Abstract
Proper development of the central nervous system (CNS) requires the establishment of appropriate connections between neurons. Recent work suggests that this process is controlled by a balance between synaptogenic molecules and proteins that negatively regulate synapse formation and plasticity. Surprisingly, many of these newly identified synapse-limiting molecules are classic “immune” proteins. In particular, major histocompatibility complex class I (MHCI) molecules regulate neurite outgrowth, the establishment and function of cortical connections, activity-dependent refinement in the visual system, and long-term and homeostatic plasticity. This review summarizes our current understanding of MHCI expression and function in the CNS, as well as the potential mechanisms used by MHCI to regulate brain development and plasticity.
Keywords: neuroimmunology, synapse formation
Introduction
Although there has been evidence for years for cross-talk between the immune and nervous systems following injury, the dogma in the field of neuroimmunology has been that the healthy CNS is “immune-privileged” because of a lack of classical immune molecules in the CNS [1–2]. Recently, however, a paradigm shift in the field of neuroimmunology has occurred due to the discovery that immune molecules, such as cytokines, complement, and major histocompatibility complex (MHC) proteins, are expressed in the developing and adult brain where they play important roles in development and plasticity [3–6].
In contrast to the extensive literature on the role for MHCI molecules in the immune system, the function of MHCI molecules in the CNS is much less well understood. Nevertheless, the past few years have witnessed significant progress on this topic. This review focuses on MHCI molecules in CNS development and plasticity. Roles for other immune molecules in the CNS, as well as for mediating injury in the peripheral nervous system, have been recently reviewed [3–4, 7–8].
MHCI and MHCI receptors in the immune system
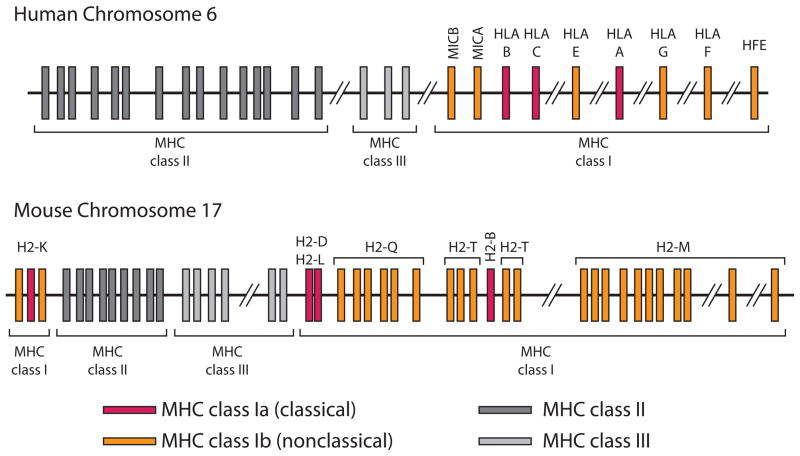
One of the defining features of MHC molecules is their complexity (Figure 1). They are both polygenic—containing multiple genes—and polymorphic—containing multiple variants of each gene [9]. MHC genes are the most polymorphic genes known [10–12]. Classical MHCI α-chains are encoded by three genes in humans, denoted HLA-A, -B and –C [11]. In mice, these genes are H2-K, -D and –L [11]. In addition, multiple genomic insertions and deletions have created many non-classical (class Ib) MHCI genes [9], many of which are not well characterized [13]. The specific genes and variants that an individual expresses comprise its MHC haplotype.
Figure 1.
Genomic map of the human and mouse major histocompatibility complex (MHC). A simplified schematic of the human and mouse MHC genomic regions (not drawn to scale). Annotations were taken from the mouse Genome Reference Consortium (GRC) m38/mm10 (2011) and human GRCh37/hg19 (2009) assemblies. The MHC spans approximately 3.6 Mb and is located on chromosome 6 of humans and 17 of mice. The classical MHCI genes (red) are highly polymorphic, whereas the non-classical MHCI genes (orange) are not. Class II and III genes are indicated by dark gray and light gray boxes, respectively, but have not been annotated here (see [9] for more detail on these regions). The light chain of MHCI molecules, β2-microglobulin, is encoded on a separate chromosome (15 in humans and 2 in mice). Classical MHC class I genes include HLA-A, HLA-B, and HLA-C in humans, and H2-K, D, L and B in mice. H2-L is very closely related to H2-D and appears to be present only in the BALB/c mouse strain. As such, H2-L is left out of current assemblies based on the C57BL/6 strain, but has been retained here for completeness. H2-B is a gut restricted classical MHCI gene. There are many nonclassical MHC class I genes that include MICA, MICB, HLA-E, HLA-G, HLA-F, and HFE in humans and MICA, MICB, Q, T, M and HFE in mice. The general arrangement of the MHC is similar between humans and rodents, with the main difference being that MHCI genes in mice have become separated at either end of the MHC by class II and III genes.
In the immune system, MHCI proteins mediate both the adaptive and innate immune responses [13]. Classical MHCI proteins consist of a transmembrane α-chain and an obligate, extracellular light chain, called β2-microglobulin (β2m) [13]. The α-chain contains a polymorphic groove that binds to proteolytically-digested peptides from intracellular proteins for presentation on the surface [13] of all nucleated cells. Usually these are self-peptides but MHCI will present non-self peptides if a cell is infected with a virus, for example. Non-self peptides are recognized by T-cell receptor complexes (TCR) on cytotoxic T cells, leading to the initiation of an immune response. Immune signaling molecules called cytokines are released early in the immune response and initiate a cascade of events including increased MHCI expression and eventual lysis of cells displaying foreign peptide [14]. In addition to TCRs, MHCI molecules also bind to receptors on natural killer (NK) cells including (in mice) paired immunoglobulin-like (Pir) and Ly49 receptors to regulate NK-mediated lysis of target cells [15–16]. PirA is an activating, and PirB is an inhibitory, NK receptor. There are numerous activating and inhibiting Ly49 receptors in mice that are expressed in a strain-specific manner [16]. When bound to MHCI molecules on target cells, PirB and Ly49 inhibitory receptors prevent NK immune synapse formation [16].
MHCI and MHCI receptor expression in the CNS
MHCI expression
MHCI molecules are found in an isoform- and region-specific manner throughout the CNS [17–18]. MHCI mRNA is expressed in marmosets, cats, rats, and mice in neurons and glial cells in the visual and olfactory systems, cerebral cortex, striatum, hippocampus, cerebellum, and spinal cord [17, 19–28]. MHCI protein is present in the developing and adult mammalian CNS, with the highest levels occurring during early postnatal development [22, 29]. Although MHCI protein was historically thought to be absent from the surface of neurons [2, 24, 30–31], recent work clearly indicates that MHCI protein is expressed on the surface of axons and dendrites and its distribution is developmentally regulated [17, 29, 32]. MHCI protein is also located at synapses both pre- and postsynaptically [17, 29, 32] (Figure 2a). MHCI proteins may also be translated locally in dendrites since MHCI mRNAs are trafficked to dendrites of hippocampal neurons [33], where they are enriched in FMRP-mRNA complexes [34]. Finally, although MHCI is not present on astrocytes and microglia in cortical tissue [35], it is found on astrocytes in culture [36], on microglia following their activation [37], and in the hippocampus of aged mice [38].
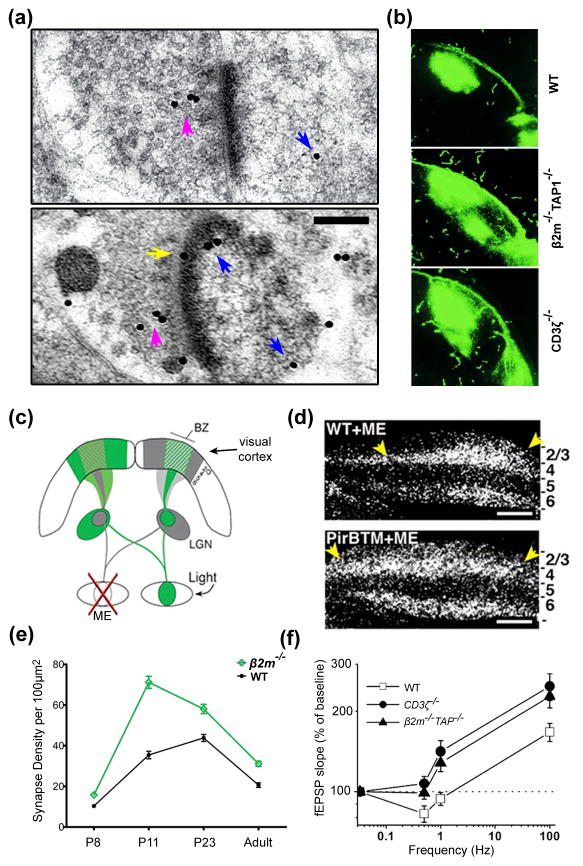
Figure 2. MHCI localization and function in the CNS.
(a) Post-embedding immuno-electron micrographs of adult rat cortex show MHCI protein labeled with gold (black dots) present presynaptically in synaptic vesicle pools (magenta arrows), postsynaptically (blue arrows), including within the postsynaptic density, and in the synaptic cleft (yellow arrow) [29]. Scale bar: 0.2 μm. (b) P13 mice deficient in sMHCI (β2m−/−TAP1−/−) or CD3ζ fail to properly refine their retinogeniculate connections. Images show the expanded ipsilateral retinogeniculate projections in the knockout mice compared to wild-type (WT) [40]. (c) Schematic showing the mouse visual system and the monocular enucleation (ME) paradigm to test ocular dominance (OD) plasticity as shown in (d). The pathway from the open eye through the lateral geniculate nucleus (LGN) to the visual cortex is colored green, while that from the enucleated eye is gray. Within visual cortex, solid shading indicates regions of monocular activity and crosshatching indicates the binocular zones (BZs). The numbers to the right of the gray area of cortex indicate cortical layers. (d) Using the paradigm described in (c), changes in OD plasticity were measured by changes in the width of Arc mRNA induction (white signal) ipsilateral to the remaining eye. Mice lacking functional PirB (PirBTM) exhibit enhanced OD plasticity after ME compared to WT, indicated by an increased width of Arc mRNA induction (between yellow arrows) [44]. Layers of visual cortex are marked on the right. Scale bar: 500 μm. (e) Synapse density is greater in layer 5 of visual cortex in mice lacking sMHCI (β2m−/−) compared to WT at all ages examined (P8 – adult), as quantified from transmission electron micrographs [32]. The greatest increase in synapse density occurs during the initial establishment of cortical connections (P11–P23). (f) Mice lacking sMHCI protein (β2m−/−TAP1−/−) or the MHCI co-receptor CD3ζ exhibit enhanced hippocampal LTP and an absence of LTD compared to WT mice, indicating a role for MHCI in restricting synaptic strength. Adapted, with permission, from [29] (a), [40] (b, f), [44] (c, d) and [32] (e).
The recognition that MHCI is expressed in the healthy CNS initially resulted from its identification as an activity-regulated gene in an unbiased differential display screen [17]. Decreasing activity through intracranial infusion of tetrodotoxin (TTX) decreases MHCI mRNA levels in the lateral geniculate nucleus (LGN). Similarly, decreasing retinogeniculate activity through monocular injection of TTX also decreases MHCI mRNA levels in the LGN. Conversely, increasing activity through intracranial kainic acid infusion increases levels of MHCI mRNA in the hippocampus and cortex [17].
MHCI expression is also regulated by activity in cultured neurons, but there are conflicting reports as to the direction of the effect. Some reports show an increase in MHCI mRNA and surface protein (sMHCI) in mature cultured hippocampal neurons after TTX [24, 30] and an increase in internal MHCI protein after activity blockade in young cortical cultures [32]. However, other studies report decreases in total [39] and sMHCI [32] after TTX treatment of hippocampal and young cortical cultures, respectively. While this discrepancy could be due to differences in culture age and experimental timeline, it is also possible that differences in immunostaining protocols could have revealed distinct subsets of MHCI. Most MHCI antibodies recognize tertiary structure, making their binding highly sensitive to conformational changes. So, the ability of most antibodies to stain sMHCI is lost following even light permeabilization [32]. Clearly, more work needs to be done to dissect the role that physiological activity plays in the regulation of sMHCI in the CNS.
MHCI receptor expression
MHCI receptors are also expressed in the CNS. Some components of the TCR complex are present in the brain. For example, CD3ζ is found throughout the CNS [40–41] and CD3ε is expressed specifically in the cerebellum [42]. However, there is no evidence for TCRα expression [43] and the TCRβ gene locus is not recombined in neurons, implying that functional TCRs are not present in the CNS [43]. Conversely, mRNA for the NK receptor, PirB, is expressed throughout the CNS and PirB protein is found in hippocampal neurons, especially in axonal growth cones and at synapses [44]. Moreover, soluble, recombinant PirB binds to cortical neurons in a partially MHCI-dependent manner [44]. PirB has clear effects on CNS development and plasticity as discussed below and is able to recruit components of the same signaling pathway it uses in NK cells to suppress immune synapse formation [35, 44]. Like PirB, Ly49 receptor protein is expressed in neurites of young cultured cortical neurons [45]. Finally, the mouse killer cell immunoglobulin-like receptor-like 1 (KIRL) gene is also expressed in the brain and produces a truncated protein similar to other inhibitory immune receptors [46]. It should be noted, however, that this receptor may not be functional in the CNS since it is missing the transmembrane and cytoplasmic motifs typically required for intracellular signaling [46].
Activity-dependent refinement and plasticity
The first report on the functional effects of MHCI in CNS development identified defects in the activity-dependent refinement of retinogeniculate projections in mice deficient in sMHCI (β2m−/− and β2m−/−TAP1−/−) (Table 1, Figure 2b) [40]. In the absence of β2m and/or TAP1, which mediates peptide loading onto the heavy chain, MHCI molecules fail to exit the endoplasmic reticulum for expression on the cell surface [13, 29, 32]. Similarly, mice that lack the classical MHCI isoforms H2-Kb and H2-Db (KbDb−/−), and mice deficient in CD3ζ, exhibit defects in LGN refinement that mimic those found in β2m−/− mice (Figure 2b) [40, 47]. However, CD3ζ−/− mice also have altered RGC dendritic structure, therefore future experiments must elucidate whether the retinogeniculate refinement defects in CD3ζ−/− mice are specific to the LGN or are a result of abnormal RGC development [48]. Interestingly, there are no defects in retinogeniculate refinement in mice engineered to express a truncated, inactive form of PirB (PirBTM mice) [44], suggesting that MHCI acts through a different signaling pathway to regulate retinogeniculate refinement. Later in development, the mammalian visual cortex is refined into specific regions called ocular dominance (OD) columns (ODCs; see Glossary & Figure 2c). Although this developmental refinement is unchanged in KbDb−/− [47] and in PirBTM mice [44], OD plasticity following monocular enucleation (ME) is enhanced in these mice (Figure 2d), suggesting that classical MHCI molecules restrict OD plasticity through PirB.
Table 1.
Roles of MHCI and its receptors in CNS development
| Manipulation | Region | Phenotype (relative to WT or control) | Experimental details | Refs |
|---|---|---|---|---|
| MHCI | ||||
| β2m−/− (sMHfCI deficient) | Retinogeniculate projection | Expanded ipsilateral projection, no change in LGN area | In vivo: P13 | [40] |
| Visual cortex | Increased synapse density (EM) | In vivo: P8, P11, P23, P60 | [32] | |
| Increased glutamatergic synapse density (ICC: vGlut1, GluN2A/B) | Cultures: 8 div | |||
| Hippocampus | No change in neurite outgrowth | Cultures: 1 div | [52] | |
| Cerebellum | Normal CF-PC synapse elimination & CF-PC EPSC amplitude | Slices: 12–16 weeks old | [20] | |
| β2m−/−TAP1−/− (sMHCI deficient) | Retinogeniculate projection | Expanded ipsilateral projection, no change in LGN area or retinal waves | In vivo: P13 | [40] |
| Expanded ipsilateral projection & abnormal segregation of inputs | In vivo: P34 | [47] | ||
| Thalamocortical projection | Enhanced OD plasticity (Arc induction) | In vivo: P22–31 | ||
| Visual cortex | Increased mEPSC frequency, no change in amplitude | Slices: P19–21, Layer 4 | [39] | |
| Hippocampus | Increased # of SVs at synapses, no change in PSD length, decreased perforated PSDs (EM) | In vivo: P44–45 | ||
| Enhanced LTP, absent LTD, no change in fEPSP slope (CA1) | Slices: P30–44 | [40] | ||
| Increased mEPSC frequency, no change in amplitude | Cultures: 14 div | [39] | ||
| Impaired synaptic scaling following TTX for 3–6 days | ||||
| No change in synapse density (ICC: synapsin, tubulin) | ||||
| Increased size of synapsin & vGlut1/2 puncta, no change in PSD-95 puncta | ||||
| No change in protein levels of GluN1, GluN3A, GluA1, GluA2, or synaptophsyin (biochemistry) | Tissue: P28–35 | [55] | ||
| Decreased GluA/GluN ratio, increased slope of GluN-mediated fEPSP, no change in proportion of silent synapses, GluN2A/B composition | Slices: P13–P16 | |||
| No change in surface GluA1, GluA2, GluN1, GluN2B, or synapse density (ICC: SV2, GluN1) | Cultures: 16–20 div | |||
| Increased surface GluA1 following NMDA treatment (ICC), blockade of NMDA-induced decrease in surface GluA1 (biochemistry), increased surface GluA2 in response to NMDA (biochemistry) | ||||
| Kb−/−Db−/− | Retinogeniculate projection | Expanded ipsilateral projection, no change in LGN area | In vivo: P34 | [47] |
| Thalamocortical projection | Enhanced OD plasticity (Arc induction, transneuronal tracing) | In vivo: P22–31 | ||
| Cerebellum | Normal CF-PC synapse elimination, PC dendritic arbors, & synapse density on CFs (ICC: vGlut2) | Slices: P19–25, 12–16 wks | [19, 20] | |
| CF-PC EPSC amplitude normal, enhanced PF PPF and reduced PPD | Slices: P19, P19–25 | |||
| Lower induction threshold for LTD at PF-PC synapses | Slices: P19–P25 | [19] | ||
| Increased rotarod learning and retention | In vivo: 8–12 week males | |||
| Hippocampus | Reduced neurite outgrowth, delayed neuronal polarization | Cultures: 1–2 div | [52] | |
| β2m RNAi (acute sMHCI knockdown) | Visual Cortex | Increased glutamatergic synapse density (ICC: vGlut1, GluN2A/B) | Cultures: 8 div (no effect, 13 div) | [32] |
| Increased GABAergic synapse density (ICC: synapsin, GABAAR) Is this GABA-A or GABA-B? | Cultures: 8 div | |||
| Increased mEPSC frequency and amplitude | ||||
| Increased mIPSC frequency, no change in amplitude | ||||
| Altered E/I balance | ||||
| NSE-H2Db (neuronal MHCI overexpression) | Retinogeniculate projection | Smaller contralateral, but not ipsilateral, projection, & decreased LGN area | In vivo: P11 | [51] |
| Reduced RGC axonal outgrowth toward thalamic explants caused by secreted (shed) MHCI | Retina-thalamic explants, 4–5 div | [53] | ||
| Hippocampus | Reduced synapsin expression in DG & CA3, but not CA1 | In vivo: P39 | [51] | |
| No change in basal synaptic transmission (CA1) | Slices: P28–42 | |||
| No change in LTP (CA1) | ||||
| Enhanced neurite outgrowth, increased numbers of neurites per cell | Cultures: 1–2 div | [52] | ||
| H2Kb-GFP (acute overexpression) | Visual Cortex | Decreased glutamatergic synapse density (ICC: vGlut1, GluN2A/B) | Cultures: 8 div (no effect, 13 div) | [32] |
| Decreased GABAergic synapse density (ICC: synapsin, GABA-A) | Cultures: 8 div | |||
| Decreased mEPSC frequency and amplitude | ||||
| Decreased mIPSC frequency, no change in amplitude | ||||
| Altered E/I balance | ||||
| Prevents the TTX-induced increase in glutamatergic synapse density | ||||
| Exogenous soluble β2m (to decrease homotypic MHCI heavy chain) | Visual Cortex | Increased glutamatergic synapse density (ICC: vGlut1, GluN2A/B) | Cultures: 8 div (no effect, 13 div) | [32] |
| Hippocampus | Decreased axon outgrowth | Cultures: 1–2 div | [52] | |
| MHCI/peptide monomers | Retina | Inhibition of neurite outgrowth by self-MHC I molecules, regardless of the peptide presented | Explants: 2 div | [54] |
| Hippocampus | Inhibition of axon outgrowth by self-, but not non-self-, MHCI monomers | Cultures: 1–2 div | [52] | |
| MHCI antibodies | Cortex | Increased neurite density & decreased synapsin levels and neuron number | Cultures: 5 div | [45] |
| PIRB | ||||
| PirBTM (functionally PIRB deficient) | Retinogeniculate projection | Normal | In vivo: P15 | [44] |
| Thalamocortical projection | Normal refinement (Arc induction) | In vivo: P19, P34 | ||
| Enhanced OD plasticity (Arc induction, transneuronal tracing) | In vivo, ME (P19–25, 22–31, 31–36); MD (P19–25, 25–40) | |||
| Extended OD plasticity after crtitical period (Arc induction) | In vivo, ME (P100–P110) | |||
| PirB−/− | Hippocampus | fEPSP amplitude normal (CA3-CA1) | Slices: age not specified | [56] |
| No change in CA3-CA1 LTP or LTD | Slices, LTP: P42–P56, LTD: P15–20 | |||
| CD3ζ | ||||
| CD3ζ−/− | Retinogeniculate projection | Expanded ipsilateral projection, no change in retinal waves | In vivo: P13, P16 | [40, 48] |
| Retina | Increased RGC dendritic branching | In vivo: P12 & P33 | [48] | |
| Altered dendritic stratification and segregation of ON/OFF inputs onto RGCs | In vivo: P33 | |||
| Reduced frequency of retinal waves in second, but not in first, week | In vivo: P3, P10 | |||
| Hippocampus | Enhanced LTP, absent LTD, no change in fEPSP slope | Slices: P30–44 | [40] | |
| CD3ζ RNAi & DN | Cortex | Increased dendritic branching and width, reduced dendritic motility | Cultures: 5 div | [41] |
| CD3ζ antibody | Hippocampus | Decreased dendritic branching | Cultures: 5–7 div | [41] |
| CD3ε | ||||
| CD3ε −/− | Cerebellum | Decreased PC dendritic branching and vGlut1 intensity; no change in PF-PC or CF-PC basal synaptic transmission or CF-PC synapse elimination; enhanced PF-PC PPF | Slices: P7 | [42] |
| Impaired rotarod performance at high speed | In vivo: adult | |||
| Ly49 | ||||
| Ly49 antibody | Cortex | Decreased neurite density, increased synapsin levels and neuron number | Cultures: 5 div | [45] |
Abbreviations: Arc: activity regulated cytoskeleton-associated protein; a gene that is upregulated by neuronal activity, CF: climbing fiber, DG: dentate gyrus, div: days in vitro, DN: dominant negative, E/I: excitatory/inhibitory, EM: electron microscopy, EPSC: evoked postsynaptic current, fEPSP: field evoked postsynaptic potential, ICC: immunocytochemistry, LGN: lateral geniculate nucleus, LTP: long term potentiation, LTD: long term depression, MD: monocular deprivation, ME: monocular enucleation, mEPSC: miniature excitatory postsynaptic current, mIPSC: miniature inhibitory postsynaptic current, OD: ocular dominance, P: postnatal day, PC: Purkinje cell, PF: parallel fiber, PPD: paired-pulse depression, PPF: paired-pulse facilitation, PSD: postsynaptic density, RGC: retinal ganglion cell, sMHCI: surface MHCI, SV: synaptic vesicle, TTX: tetrodotoxin.
An important consideration in interpreting all of these results involving knockout mice is that these genes are knocked out in all cells throughout development, including neurons and glial cells. Although several lines of evidence described below indicate that neuronal MHCI plays an important role in plasticity, the role for glial MHCI during CNS development is unknown. One intriguing possibility is that MHCI expression on microglia may be involved in their critical role in synaptic pruning [49].
While β2m−/−TAP1−/− mice lack sMHCI in all cells, the development of NSE-Db mice has allowed researchers to investigate MHCI gain-of-function specifically in neurons [50]. These mice also exhibit altered retinogeniculate refinement, but in the direction opposite to MHCI loss-of-function mice. However, the way in which LGN refinement is altered is distinct. In NSE-Db mice, total LGN area is smaller and projections from the contralateral eye are more restricted than in wild-type mice, with no change in the ipsilateral projection [51]. In contrast, in the β2m−/−TAP1−/− mice, the ipsilateral projection normalized to total dLGN area is significantly increased [40]. Although the reasons for these differences need to be clarified, in general these results reinforce the conclusion that MHCI mediates the elimination of inappropriate connections in the developing visual system [5].
In addition to regulating visual system development, MHCI also modulates development of the cerebellum and olfactory system. MHCI molecules are expressed throughout the cerebellum, but are not required for activity-dependent refinement of climbing fiber projections to Purkinje cells [19–20]. As discussed below, MHCI instead appears to regulate synaptic plasticity and motor learning in the cerebellum [19–20]. In the olfactory system, the non-classical H2-Mv proteins are expressed in vomeronasal sensory neurons (VSN) [25]. Consistent with a possible role for MHCI in axon guidance, VSN axons are targeted to different areas of the posterior accessory bulb (AOB) depending on whether they express H2-Mv proteins [25]. Moreover, H2-M1 and H2-M10 (from the H2-Mv family) are required for trafficking of the vomeronasal pheromone receptor V2R to the surface of neurons in the vomeronasal organ (VNO). These V2Rs are mislocalized in the VNO dendrites of β2m−/− mice, leading to behavioral changes related to pheromone detection [21]. It is currently unknown if MHCI regulates activity-dependent refinement or plasticity in the olfactory system.
The establishment and function of CNS connections
Axonal and dendritic growth
In addition to controlling activity-dependent refinement of connections, MHCI also controls axonal and dendritic outgrowth. Neurite outgrowth and polarization is regulated by MHCI in very young, 1–2 days in vitro (div), cultures from embryonic day 15 (E15) mouse hippocampus [52]. MHCI limits the growth of axons from at least two kinds of neurons. Extension of axons from retinal explants toward thalamic explants from NSE-Db mice is stunted [53] and addition of recombinant MHCI similarly inhibits neurite outgrowth from retinal explants [54] and cultured dorsal root ganglion cells [51]. MHCI may also limit dendritic complexity since genetic loss of CD3ζ increases the dendritic complexity of retinal ganglion cells in vivo [48]. Similarly, RNA interference (RNAi)-mediated knockdown of CD3ζ increases dendritic complexity, while activation of CD3ζ reduces it, in young, cultured cortical neurons [41]. Ly49 signaling may also regulate neurite outgrowth since treatment of cultured cortical neurons with a Ly49 antibody inhibits neurite outgrowth and promotes cell survival, while an anti-MHCI antibody has the opposite effects [45]. These MHCI or Ly49 antibodies decrease or increase, respectively, levels of synapsin in cultured cortical neurons [45]. Together, these reports indicate a potential role for MHCI in neuronal differentiation but many questions remain unanswered, including whether endogenous MHCI regulates axonal and dendritic growth directly or indirectly through effects on synaptic activity (discussed in further detail below).
Synapse density
MHCI also negatively regulates the initial establishment of connections in the CNS. Although there is no change in the density of synapsin-positive puncta in 14 div cultured hippocampal neurons from β2m−/−TAP1−/− mice [39], synapse density is increased in layer 5 of visual cortex throughout postnatal development and into adulthood in mice deficient in sMHCI (β2m−/−) [32] (Figure 2e). The greatest increase in synapse density in β2m−/− cortex relative to wild-type occurs between postnatal day 11 (P11) and P23, a period of rapidly increasing cortical connectivity [32]. Synapse density is also increased in young cultured cortical neurons in which sMHCI is acutely knocked down, but is decreased in cells that overexpress H2-Kb [32]. Similarly, levels of presynaptic proteins in the dentate gyrus and CA3, but not CA1, regions of the hippocampus are reduced in H2-Db overexpressing mice at P39 [51]. Finally, MHCI negatively regulates the density of GABAergic synapses in young cortical cultures [32]. Together, these results indicate that MHCI restricts synapse density within the visual cortex and possibly also in the hippocampus of NSE-Db mice, but not in the hippocampus of β2m−/−TAP1−/− mice. It will be important in the future to determine whether MHCI negatively regulates synapse density by limiting the formation of synapses, promoting their elimination, or a combination of both.
Synaptic transmission
MHCI molecules also regulate synaptic transmission in hippocampal and cortical neurons, but in distinct ways. Although there is no change in the amplitude of field excitatory postsynaptic potentials (fEPSPs) [40] or pharmacologically isolated AMPA receptor (AMPAR)-mediated fEPSPs [55] from hippocampal slices, miniature excitatory postsynaptic current (mEPSC) frequency, but not amplitude, is increased by 40% in mature cultured hippocampal neurons from β2m−/−TAP1−/− mice [39]. Combined with the increase in synaptic vesicle number measured from electron micrographs, these findings suggest that loss of MHCI selectively alters presynaptic release properties, but not synapse density in the hippocampus [39]. In contrast, MHCI clearly alters synapse number and function in the visual cortex. mEPSC frequency, but not amplitude, is doubled in cortical slices from P20 β2m−/−TAP1−/− mice [39], consistent with increases in synapse density and/or presynaptic release properties. Similarly, mEPSC frequency is increased in young cortical cultures transfected with β2m siRNA, and decreased in neurons overexpressing H2-Kb. In these cortical cultures, mEPSC amplitude is also altered following both manipulations, indicating that MHCI negatively regulates synaptic strength as well as synapse density [32]. In young cortical neurons, MHCI also regulates inhibitory synaptic transmission; mIPSC frequency, but not amplitude, is increased following β2m knockdown and is decreased in neurons overexpressing H2-Kb [32]. Because glutamatergic transmission is affected to a greater extent than GABAergic transmission, the balance of excitation to inhibition on cortical neurons is altered by changing MHCI levels [32].
Recent work suggests that MHCI molecules normally inhibit NMDA receptor (NMDAR) function and regulate trafficking of AMPARs after NMDAR stimulation [55]. Loss of MHCI leads to de-repression of NMDAR function, reflected as a decrease in the AMPAR/NMDAR ratio in hippocampal slices from β2m−/−TAP1−/− mice. Following NMDA application, AMPAR trafficking is also altered in β2m−/−TAP1−/− mice. Basal NMDAR expression, GluN2B/3A subunit composition, and synaptic localization of NMDARs are not changed, suggesting that MHCI may regulate channel properties to repress NMDARs [55]. Taken together, these studies show that MHCI proteins bidirectionally regulate both the initial establishment and strength of synapses in the CNS in a region-specific manner.
Synaptic Plasticity
There are primarily two types of plasticity that govern the formation and maintenance of connections in the CNS—Hebbian and homeostatic plasticity (see Glossary). MHCI molecules are critical for Hebbian plasticity. In β2m−/−TAP1−/− mice, hippocampal long-term depression (LTD) is absent and long-term plasticity (LTP) is enhanced, suggesting that endogenous MHCI limits synaptic strength [40] (Figure 2f). MHCI does not regulate hippocampal plasticity through PirB since LTP/LTD is normal in PirB−/− mice [56]. However, it may act through a receptor complex that contains CD3ζ, since CD3ζ−/− mice exhibit the same defects in LTP/LTD as β2m−/−TAP1−/− mice [40] (Figure 2f). Interestingly, hippocampal LTP appears to be normal in NSE-Db mice; changes in LTD in these mice have not been reported [51]. MHCI also regulates synaptic plasticity in the cerebellum. KbDb−/− mice exhibit a lower induction threshold for LTD, consistent with enhanced rotarod learning [19]. Finally, MHCI may act downstream of late-LTP induction, since its levels are increased in mice expressing constitutively active cAMP response element-binding (CREB) [57].
LTP- and LTD-induced changes in synaptic strength often require NMDAR -dependent alterations in AMPAR function or trafficking [58]. The LTP/LTD phenotypes of β2m−/−TAP1−/− mice suggest that MHCI molecules may alter the trafficking or function of NMDARs and AMPARs, an idea supported by the observed effects of MHCI on mEPSC amplitude and NMDAR function discussed above [55]. Additional experiments are needed to determine if LTP in the visual cortex is altered following manipulation of MHCI or its receptors and how MHCI might act downstream of late-LTP induction.
MHCI also mediates homeostatic plasticity. In mature hippocampal neurons, application of TTX for 3–6 days increases both mEPSC frequency and amplitude [39]. This homeostatic increase in synaptic transmission is impaired in β2m−/−TAP1−/− neurons, which fail to scale their responses appropriately [39]. Presynaptic terminals in β2m−/− TAP1−/− neurons are already enlarged prior to, and fail to increase after, TTX treatment, as indicated by synapsin immunoreactivity [39]. Moreover, postsynaptic density protein 95 (PSD-95) puncta size is normal prior to TTX treatment, but also fails to scale up in these mice [39]. This suggests that synaptic scaling in β2m−/−TAP1−/− synapses is impaired. A shorter, 24hr application of TTX in young cortical neurons increases glutamatergic synapse density and this increase is blocked by overexpression of MHCI, suggesting that MHCI mediates the TTX-induced increase in synapse density [32]. Given the conflicting reports on the effect of TTX on sMHCI expression discussed above, additional work is necessary to dissect the role of MHCI in synaptic scaling and determine how this role changes depending on the developmental age and brain region. Additionally, it will be important to determine if local synaptic activity regulates the insertion of MHCI in the membrane on a rapid time-scale.
MHCI signaling
Despite the clear importance of MHCI in modulating synaptic connectivity, function, and plasticity during CNS development, the mechanisms that underlie these effects remain mostly unknown. The varied phenotypes resulting from perturbation of MHCI and its receptors in the CNS suggest that MHCI likely utilizes different binding partners and signaling pathways depending on the environmental stimulus, cell type and developmental stage –much like its interactions with receptors in the immune system. It is generally assumed that MHCI itself does not initiate much intracellular signaling because its cytoplasmic tail is so short; thus, MHCI likely functions through binding to other proteins. In the CNS, MHCI activity may be mediated through transmembrane immune proteins, such as components of the TCR complex and the NK cell receptors PirB and Ly49, on neighboring neurons [59] (Figure 3a). Indeed, PirB binds to neurons in a partially MHCI-dependent manner and PirBTM mice exhibit similar changes in OD plasticity as KbDb−/− mice [44, 47], suggesting that MHCI signaling through PirB may regulate OD plasticity. Similarly, defects in retinogeniculate refinement and hippocampal plasticity in CD3ζ−/− mice phenocopy those seen in β2m−/−TAP1−/− mice, suggesting that a receptor complex containing CD3ζ may mediate the effects of MHCI molecules in these processes [40]. However, the enhanced motor learning in the cerebellum observed in β2m−/−TAP1−/− mice is opposite that found in CD3ε−/− mice [19, 42]. CD3ζ and CD3ε typically function as co-receptors in the TCR complex, yet functional TCRs are not expressed in the CNS [43]. Thus, defining how these co-receptors alter brain development, and if their functions are MHCI-dependent, are important questions. Generation of conditional and inducible transgenic mice to knock out MHCI, PirB, and the many Ly49 receptors, specifically in the CNS at identified time-points during postnatal development, is also needed to clarify these issues.
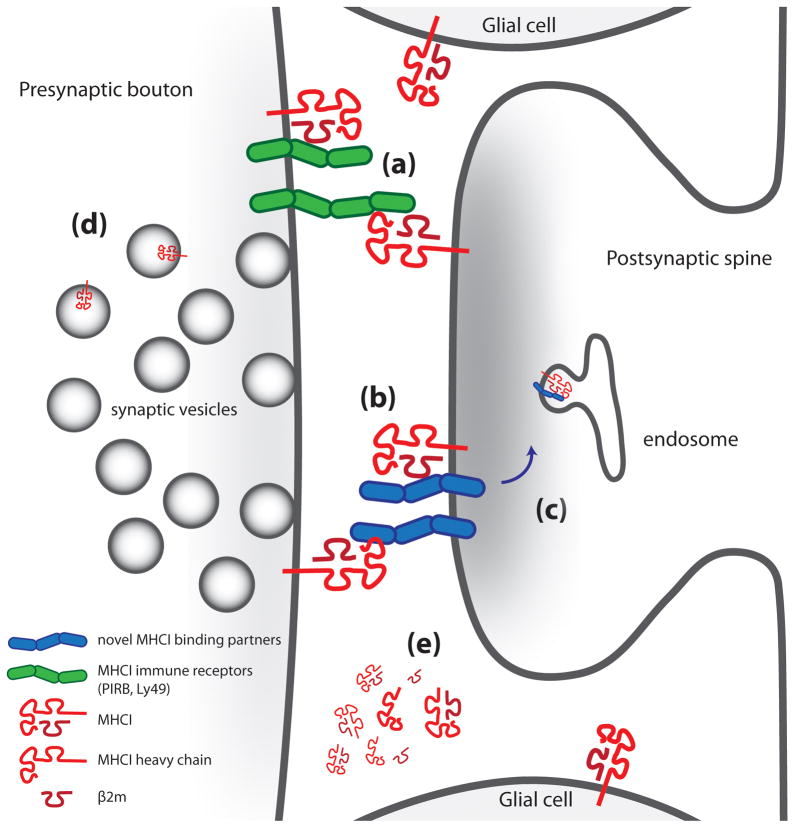
Figure 3. Schematic of potential mechanisms for MHCI signaling.
The presence of MHCI protein both pre- and postsynaptically, as well as the potential for in-cis (same cell) or in-trans (between cell) interactions with receptors and other nearby proteins, must be considered in models of MHCI function. Both types of interactions could occur with MHCI pre- or postsynaptically, but have been drawn in only one orientation for simplicity. The pathways illustrated in this figure are based on data from the CNS described in this review and from roles for MHCI in the immune system, since several of these possibilities have not yet been confirmed in neurons or glial cells. (a) MHCI can interact with immune receptors like PirB or Ly49 in-cis or in-trans [101–102]. MHCI also appears to signal through the TCR coreceptor CD3ζ in the brain, but the presumed additional members of the receptor complex containing CD3ζ in the brain remain unknown [43]. (b,c) MHCI proteins can interact with other plasma membrane proteins in-cis and alter their trafficking, surface expression, and/or sensitivity to ligand [103]. (d) MHCI proteins are associated with synaptic vesicle pools as determined by immuno-electron microscopy and biochemical fractionation [29, 35]. (e) MHCI can also be shed from the plasma membrane of neurons and may initiate signaling on nearby cells [54]. The differing sizes of MHCI are meant to illustrate MHCI diffusing away from the synapse.
In addition to signaling in-trans through binding to immune receptors, MHCI may also alter neuronal function in the same cell by signaling through in-cis interactions with adjacent proteins. MHCI binds to NK receptors both in-cis and in-trans [11, 44, 60] (Figure 3a) and in-cis interactions of MHCI with other transmembrane receptors (Figure 3b) have been documented in non-neuronal cell types [61–64]. The MHCIb molecule, hemochromatosis (HFE), regulates trafficking of the transferrin receptor [65]. MHCI also binds to insulin-like growth factor receptors (IGFI and II), the interleukin-2 receptor (IL2R), intercellular adhesion molecule (ICAM), and the epidermal growth factor receptor (EGFR) [62, 66]. MHCI binding to the insulin receptor alters its surface expression and affinity for ligand [63–64, 67]. It remains to be determined if most of these interactions, or novel ones, occur in the CNS (Figure 3c). Future work in this field will focus on identifying novel binding partners for MHCI proteins and elucidating immune receptor signaling in neurons.
Adding further complexity to any potential model of MHCI signaling is the lack of understanding of where the MHCI proteins are located that mediate each of its functions in the developing brain. MHCI protein is present in most compartments of neurons—axons, axonal growth cones, and dendrites—throughout development. At synapses, MHCI molecules are present both pre- and postsynaptically [29]. Although postsynaptic MHCI clearly controls synapse density [32], the function of presynaptic MHCI has not yet been identified. The expression of MHCI [32] and PirB [44] on axonal growth cones and the role of H2-Mv proteins in axon targeting in the olfactory system [25] suggests that MHCI molecules could regulate axon guidance, in addition to their role in inhibiting axonal outgrowth [53–54]. MHCI is also closely associated with synaptic vesicles in presynaptic terminals in the cerebral cortex [29](Figure 2a), where it may regulate synaptic vesicle number and recycling through as yet undefined mechanisms [32, 39] (Figure 3d). MHCI protein can be shed from the cell surface or cleaved by membrane-bound metalloproteases and released as soluble, fully conformed MHCI [53, 68] (Figure 3e). Addition of recombinant MHCI at picomolar levels inhibits neurite outgrowth from retinal explants; supporting the idea that secreted MHCI molecules are capable of activating receptors on nearby neurons [54], but the function of MHCI shedding in most aspects of brain development and function remain unknown. Finally, glial cells may also contribute to the role for MHCI in the CNS since they may also express MHCI proteins [35–37], but the function of glial MHCI in CNS development and plasticity has not been elucidated.
Concluding Remarks
Over the past ten years it has become clear that MHCI molecules play a significant non-immune role in the development and plasticity of the CNS. A common theme to date is that MHCI and its receptors act to inhibit neural development. They inhibit axonal and dendritic growth [45, 51, 53–54], limit the initial establishment of cortical connections [32], and mediate synaptic weakening through LTD, as well as activity-dependent refinement of connections in the developing visual system [17, 19, 39–40, 44, 47]. The similarity of this negative regulation to that recently identified for another class of immune proteins—those in the complement cascade system--which promote microglial-mediated synapse elimination in the CNS, suggests a broad function for “immune” molecules in the brain to limit connectivity during development [7, 69]. Nevertheless, despite recent progress in understanding the roles for MHCI in CNS development and plasticity, many basic questions about the mechanism through which MHCI exerts its functions remain unknown (Box 1).
Box 1. Outstanding Questions.
What are the receptors and other binding partners for MHCI proteins in the brain?
How do these interactions mediate the effects of MHCI on neuronal differentiation, synapse formation, function, and plasticity?
Given the diversity of MHCI family members, are there specific functions for individual classical and non-classical MHCI proteins?
How does localized synaptic activity alter MHCI protein expression on neurons, and within what time-scale?
What role does MHCI in CNS glia play in brain development and function?
What role does antigen presentation by MHCI molecules play during normal CNS development?
The role for MHCI in limiting CNS connectivity has important implications for injury and repair, in that blocking the growth-inhibition effects of MHCI might promote regeneration in the CNS. Indeed, MHCI and PirB limit axonal outgrowth following injury in vitro and in vivo [70]. PirB binds to myelin-derived axon growth inhibitory molecules, including Nogo-66, myelin-associated glycoprotein (MAG) and oligodendrocyte-myelin glycoprotein (OMgp), and is essential for the effects of myelin in inhibiting neurite growth [71]. PirB signaling also inhibits Trk receptors that function to promote axonal outgrowth [70]. Compensatory neuronal sprouting following hippocampal lesion is also impaired in NSE-Db mice [51]. Interestingly, MHCI has been reported to either have no effect on [72], or to enhance [73], corticospinal tract (CST) regeneration and locomotor function following spinal cord injury. Finally, the absence of MHCI and PirB enhances neuroprotection following stroke [35]. Stroke increases neuronal expression of PirB and MHCI and mice lacking these proteins exhibit smaller infarcts, enhanced corticospinal axonal projections, and decreased numbers of reactive astrocytes, supporting the conclusion that MHCI signaling contributes to brain injury after ischemia [35]. Interestingly, the function of MHCI in the spinal cord may be cell-type-dependent since MHCI molecules maintain synapses onto sciatic motor neurons following injury, rather than limit their density as in the brain [74–75].
The role for MHCI in limiting neural connectivity and function also has potentially profound implications for neurodevelopmental disorders and neurological and psychiatric diseases. Specific MHCI haplotypes and mutations in MHCI genes have been implicated in autism spectrum disorders [76–78] and schizophrenia [79–83]. Moreover, MHCI levels on neurons are regulated by cytokines [30, 84–86] and cytokine levels are altered in the blood, brain, and cerebrospinal fluid (CSF) in many neurodevelopmental and neurodegenerative disorders [87–99]. This suggests that a peripheral immune response could alter brain cytokines through a porous blood-brain barrier in early development, following injury, or in disease [3, 93, 100] and thereby alter connectivity and/or function in the CNS through changes in MHCI levels. Because MHCI molecules and its receptors play critical roles in CNS function, understanding MHCI signaling in the CNS may illuminate not only novel mechanisms of neural development, but also new pathways to target for treating injury and many diverse psychiatric and neurological disorders.
Acknowledgments
We would like to thank Myka Estes for comments on the manuscript. Our work on immune molecules in cortical development is supported by National Institute of Neurological Disorders and Stroke (R01NS060125) and National Institute of Mental Health (R01MH088879) to A.K.M. We are also grateful to the Higgins and Gassin Family Foundations for their invaluable support through Autism Speaks of basic science research into the role of immune molecules in brain development.
Glossary
- Activity-dependent retinogeniculate refinement
A classic experimental model for the activity-dependent refinement of connections in the CNS is the mammalian visual system. Retinal ganglion cell (RGC) axons from each eye overlap in the lateral geniculate nucleus (LGN) early in development, but are later refined in an activity-dependent manner into eye-specific regions[105]. This mechanism of activity-dependent refinement is not restricted to the visual system. Regions throughout the CNS use this mechanism to prune their circuits
- Homeostatic plasticity/synaptic scaling
Refers to the ability of neurons to adjust to chronic changes in extrinsic network activity so that their overall excitability remains stable over time[104]. Chronic application of TTX to neurons increases basal activity following drug removal, whereas incubation with picrotoxin results in a compensatory decrease in basal synaptic transmission [104]
- Ocular dominance (OD) plasticity
In adult mice, most of the primary visual cortex receives input from the contralateral eye; this area is called the monocular zone. A smaller region receives input from both eyes, and is called the binocular zone (BZ). Early in development inputs from both eyes innervate a wide area of visual cortex and these inputs are refined in an activity-dependent manner into the adult BZ. Monocular enucleation (ME, removal of one eye), or monocular deprivation (MD, eyelid suture), shifts activity to that only coming from the available/open eye. If this occurs during a specific time early in development termed a “critical period”, OD will shift to represent inputs that favor the remaining eye, forming much wider binocular zones. This process is called OD plasticity (Figure 2c)[106]
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Murphy JB, Sturm E. Conditions Determining the Transplantability of Tissues in the Brain. J Exp Med. 1923;38:183–197. doi: 10.1084/jem.38.2.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Joly E, et al. Viral persistence in neurons explained by lack of major histocompatibility class I expression. Science. 1991;253:1283–1285. doi: 10.1126/science.1891717. [DOI] [PubMed] [Google Scholar]
- 3.Garay PA, McAllister AK. Novel roles for immune molecules in neural development: implications for neurodevelopmental disorders. Frontiers in synaptic neuroscience. 2010;2:136. doi: 10.3389/fnsyn.2010.00136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boulanger LM. Immune proteins in brain development and synaptic plasticity. Neuron. 2009;64:93–109. doi: 10.1016/j.neuron.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 5.Shatz CJ. MHC class I: an unexpected role in neuronal plasticity. Neuron. 2009;64:40–45. doi: 10.1016/j.neuron.2009.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stephan AH, et al. The complement system: an unexpected role in synaptic pruning during development and disease. Annu Rev Neurosci. 2012;35:369–389. doi: 10.1146/annurev-neuro-061010-113810. [DOI] [PubMed] [Google Scholar]
- 7.Schafer DP, Stevens B. Synapse elimination during development and disease: immune molecules take centre stage. Biochem Soc Trans. 2010;38:476–481. doi: 10.1042/BST0380476. [DOI] [PubMed] [Google Scholar]
- 8.Cullheim S, Thams S. Classic major histocompatibility complex class I molecules: new actors at the neuromuscular junction. The Neuroscientist : a review journal bringing neurobiology, neurology and psychiatry. 2010;16:600–607. doi: 10.1177/1073858410381534. [DOI] [PubMed] [Google Scholar]
- 9.Shiina T, et al. The HLA genomic loci map: expression, interaction, diversity and disease. J Hum Genet. 2009;54:15–39. doi: 10.1038/jhg.2008.5. [DOI] [PubMed] [Google Scholar]
- 10.Thorsby E. Invited anniversary review: HLA associated diseases. Human immunology. 1997;53:1–11. doi: 10.1016/S0198-8859(97)00024-4. [DOI] [PubMed] [Google Scholar]
- 11.Murphy KP, et al. Garland Science. 2008. Janeway’s immunobiology. [Google Scholar]
- 12.Chao YL, et al. Association study of HLA-A gene and schizophrenia in Han Chinese from Taiwan. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:1834–1837. doi: 10.1016/j.pnpbp.2008.08.009. [DOI] [PubMed] [Google Scholar]
- 13.Peaper DR, Cresswell P. Regulation of MHC class I assembly and peptide binding. Annu Rev Cell Dev Biol. 2008;24:343–368. doi: 10.1146/annurev.cellbio.24.110707.175347. [DOI] [PubMed] [Google Scholar]
- 14.Abbas AK, et al. Cellular and molecular immunology. Saunders/Elsevier; 2010. [Google Scholar]
- 15.Krzewski K, Strominger JL. The killer’s kiss: the many functions of NK cell immunological synapses. Curr Opin Cell Biol. 2008;20:597–605. doi: 10.1016/j.ceb.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Long EO. Negative signaling by inhibitory receptors: the NK cell paradigm. Immunological reviews. 2008;224:70–84. doi: 10.1111/j.1600-065X.2008.00660.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Corriveau R, et al. Regulation of class I MHC gene expression in the developing and mature CNS by neural activity. Neuron. 1998;21:505–520. doi: 10.1016/s0896-6273(00)80562-0. [DOI] [PubMed] [Google Scholar]
- 18.Belgard TG, et al. A Transcriptomic Atlas of Mouse Neocortical Layers. Neuron. 2011;71:605–616. doi: 10.1016/j.neuron.2011.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McConnell MJ, et al. H2-Kb and H2-Db regulate cerebellar long-term depression and limit motor learning. Proc Natl Acad Sci U S A. 2009;106:6784–6789. doi: 10.1073/pnas.0902018106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Letellier M, et al. Normal adult climbing fiber monoinnervation of cerebellar Purkinje cells in mice lacking MHC class I molecules. Developmental neurobiology. 2008;68:997–1006. doi: 10.1002/dneu.20639. [DOI] [PubMed] [Google Scholar]
- 21.Loconto J, et al. Functional expression of murine V2R pheromone receptors involves selective association with the M10 and M1 families of MHC class Ib molecules. Cell. 2003;112:607–618. doi: 10.1016/s0092-8674(03)00153-3. [DOI] [PubMed] [Google Scholar]
- 22.Ribic A, et al. Activity-dependent regulation of MHC class I expression in the developing primary visual cortex of the common marmoset monkey. Behavioral and brain functions : BBF. 2011;7:1. doi: 10.1186/1744-9081-7-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cahoy JD, et al. A transcriptome database for astrocytes, neurons, and oligodendrocytes: a new resource for understanding brain development and function. J Neurosci. 2008;28:264–278. doi: 10.1523/JNEUROSCI.4178-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Neumann H, et al. Major histocompatibility complex (MHC) class I gene expression in single neurons of the central nervous system: differential regulation by interferon (IFN)-gamma and tumor necrosis factor (TNF)-alpha. J Exp Med. 1997;185:305–316. doi: 10.1084/jem.185.2.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ishii T, Mombaerts P. Expression of nonclassical class I major histocompatibility genes defines a tripartite organization of the mouse vomeronasal system. J Neurosci. 2008;28:2332–2341. doi: 10.1523/JNEUROSCI.4807-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miralves J, et al. High levels of MeCP2 depress MHC class I expression in neuronal cells. PLoS One. 2007;2:e1354. doi: 10.1371/journal.pone.0001354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thams S, et al. MHC class I expression and synaptic plasticity after nerve lesion. Brain Res Rev. 2008;57:265–269. doi: 10.1016/j.brainresrev.2007.06.016. [DOI] [PubMed] [Google Scholar]
- 28.Linda H, et al. Expression of MHC class I and beta2-microglobulin in rat spinal motoneurons: regulatory influences by IFN-gamma and axotomy. Experimental neurology. 1998;150:282–295. doi: 10.1006/exnr.1997.6768. [DOI] [PubMed] [Google Scholar]
- 29.Needleman LA, et al. MHC class I molecules are present both pre- and postsynaptically in the visual cortex during postnatal development and in adulthood. Proc Natl Acad Sci U S A. 2010;107:16999–17004. doi: 10.1073/pnas.1006087107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Neumann H, et al. Induction of MHC class I genes in neurons. Science. 1995;269:549–552. doi: 10.1126/science.7624779. [DOI] [PubMed] [Google Scholar]
- 31.Rall G, et al. Consequences of cytotoxic T lymphocyte interaction with major histocompatibility complex class I-expressing neurons in vivo. J Exp Med. 1995;182:1201–1212. doi: 10.1084/jem.182.5.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Glynn MW, et al. MHCI negatively regulates synapse density during the establishment of cortical connections. Nat Neurosci. 2011;14:442–451. doi: 10.1038/nn.2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhong J, et al. Dendritic mRNAs encode diversified functionalities in hippocampal pyramidal neurons. BMC Neurosci. 2006;7:17. doi: 10.1186/1471-2202-7-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brown V, et al. Microarray identification of FMRP-associated brain mRNAs and altered mRNA translational profiles in fragile X syndrome. Cell. 2001;107:477–487. doi: 10.1016/s0092-8674(01)00568-2. [DOI] [PubMed] [Google Scholar]
- 35.Adelson JD, et al. Neuroprotection from Stroke in the Absence of MHCI or PirB. Neuron. 2012;73:1100–1107. doi: 10.1016/j.neuron.2012.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Massa PT, et al. Cell type-specific regulation of major histocompatibility complex (MHC) class I gene expression in astrocytes, oligodendrocytes, and neurons. Glia. 1993;8:201–207. doi: 10.1002/glia.440080307. [DOI] [PubMed] [Google Scholar]
- 37.Ling EA, et al. Expression of major histocompatibility complex antigens and CR3 complement receptors in activated microglia following an injection of ricin into the sciatic nerve in rats. Histology and histopathology. 1992;7:93–100. [PubMed] [Google Scholar]
- 38.Starkey HD, et al. Neuroglial Expression of the MHCI Pathway and PirB Receptor Is Upregulated in the Hippocampus with Advanced Aging. Journal of molecular neuroscience. 2012 doi: 10.1007/s12031-012-9783-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Goddard C, et al. Regulation of CNS synapses by neuronal MHC class I. Proc Natl Acad Sci U S A. 2007;104:6828–6833. doi: 10.1073/pnas.0702023104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huh G, et al. Functional requirement for class I MHC in CNS development and plasticity. Science. 2000;290:2155–2159. doi: 10.1126/science.290.5499.2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Baudouin SJ, et al. The signaling adaptor protein CD3zeta is a negative regulator of dendrite development in young neurons. Mol Biol Cell. 2008;19:2444–2456. doi: 10.1091/mbc.E07-09-0947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nakamura K, et al. CD3 and immunoglobulin G Fc receptor regulate cerebellar functions. Molecular and cellular biology. 2007;27:5128–5134. doi: 10.1128/MCB.01072-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Syken J, Shatz CJ. Expression of T cell receptor beta locus in central nervous system neurons. Proc Natl Acad Sci U S A. 2003;100:13048–13053. doi: 10.1073/pnas.1735415100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Syken J, et al. PirB restricts ocular-dominance plasticity in visual cortex. Science. 2006;313:1795–1800. doi: 10.1126/science.1128232. [DOI] [PubMed] [Google Scholar]
- 45.Zohar O, et al. Cutting edge: MHC class I-Ly49 interaction regulates neuronal function. Journal of immunology. 2008;180:6447–6451. doi: 10.4049/jimmunol.180.10.6447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bryceson YT, et al. Expression of a killer cell receptor-like gene in plastic regions of the central nervous system. J Neuroimmunol. 2005;161:177–182. doi: 10.1016/j.jneuroim.2004.11.018. [DOI] [PubMed] [Google Scholar]
- 47.Datwani A, et al. Classical MHCI molecules regulate retinogeniculate refinement and limit ocular dominance plasticity. Neuron. 2009;64:463–470. doi: 10.1016/j.neuron.2009.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xu HP, et al. The immune protein CD3zeta is required for normal development of neural circuits in the retina. Neuron. 2010;65:503–515. doi: 10.1016/j.neuron.2010.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schafer DP, et al. Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron. 2012;74:691–705. doi: 10.1016/j.neuron.2012.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rall GF, et al. Consequences of cytotoxic T lymphocyte interaction with major histocompatibility complex class I-expressing neurons in vivo. J Exp Med. 1995;182:1201–1212. doi: 10.1084/jem.182.5.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wu ZP, et al. Enhanced neuronal expression of major histocompatibility complex class I leads to aberrations in neurodevelopment and neurorepair. J Neuroimmunol. 2011;232:8–16. doi: 10.1016/j.jneuroim.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bilousova T, et al. Major histocompatibility complex class I molecules modulate embryonic neuritogenesis and neuronal polarization. J Neuroimmunol. 2012;247:1–8. doi: 10.1016/j.jneuroim.2012.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Washburn LR, et al. A potential role for shed soluble major histocompatibility class I molecules as modulators of neurite outgrowth. PLoS One. 2011;6:e18439. doi: 10.1371/journal.pone.0018439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Escande-Beillard N, et al. Neurons preferentially respond to self-MHC class I allele products regardless of peptide presented. J Immunol. 2010;184:816–823. doi: 10.4049/jimmunol.0902159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fourgeaud L, et al. MHC class I modulates NMDA receptor function and AMPA receptor trafficking. Proc Natl Acad Sci U S A. 2010;107:22278–22283. doi: 10.1073/pnas.0914064107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Raiker SJ, et al. Oligodendrocyte-myelin glycoprotein and Nogo negatively regulate activity-dependent synaptic plasticity. J Neurosci. 2010;30:12432–12445. doi: 10.1523/JNEUROSCI.0895-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Barco A, et al. Gene expression profiling of facilitated L-LTP in VP16-CREB mice reveals that BDNF is critical for the maintenance of LTP and its synaptic capture. Neuron. 2005;48:123–137. doi: 10.1016/j.neuron.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 58.Malenka RC, Bear MF. LTP and LTD: an embarrassment of riches. Neuron. 2004;44:5–21. doi: 10.1016/j.neuron.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 59.Boulanger L, Shatz C. Immune signalling in neural development, synaptic plasticity and disease. Nat Rev Neurosci. 2004;5:521–531. doi: 10.1038/nrn1428. [DOI] [PubMed] [Google Scholar]
- 60.Doucey MA, et al. Cis association of Ly49A with MHC class I restricts natural killer cell inhibition. Nat Immunol. 2004;5:328–336. doi: 10.1038/ni1043. [DOI] [PubMed] [Google Scholar]
- 61.Fishman D, et al. Non-immune functions of MHC class I glycoproteins in normal and malignant cells. Folia biologica. 2004;50:35–42. doi: 10.14712/fb2004050020035. [DOI] [PubMed] [Google Scholar]
- 62.Hsu D, Olefsky J. Effect of a major histocompatibility complex class I peptide on insulin-like growth factor-I receptor internalization and biological signaling. Endocrinology. 1993;133:1247–1251. doi: 10.1210/endo.133.3.8365366. [DOI] [PubMed] [Google Scholar]
- 63.Olsson L, et al. Regulation of receptor internalization by the major histocompatibility complex class I molecule. Proc Natl Acad Sci U S A. 1994;91:9086–9090. doi: 10.1073/pnas.91.19.9086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Stagsted J, et al. Regulation of insulin receptor functions by a peptide derived from a major histocompatibility complex class I antigen. Cell. 1990;62:297–307. doi: 10.1016/0092-8674(90)90367-n. [DOI] [PubMed] [Google Scholar]
- 65.Bennett MJ, et al. Crystal structure of the hereditary haemochromatosis protein HFE complexed with transferrin receptor. Nature. 2000;403:46–53. doi: 10.1038/47417. [DOI] [PubMed] [Google Scholar]
- 66.Stagsted J, et al. Inhibition of internalization of glucose transporters and IGF-II receptors. Mechanism of action of MHC class I-derived peptides which augment the insulin response in rat adipose cells. The Journal of biological chemistry. 1993;268:22809–22813. [PubMed] [Google Scholar]
- 67.Chvatchko Y, et al. Immunoprecipitation of insulin receptors by antibodies against Class 1 antigens of the murine H-2 major histocompatibility complex. FEBS Lett. 1983;163:207–211. doi: 10.1016/0014-5793(83)80820-5. [DOI] [PubMed] [Google Scholar]
- 68.Demaria S, et al. Soluble beta 2-microglobulin-free class I heavy chains are released from the surface of activated and leukemia cells by a metalloprotease. The Journal of biological chemistry. 1994;269:6689–6694. [PubMed] [Google Scholar]
- 69.Stevens B, et al. The classical complement cascade mediates CNS synapse elimination. Cell. 2007;131:1164–1178. doi: 10.1016/j.cell.2007.10.036. [DOI] [PubMed] [Google Scholar]
- 70.Fujita Y, et al. Myelin suppresses axon regeneration by PIR-B/SHP-mediated inhibition of Trk activity. EMBO J. 2011;30:1389–1401. doi: 10.1038/emboj.2011.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Atwal JK, et al. PirB is a functional receptor for myelin inhibitors of axonal regeneration. Science. 2008;322:967–970. doi: 10.1126/science.1161151. [DOI] [PubMed] [Google Scholar]
- 72.Nakamura Y, et al. Paired immunoglobulin-like receptor B knockout does not enhance axonal regeneration or locomotor recovery after spinal cord injury. J Biol Chem. 2011;286:1876–1883. doi: 10.1074/jbc.M110.163493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Joseph MS, et al. Transgenic mice with enhanced neuronal major histocompatibility complex class I expression recover locomotor function better after spinal cord injury. J Neurosci Res. 2011;89:365–372. doi: 10.1002/jnr.22557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Oliveira AL, et al. A role for MHC class I molecules in synaptic plasticity and regeneration of neurons after axotomy. Proc Natl Acad Sci U S A. 2004;101:17843–17848. doi: 10.1073/pnas.0408154101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zanon RG, Oliveira AL. MHC I upregulation influences astroglial reaction and synaptic plasticity in the spinal cord after sciatic nerve transection. Experimental neurology. 2006;200:521–531. doi: 10.1016/j.expneurol.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 76.Torres AR, et al. The association and linkage of the HLA-A2 class I allele with autism. Hum Immunol. 2006;67:346–351. doi: 10.1016/j.humimm.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 77.Stubbs EG, et al. Autism and shared parental HLA antigens. J Am Acad Child Psychiatry. 1985;24:182–185. doi: 10.1016/s0002-7138(09)60445-3. [DOI] [PubMed] [Google Scholar]
- 78.Stubbs G. Shared parental HLA antigens and autism. Lancet. 1981;2:534. doi: 10.1016/s0140-6736(81)90926-0. [DOI] [PubMed] [Google Scholar]
- 79.Shi J, et al. Common variants on chromosome 6p22.1 are associated with schizophrenia. Nature. 2009;460:753–757. doi: 10.1038/nature08192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Stefansson H, et al. Common variants conferring risk of schizophrenia. Nature. 2009;460:744–747. doi: 10.1038/nature08186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Purcell SM, et al. Common polygenic variation contributes to risk of schizophrenia and bipolar disorder. Nature. 2009;460:748–752. doi: 10.1038/nature08185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jia P, et al. A bias-reducing pathway enrichment analysis of genome-wide association data confirmed association of the MHC region with schizophrenia. J Med Genet. 2012;49:96–103. doi: 10.1136/jmedgenet-2011-100397. [DOI] [PubMed] [Google Scholar]
- 83.Kano S, et al. Altered MHC class I expression in dorsolateral prefrontal cortex of nonsmoker patients with schizophrenia. Neurosci Res. 2011;71:289–293. doi: 10.1016/j.neures.2011.07.1818. [DOI] [PubMed] [Google Scholar]
- 84.Drew PD, et al. Regulation of MHC class I and beta 2-microglobulin gene expression in human neuronal cells. Factor binding to conserved cis-acting regulatory sequences correlates with expression of the genes. J Immunol. 1993;150:3300–3310. [PubMed] [Google Scholar]
- 85.Fujimaki H, et al. IFN-gamma induces expression of MHC class I molecules in adult mouse dorsal root ganglion neurones. Neuroreport. 1996;7:2951–2955. doi: 10.1097/00001756-199611250-00030. [DOI] [PubMed] [Google Scholar]
- 86.Chevalier G, et al. Neurons are MHC class I-dependent targets for CD8 T cells upon neurotropic viral infection. PLoS Pathog. 2011;7:e1002393. doi: 10.1371/journal.ppat.1002393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Vargas DL, et al. Neuroglial activation and neuroinflammation in the brain of patients with autism. Ann Neurol. 2005;57:67–81. doi: 10.1002/ana.20315. [DOI] [PubMed] [Google Scholar]
- 88.Chez MG, et al. Elevation of tumor necrosis factor-alpha in cerebrospinal fluid of autistic children. Pediatric neurology. 2007;36:361–365. doi: 10.1016/j.pediatrneurol.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 89.Ashwood P, et al. Associations of impaired behaviors with elevated plasma chemokines in autism spectrum disorders. J Neuroimmunol. 2011;232:196–199. doi: 10.1016/j.jneuroim.2010.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ashwood P, et al. Elevated plasma cytokines in autism spectrum disorders provide evidence of immune dysfunction and are associated with impaired behavioral outcome. Brain Behav Immun. 2011;25:40–45. doi: 10.1016/j.bbi.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ashwood P, et al. Decreased transforming growth factor beta1 in autism: a potential link between immune dysregulation and impairment in clinical behavioral outcomes. J Neuroimmunol. 2008;204:149–153. doi: 10.1016/j.jneuroim.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Nawa H, et al. Cytokine and growth factor involvement in schizophrenia--support for the developmental model. Molecular psychiatry. 2000;5:594–603. doi: 10.1038/sj.mp.4000730. [DOI] [PubMed] [Google Scholar]
- 93.Deverman BE, Patterson PH. Cytokines and CNS development. Neuron. 2009;64:61–78. doi: 10.1016/j.neuron.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 94.Brown AS, Patterson PH. Maternal infection and schizophrenia: implications for prevention. Schizophr Bull. 2011;37:284–290. doi: 10.1093/schbul/sbq146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bauer S, et al. The neuropoietic cytokine family in development, plasticity, disease and injury. Nature reviews. 2007;8:221–232. doi: 10.1038/nrn2054. [DOI] [PubMed] [Google Scholar]
- 96.Patterson PH. Neuroscience. Maternal effects on schizophrenia risk. Science. 2007;318:576–577. doi: 10.1126/science.1150196. [DOI] [PubMed] [Google Scholar]
- 97.Molloy CA, et al. Elevated cytokine levels in children with autism spectrum disorder. Journal of neuroimmunology. 2006;172:198–205. doi: 10.1016/j.jneuroim.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 98.Careaga M, et al. Immune dysfunction in autism: a pathway to treatment. Neurotherapeutics. 2010;7:283–292. doi: 10.1016/j.nurt.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Miller BJ, et al. Meta-Analysis of Cytokine Alterations in Schizophrenia: Clinical Status and Antipsychotic Effects. Biol Psychiatry. 2011;70:663–71. doi: 10.1016/j.biopsych.2011.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.McAllister AK, van de Water J. Breaking boundaries in neural-immune interactions. Neuron. 2009;64:9–12. doi: 10.1016/j.neuron.2009.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Held W, Mariuzza RA. Cis interactions of immunoreceptors with MHC and non-MHC ligands. Nat Rev Immunol. 2008;8:269–278. doi: 10.1038/nri2278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Scarpellino L, et al. Interactions of Ly49 family receptors with MHC class I ligands in trans and cis. J Immunol. 2007;178:1277–1284. doi: 10.4049/jimmunol.178.3.1277. [DOI] [PubMed] [Google Scholar]
- 103.Stagsted J. Journey beyond immunology. Regulation of receptor internalization by major histocompatibility complex class I (MHC-I) and effect of peptides derived from MHC-I. APMIS Suppl. 1998;85:1–40. [PubMed] [Google Scholar]
- 104.Turrigiano GG, Nelson SB. Homeostatic plasticity in the developing nervous system. Nature reviews Neuroscience. 2004;5:97–107. doi: 10.1038/nrn1327. [DOI] [PubMed] [Google Scholar]
- 105.Katz LC, Shatz CJ. Synaptic activity and the construction of cortical circuits. Science. 1996;274:1133–1138. doi: 10.1126/science.274.5290.1133. [DOI] [PubMed] [Google Scholar]
- 106.Hensch TK. Critical period plasticity in local cortical circuits. Nat Rev Neurosci. 2005;6:877–888. doi: 10.1038/nrn1787. [DOI] [PubMed] [Google Scholar]