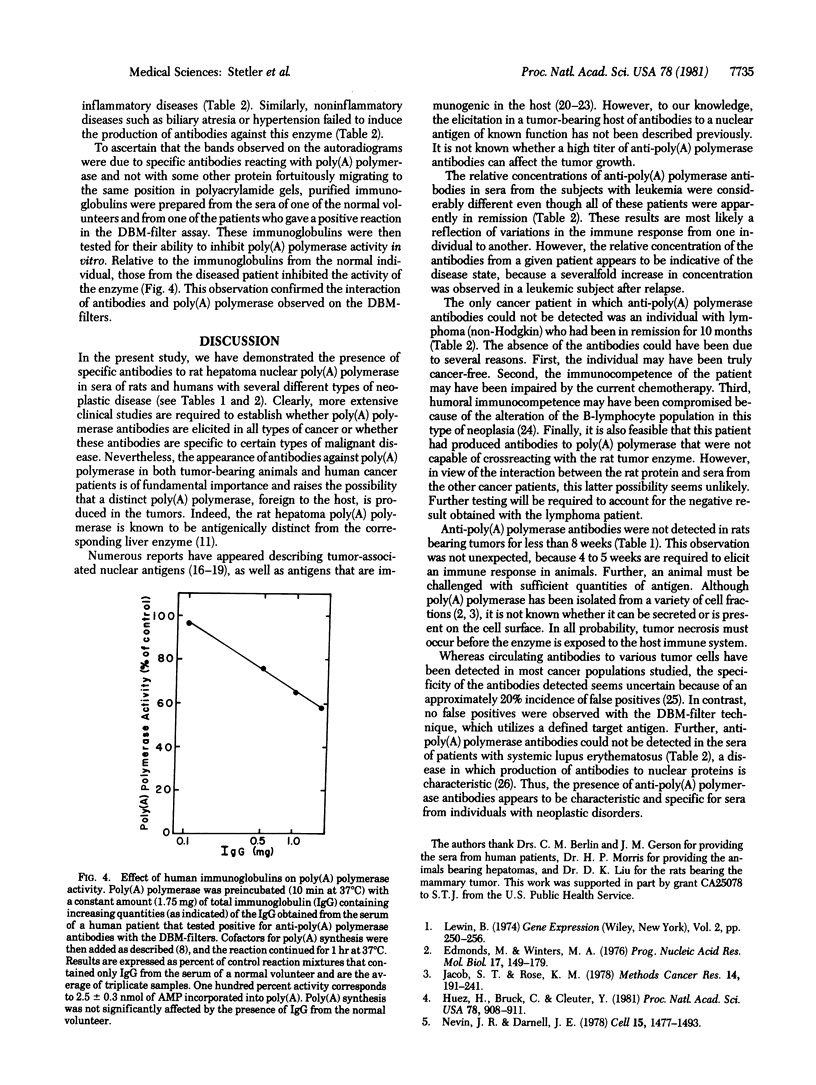
Abstract
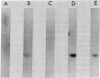
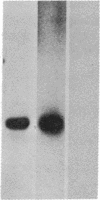
Poly(A) polymerase (polynucleotide adenylyltransferase; ATP:polynucleotide adenylyltransferase, EC 2.7.7.19) was covalently linked to diazobenzyloxymethyl-filters and used to screen the sera from a number of tumor-bearing rats and human cancer patients for antibodies to poly(A) polymerase. Sera from rats that had been inoculated with any of several Morris hepatomas or a mammary adenocarcinoma contained immunoglobulins capable of complexing with poly(A) polymerase. No antibodies to the enzyme could be detected in sera from control animals or from those bearing tumors for short periods of time. Antibodies to poly(A) polymerase were also observed in sera from human patients with leukemia, polycythemia vera, and Wilms tumor. The antibodies were not evident in sera from normal volunteers or from patients with nonneoplastic diseases. These included lupus erythematosus, a disorder in which antibodies are produced against an array of nuclear proteins. Immunoglobulins from the serum of one of the human patients were capable of inhibiting poly(A) polymerase activity in vitro, whereas those prepared from the serum of a normal volunteer did not affect enzyme activity. As determined by the diazobenzyloxymethyl-filter technique, the relative concentration of antibodies in the sera of an individual with leukemia (in remission) increased severalfold during a relapse. These data suggest that the presence of antibodies to poly(A) polymerase may be characteristic of sera from cancer patients and that the relative concentration of these antibodies may be indicative of the disease state.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alwine J. C., Kemp D. J., Stark G. R. Method for detection of specific RNAs in agarose gels by transfer to diazobenzyloxymethyl-paper and hybridization with DNA probes. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5350–5354. doi: 10.1073/pnas.74.12.5350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey T. E., Takahashi T., Resnick L. A., Oettgen H. F., Old L. J. Cell surface antigens of human malignant melanoma: mixed hemadsorption assays for humoral immunity to cultured autologous melanoma cells. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3278–3282. doi: 10.1073/pnas.73.9.3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmonds M., Winters M. A. Polyadenylate polymerases. Prog Nucleic Acid Res Mol Biol. 1976;17:149–179. doi: 10.1016/s0079-6603(08)60069-0. [DOI] [PubMed] [Google Scholar]
- Fujitani H., Chiu J. F., Hnilica L. S. Purification of nuclear antigens in Novikoff hepatoma. Proc Natl Acad Sci U S A. 1978 Apr;75(4):1943–1946. doi: 10.1073/pnas.75.4.1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herberman R. B. Immunogenicity of tumor antigens. Biochim Biophys Acta. 1977 Dec 23;473(2):93–119. doi: 10.1016/0304-419x(77)90002-6. [DOI] [PubMed] [Google Scholar]
- Huez G., Bruck C., Cleuter Y. Translational stability of native and deadenylylated rabbit globin mRNA injected into HeLa cells. Proc Natl Acad Sci U S A. 1981 Feb;78(2):908–911. doi: 10.1073/pnas.78.2.908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lewin B. Alternatives for splicing: recognizing the ends of introns. Cell. 1980 Nov;22(2 Pt 2):324–326. doi: 10.1016/0092-8674(80)90340-2. [DOI] [PubMed] [Google Scholar]
- Lewis M. G., Avis P. J., Phillips T. M., Sheikh K. M. Tumor-associated antigens in human malignant melanoma. Yale J Biol Med. 1973 Dec;46(5):661–668. [PMC free article] [PubMed] [Google Scholar]
- Marashi F., Davis F. M., Busch R. K., Savage H. E., Busch H. Purification and partial characterization of nucleolar antigen-1 of the Novikoff hepatoma. Cancer Res. 1979 Jan;39(1):59–66. [PubMed] [Google Scholar]
- Nevins J. R., Darnell J. E., Jr Steps in the processing of Ad2 mRNA: poly(A)+ nuclear sequences are conserved and poly(A) addition precedes splicing. Cell. 1978 Dec;15(4):1477–1493. doi: 10.1016/0092-8674(78)90071-5. [DOI] [PubMed] [Google Scholar]
- Notman D. D., Kurata N., Tan E. M. Profiles of antinuclear antibodies in systemic rheumatic diseases. Ann Intern Med. 1975 Oct;83(4):464–469. doi: 10.7326/0003-4819-83-4-464. [DOI] [PubMed] [Google Scholar]
- Ojala D., Montoya J., Attardi G. tRNA punctuation model of RNA processing in human mitochondria. Nature. 1981 Apr 9;290(5806):470–474. doi: 10.1038/290470a0. [DOI] [PubMed] [Google Scholar]
- Renart J., Reiser J., Stark G. R. Transfer of proteins from gels to diazobenzyloxymethyl-paper and detection with antisera: a method for studying antibody specificity and antigen structure. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3116–3120. doi: 10.1073/pnas.76.7.3116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose K. M., Allen M. S., Crawford I. L., Jacob S. T. Functional role of zinc in poly(A) synthesis catalyzed by nuclear poly(A) polymerase. Eur J Biochem. 1978 Jul 17;88(1):29–36. doi: 10.1111/j.1432-1033.1978.tb12419.x. [DOI] [PubMed] [Google Scholar]
- Rose K. M., Jacob S. T. Nuclear poly(A) polymerase from rat liver and a hepatoma. Comparison of properties, molecular weights and amino acid compositions. Eur J Biochem. 1976 Aug 1;67(1):11–21. doi: 10.1111/j.1432-1033.1976.tb10626.x. [DOI] [PubMed] [Google Scholar]
- Rose K. M., Jacob S. T. Phosphorylation of nuclear poly(A) polymerase. Comparison of liver and hepatoma enzymes. J Biol Chem. 1979 Oct 25;254(20):10256–10261. [PubMed] [Google Scholar]
- Rose K. M., Stetler D. A., Jacob S. T. Protein kinase activity of RNA polymerase I purified from a rat hepatoma: probable function of Mr 42,000 and 24,600 polypeptides. Proc Natl Acad Sci U S A. 1981 May;78(5):2833–2837. doi: 10.1073/pnas.78.5.2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiku H., Takahashi T., Oettgen H. F. Cell surface antigens of human malignant melanoma. II. Serological typing with immune adherence assays and definition of two new surface antigens. J Exp Med. 1976 Oct 1;144(4):873–881. doi: 10.1084/jem.144.4.873. [DOI] [PMC free article] [PubMed] [Google Scholar]