eimert K, Watillon B, Sangwan RS, Gleissberg S. KNOX overexpression in transgenic Kohleria (Gesneriaceae) prolongs the activity of proximal leaf blastozones and drastically alters segment fate. Planta. 2009;230:1081–91. doi: 10.1007/s00425-009-0997-0. [DOI] [PubMed] [Google Scholar]
- 5.Hareven D, Gutfinger T, Parnis A, Eshed Y, Lifschitz E. The making of a compound leaf: genetic manipulation of leaf architecture in tomato. Cell. 1996;84:735–44. doi: 10.1016/S0092-8674(00)81051-X. [DOI] [PubMed] [Google Scholar]
- 6.Hay A, Tsiantis M. The genetic basis for differences in leaf form between Arabidopsis thaliana and its wild relative Cardamine hirsuta. Nat Genet. 2006;38:942–7. doi: 10.1038/ng1835. [DOI] [PubMed] [Google Scholar]
- 7.Janssen BJ, Lund L, Sinha N. Overexpression of a homeobox gene, LeT6, reveals indeterminate features in the tomato compound leaf. Plant Physiol. 1998;117:771–86. doi: 10.1104/pp.117.3.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shani E, Burko Y, Ben-Yaakov L, Berger Y, Amsellem Z, Goldshmidt A, et al. Stage-specific regulation of Solanum lycopersicum leaf maturation by class 1 KNOTTED1-LIKE HOMEOBOX proteins. Plant Cell. 2009;21:3078–92. doi: 10.1105/tpc.109.068148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shani E, Ben-Gera H, Shleizer-Burko S, Burko Y, Weiss D, Ori N. Cytokinin regulates compound leaf development in tomato. Plant Cell. 2010;22:3206–17. doi: 10.1105/tpc.110.078253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Efroni I, Blum E, Goldshmidt A, Eshed Y. A protracted and dynamic maturation schedule underlies Arabidopsis leaf development. Plant Cell. 2008;20:2293–306. doi: 10.1105/tpc.107.057521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nath U, Crawford BC, Carpenter R, Coen E. Genetic control of surface curvature. Science. 2003;299:1404–7. doi: 10.1126/science.1079354. [DOI] [PubMed] [Google Scholar]
- 12.Ori N, Cohen AR, Etzioni A, Brand A, Yanai O, Shleizer S, et al. Regulation of LANCEOLATE by miR319 is required for compound-leaf development in tomato. Nat Genet. 2007;39:787–91. doi: 10.1038/ng2036. [DOI] [PubMed] [Google Scholar]
- 13.Palatnik JF, Allen E, Wu X, Schommer C, Schwab R, Carrington JC, et al. Control of leaf morphogenesis by microRNAs. Nature. 2003;425:257–63. doi: 10.1038/nature01958. [DOI] [PubMed] [Google Scholar]
- 14.Shleizer-Burko S, Burko Y, Ben-Herzel O, Ori N. Dynamic growth program regulated by LANCEOLATE enables flexible leaf patterning. Development. 2011;138:695–704. doi: 10.1242/dev.056770. [DOI] [PubMed] [Google Scholar]
- 15.Bassel GW, Mullen RT, Bewley JD. Procera is a putative DELLA mutant in tomato (Solanum lycopersicum): effects on the seed and vegetative plant. J Exp Bot. 2008;59:585–93. doi: 10.1093/jxb/erm354. [DOI] [PubMed] [Google Scholar]
- 16.Fleishon S, Shani E, Ori N, Weiss D. Negative reciprocal interactions between gibberellin and cytokinin in tomato. New Phytol. 2011;190:609–17. doi: 10.1111/j.1469-8137.2010.03616.x. [DOI] [PubMed] [Google Scholar]
- 17.Hay A, Kaur H, Phillips A, Hedden P, Hake S, Tsiantis M. The gibberellin pathway mediates KNOTTED1-type homeobox function in plants with different body plans. Curr Biol. 2002;12:1557–65. doi: 10.1016/S0960-9822(02)01125-9. [DOI] [PubMed] [Google Scholar]
- 18.Jasinski S, Tattersall A, Piazza P, Hay A, Martinez-Garcia JF, Schmitz G, et al. PROCERA encodes a DELLA protein that mediates control of dissected leaf form in tomato. Plant J. 2008;56:603–12. doi: 10.1111/j.1365-313X.2008.03628.x. [DOI] [PubMed] [Google Scholar]
- 19.Gray RA. Alteration of leaf size and shape and other changes caused by gibberellins in plants. Am J Bot. 1957;44:674–82. doi: 10.2307/2438632. [DOI] [Google Scholar]
- 20.Yanai O, Shani E, Russ D, Ori N. Gibberellin partly mediates LANCEOLATE activity in tomato. Plant J. 2011;68:571–82. doi: 10.1111/j.1365-313X.2011.04716.x. [DOI] [PubMed] [Google Scholar]
- 21.Barkoulas M, Hay A, Kougioumoutzi E, Tsiantis M. A developmental framework for dissected leaf formation in the Arabidopsis relative Cardamine hirsuta. Nat Genet. 2008;40:1136–41. doi: 10.1038/ng.189. [DOI] [PubMed] [Google Scholar]
- 22.Ben-Gera H, Shwartz I, Shao MR, Shani E, Estelle M, Ori N. ENTIRE and GOBLET promote leaflet development in tomato by modulating auxin response. Plant J. 2012;70:903–15. doi: 10.1111/j.1365-313X.2012.04939.x. [DOI] [PubMed] [Google Scholar]
- 23.Blein T, Hasson A, Laufs P. Leaf development: what it needs to be complex. Curr Opin Plant Biol. 2010;13:75–82. doi: 10.1016/j.pbi.2009.09.017. [DOI] [PubMed] [Google Scholar]
- 24.DeMason DA, Polowicky PL. Patterns of DR5:GUS expression in organs of Pea (Pisum sativum) Int J Plant Sci. 2009;170:1–11. doi: 10.1086/593046. [DOI] [Google Scholar]
- 25.Koenig D, Bayer E, Kang J, Kuhlemeier C, Sinha N. Auxin patterns Solanum lycopersicum leaf morphogenesis. Development. 2009;136:2997–3006. doi: 10.1242/dev.033811. [DOI] [PubMed] [Google Scholar]
- 26.Zhou C, Han L, Hou C, Metelli A, Qi L, Tadege M, et al. Developmental analysis of a Medicago truncatula smooth leaf margin1 mutant reveals context-dependent effects on compound leaf development. Plant Cell. 2011;23:2106–24. doi: 10.1105/tpc.111.085464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Berger Y, Harpaz-Saad S, Brand A, Melnik H, Sirding N, Alvarez JP, et al. The NAC-domain transcription factor GOBLET specifies leaflet boundaries in compound tomato leaves. Development. 2009;136:823–32. doi: 10.1242/dev.031625. [DOI] [PubMed] [Google Scholar]
- 28.Wang H, Jones B, Li Z, Frasse P, Delalande C, Regad F, et al. The tomato Aux/IAA transcription factor IAA9 is involved in fruit development and leaf morphogenesis. Plant Cell. 2005;17:2676–92. doi: 10.1105/tpc.105.033415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang H, Schauer N, Usadel B, Frasse P, Zouine M, Hernould M, et al. Regulatory features underlying pollination-dependent and -independent tomato fruit set revealed by transcript and primary metabolite profiling. Plant Cell. 2009;21:1428–52. doi: 10.1105/tpc.108.060830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang J, Chen R, Xiao J, Qian C, Wang T, Li H, et al. A single-base deletion mutation in SlIAA9 gene causes tomato (Solanum lycopersicum) entire mutant. J Plant Res. 2007;120:671–8. doi: 10.1007/s10265-007-0109-9. [DOI] [PubMed] [Google Scholar]
- 31.Dengler NG. Comparison of leaf development in Normal (+/+), Entire (E/E), and Lanceolate (La/+) plants of tomato, Lycopersicon esculentum Ailsa Craig. Bot Gaz. 1984;145:66–77. doi: 10.1086/337428. [DOI] [Google Scholar]
- 32.Parnis A, Cohen O, Gutfinger T, Hareven D, Zamir D, Lifschitz E. The dominant developmental mutants of tomato, Mouse-ear and Curl, are associated with distinct modes of abnormal transcriptional regulation of a Knotted gene. Plant Cell. 1997;9:2143–58. doi: 10.1105/tpc.9.12.2143. [DOI] [PMC free article] [PubMed] [Google Scholar]