Abstract
Plant tropisms are decisively influenced by dynamic adjustments in spatiotemporal distribution of the growth regulators auxin. Polar auxin transport requires activity of PIN-type auxin carrier proteins, with their distribution at the plasma membrane significantly contributing to the directionality of auxin flow. Control of PIN protein distribution involves regulation of their endocytosis and further sorting into the lytic vacuole for degradation and recently, protein ubiquitylation has been demonstrated to control degradative sorting of plasma membrane proteins in plants.1-6 Here we show dynamic adjustments in PIN2 ubiquitylation in gravity-stimulated roots, a response that coincides with establishment of a lateral PIN2 expression gradient. Our results imply that perception and transduction of gravity signals triggers differential ubiquitylation of PIN2, which might feed back on the coordination of auxin distribution in root meristems.
Keywords: Auxin transport, PIN protein, ubiquitin, root gravitropism, protein degradation
Results and Discussion
Control of abundance and distribution of plasma membrane proteins determines crosstalk of cells with their environment. This involves adjustments in the amounts of proteins at the plasma membrane, which in turn modulate the amplitude of signal perception as well as rates of solute transport across membrane borders.5,7
Recently, adjustments in sorting and proteolytic turnover of Arabidopsis PIN2 auxin carrier protein have been demonstrated to coincide with variations in its ubiquitylation. Specifically, reduced PIN2 ubiquitylation could be mimicked by mutagenesis of various lysines found in its central hydrophilic loop, which caused protein stabilization.3 Notably, such defects in ubiquitylation were apparent only upon combined replacement of several loop-residing lysines by arginines. This suggested that these various lysines in the loop could be recognized by the plant's ubiquitylation machinery, potentially highlighting functional redundancies of these residues. In contrast, constitutive ubiquitylation, as mimicked by expressing a PIN2-ubiquitin fusion protein, resulted in enhanced internalization and vacuolar targeting, which seemingly depended on recognition of the ubiquitin moiety by so far elusive adaptor proteins.3
Apart from cis-acting elements, mediating intracellular sorting of PIN2, RGLG RING-finger E3 ligases have been identified to modulate levels of PIN2 ubiquitylation and steady-state levels, suggestive of their active involvement in the regulation of PIN2 turnover.3,8 In addition, application of excess amounts of a synthetic auxin analog was demonstrated to trigger PIN2 degradation, a response that coincided with an increase of ubiquitylated PIN2, indicating that protein ubiquitylation could indeed function as determinant of PIN2 stability.3 However, it remained to be established whether or not dynamic changes in overall ubiquitylation could influence PIN2 protein fate in a developmental context.
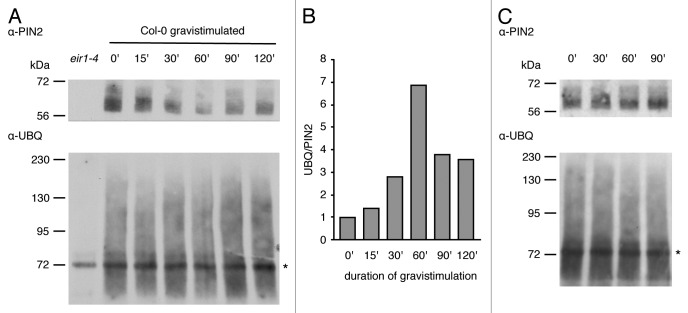
Gravitropic root bending was suggested to depend on establishment of a lateral auxin gradient, resulting in differential cell elongation at the upper vs. the lower side of the root meristem.9,10 Dynamic variations in PIN2 stability could reinforce hormone gradient establishment, since this protein has been demonstrated to undergo more pronounced endocytosis and vacuolar targeting at the upper side of the root meristem, whereas the protein is seemingly stabilized at its lower side.11-13 Mutant pin2 alleles deficient in their ubiquitylation no longer efficiently establish a lateral PIN2 expression gradient in gravistimulated root meristems suggesting that differential protein ubiquitylation could be involved.3 We further tested this possibility and determined PIN2 ubiquitylation in gravistimulated roots over time. To this end, 6-d-old Col-0 wild type seedlings germinated on vertically oriented plates were gravistimulated by turning plates at an angle of 90 degrees. Roots were then harvested at indicated time points and used for membrane protein extraction followed by immunoprecipitation as described.3,10 Precipitated proteins were separated, blotted and probed with PIN2- and ubiquitin-specific antibodies (Fig. 1A). The experiment revealed a transient decrease in immunoprecipitated PIN2 levels upon gravistimulation, which was not observed in non-stimulated controls (Fig. 1A and C), suggestive of enhanced degradation of the auxin carrier protein in response to gravity. Normalization of ubiquitin-specific signals to amounts of precipitated PIN2 allowed us to determine variations in ubiquitylation of membrane-associated PIN2 (Fig. 1B). These experiments revealed a transient increase in protein ubiquitylation starting at around 30 min after gravistimulation. Relative PIN2 ubiquitylation peaked at 60 min of gravistimulation and dropped again afterwards (Fig. 1B), whereas no comparable kinetics could be observed in non-stimulated control samples (Fig. 1C). This indicates that gravitropic root bending coincides with dynamic adjustments in PIN2 ubiquitylation.
Figure 1. PIN2 ubiquitylation upon gravistimulation (A) western blots performed with PIN2 immunoprecipitates derived from 6-d-old Col-0 roots grown on horizontally positioned roots that were probed with either anti-PIN2 (top) or anti-ubiquitin (bottom) antibodies. Samples were gravistimulated by turning plates clockwise at an angle of 90 degrees. A first sample was taken at time point 0' immediately before gravistimulation. The duration of gravistimulation is indicated below the blots. All details on protein extraction as well as reagents and conditions used, were according to Leitner et al.3 Two biological repetitions have been performed. Ig-specific signals are indicated by an asterisk. (B) Quantification of signal intensities performed with ImageJ software. Signals on scanned blots (A) were quantified and ubiquitin-specific signals were normalized to PIN2 signals (signals corresponding to PIN2 and ubiquitin-specific signals ranging from 75 kDa to 230 kDa were considered for quantification; Ig-specific bands visible on the ubiquitin blot were omitted). (C) Control immunoprecipitation performed as described in (A), but without gravistimulation. Samples were extracted from roots of vertically positioned Col-0 seedlings at time points indicated on top of the blots. Ig-specific signals are indicated by an asterisk.
Variations in auxin distribution have been intimately connected to the regulation of gravitropic root bending.9,14 This might involve translocation of the growth regulator from the very root tip into auxin responsive cells in the transition zone of the root meristem, ultimately resulting in differential expansion of root cells.9 Variations in PIN2 ubiquitylation might further enhance this kind of response. In one possible scenario, increased ubiquitylation in cell files at the upper side of the root, could trigger enhanced vacuolar targeting, thereby promoting establishment of a PIN2 expression gradient that would facilitate differential auxin transport. Mechanisms and molecular determinants mediating such differential protein ubiquitylation, however, remain to be determined.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
The authors acknowledge funding by the Austrian Science Funds (FWF) to B.K. (T941008324) and C.L. (P19585).
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/21715
References
- 1.Barberon M, Zelazny E, Robert S, Conéjéro G, Curie C, Friml J, et al. Monoubiquitin-dependent endocytosis of the iron-regulated transporter 1 (IRT1) transporter controls iron uptake in plants. Proc Natl Acad Sci U S A. 2011;108:E450–8. doi: 10.1073/pnas.1100659108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kasai K, Takano J, Miwa K, Toyoda A, Fujiwara T. High boron-induced ubiquitination regulates vacuolar sorting of the BOR1 borate transporter in Arabidopsis thaliana. J Biol Chem. 2011;286:6175–83. doi: 10.1074/jbc.M110.184929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leitner J, Petrášek J, Tomanov K, Retzer K, Pařezová M, Korbei B, et al. Lysine63-linked ubiquitylation of PIN2 auxin carrier protein governs hormonally controlled adaptation of Arabidopsis root growth. Proc Natl Acad Sci U S A. 2012;109:8322–7. doi: 10.1073/pnas.1200824109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lu D, Lin W, Gao X, Wu S, Cheng C, Avila J, et al. Direct ubiquitination of pattern recognition receptor FLS2 attenuates plant innate immunity. Science. 2011;332:1439–42. doi: 10.1126/science.1204903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reyes FC, Buono R, Otegui MS. Plant endosomal trafficking pathways. Curr Opin Plant Biol. 2011;14:666–73. doi: 10.1016/j.pbi.2011.07.009. [DOI] [PubMed] [Google Scholar]
- 6.Roberts D, Pedmale UV, Morrow J, Sachdev S, Lechner E, Tang X, et al. Modulation of phototropic responsiveness in Arabidopsis through ubiquitination of phototropin 1 by the CUL3-Ring E3 ubiquitin ligase CRL3(NPH3) Plant Cell. 2011;23:3627–40. doi: 10.1105/tpc.111.087999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lauwers E, Erpapazoglou Z, Haguenauer-Tsapis R, André B. The ubiquitin code of yeast permease trafficking. Trends Cell Biol. 2010;20:196–204. doi: 10.1016/j.tcb.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 8.Yin XJ, Volk S, Ljung K, Mehlmer N, Dolezal K, Ditengou F, et al. Ubiquitin lysine 63 chain forming ligases regulate apical dominance in Arabidopsis. Plant Cell. 2007;19:1898–911. doi: 10.1105/tpc.107.052035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ottenschläger I, Wolff P, Wolverton C, Bhalerao RP, Sandberg G, Ishikawa H, et al. Gravity-regulated differential auxin transport from columella to lateral root cap cells. Proc Natl Acad Sci U S A. 2003;100:2987–91. doi: 10.1073/pnas.0437936100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abas L, Luschnig C. Maximum yields of microsomal-type membranes from small amounts of plant material without requiring ultracentrifugation. Anal Biochem. 2010;401:217–27. doi: 10.1016/j.ab.2010.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kleine-Vehn J, Leitner J, Zwiewka M, Sauer M, Abas L, Luschnig C, et al. Differential degradation of PIN2 auxin efflux carrier by retromer-dependent vacuolar targeting. Proc Natl Acad Sci U S A. 2008;105:17812–7. doi: 10.1073/pnas.0808073105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Paciorek T, Zazímalová E, Ruthardt N, Petrásek J, Stierhof YD, Kleine-Vehn J, et al. Auxin inhibits endocytosis and promotes its own efflux from cells. Nature. 2005;435:1251–6. doi: 10.1038/nature03633. [DOI] [PubMed] [Google Scholar]
- 13.Abas L, Benjamins R, Malenica N, Paciorek T, Wiśniewska J, Moulinier-Anzola JC, et al. Intracellular trafficking and proteolysis of the Arabidopsis auxin-efflux facilitator PIN2 are involved in root gravitropism. Nat Cell Biol. 2006;8:249–56. doi: 10.1038/ncb1369. [DOI] [PubMed] [Google Scholar]
- 14.Peer WA, Blakeslee JJ, Yang H, Murphy AS. Seven things we think we know about auxin transport. Mol Plant. 2011;4:487–504. doi: 10.1093/mp/ssr034. [DOI] [PubMed] [Google Scholar]