Abstract
Persimmon Leaf (PL), commonly consumed as herbal tea and traditional medicines, contains a variety of compounds that exert antioxidant, α-amylase and α-glucosidase inhibitory activity. However, little is known about the in vivo effects and underlying mechanisms of PL on hyperglycemia, hyperlipidemia and hepatic steatosis in type 2 diabetes. Powered PL (5%, w/w) was supplemented with a normal diet to C57BL/KsJ-db/db mice for 5 weeks. PL decreased blood glucose, HOMA-IR, plasma triglyceride and total cholesterol levels, as well as liver weight, hepatic lipid droplets, triglycerides and cholesterol contents, while increasing plasma HDL-cholesterol and adiponectin levels. The anti-hyperglycemic effect was linked to decreased activity of gluconeogenic enzymes as well as increased glycogen content, glucokinase activity and its mRNA level in the liver. PL also led to a decrease in lipogenic transcriptional factor PPARγ as well as gene expression and activity of enzymes involved in lipogenesis, with a simultaneous increase in fecal lipids, which are seemingly attributable to the improved hyperlipidemia and hepatic steatosis and decreased hepatic fatty acid oxidation. Furthermore, PL ameliorated plasma and hepatic oxidative stress. Supplementation with PL may be an effective dietary strategy to improve type 2 diabetes accompanied by dyslipidemia and hepatic steatosis by partly modulating the activity or gene expression of enzymes related to antioxidant, glucose and lipid homeostasis.
Introduction
Persimmon (diospyros kaki) is a widely cultivated fruit in East Asia, and the persimmon leaf (PL) is commonly used for herbal tea and traditional medicines in South Korea, Japan and China. PL contains proanthocyanidins (also called condensed tannins), flavonoids and other compounds, which have a variety of pharmacological actions [1]–[7]. Flavonoids isolated from PL have antioxidant [1], hypotensive [2] and anti-allergic effects [3] and proanthocyanidins, the major polyphenol in PL, have anti-hypertensive and vasorelaxant effects [4]. Some in vitro studies have suggested that PL may also have beneficial effects on diabetes. For example, components from PL inhibited α-amylase, α-glucosidase and protein tyrosine phosphatase 1B activity [5]–[7], and stimulated glucose uptake in HepG2 cells and 3T3-L1 adipocytes [6]. However, there is relatively little known regarding the in vivo efficacy of PL for diabetes.
Type 2 diabetes, the most common type of diabetes, is one of the fastest growing and most costly metabolic disorders in the world. It is accepted that changes in lifestyle, such as increased fat intake and/or physical inactivity and decreased vegetable, fruits and whole grain intake, are responsible for the increasing the incidence of type 2 diabetes. Insulin resistance is a major underlying factor contributing to the development of type 2 diabetes, which can result in hyperglycemia, dyslipidemia, or hepatic steatosis. The liver is a key metabolic buffering organ that controls glucose and lipid homeostasis, and, in particular, hepatic insulin resistance can cause hyperglycemia through reduced glycogen synthesis and storage and a failure to suppress glucose production and release into the blood. In addition, hepatic steatosis is linked to insulin resistance, although whether the excessive accumulation of lipids in the liver is a cause or a consequence of insulin resistance still remains unclear [8]. Since hyperglycemia- and hyperlipidemia-induced reactive oxygen species is one of the major contributors of insulin resistance [9], the identification of antioxidant foods, which can improve insulin sensitivity, is crucial for the amelioration of type 2 diabetes and its complications.
We previously reported that powdered PL improved lipid profiles and suppressed body weight gain in rats fed a high-fat diet [10]. In this study, we investigated the anti-diabetic effect of PL in type 2 diabetic db/db mice that have an exacerbation of insulin resistance and hepatic steatosis [11]. We also examined the potential mechanisms of action, particularly focusing on the gene expression and activity of enzymes involved in glucose and lipid metabolism and oxidative stress in the liver as well as the lipid content in the feces.
Materials and Methods
Preparation of Powdered Persimmon Leaf and its Composition Analysis
PL was prepared as described previously [10]. PL was harvested in Sangju (Korea) and dried in the shade for a week. The leaf was powdered and passed through 60 mesh sieves. The total fiber and phenolic contents were analyzed by AOAC method and a modified Folin-Ciocaleu colorimetric method, respectively [12], [13]. For determination of total phenolic content, a solution containing extract or standard solution of gallic acid was mixed with Folin-Ciocalten reagent. After 6 min, 7% sodium carbonate solution was added to the mixture. The resulting solution was incubated for a further 90 min before absorbance reading was spectrophotometrically read at 760 nm. The total phenolic content was expressed as mg of gallic acid equivalents/g of powdered PL. The total flavonoid content was determined according to Moreno et al. [14] and was expressed as mg of quercetin equivalents/g of powdered PL. The total fiber, phenolic and flavonoid contents in the PL were 630 mg/g, 11.49 mg/g, 1.59 mg/g respectively.
Animals and Diets
Male C57BL/KsJ-db/db (db/db) mice were purchased from Jackson Laboratory (Bar Harbor, ME) at 5 weeks of age and maintained under standard light (12 h light/dark) and temperature conditions (22±2°C). The twenty db/db mice were fed a pelletized commercial chow diet for 2 weeks after arrival, and then the db/db mice were divided into two groups (n = 10). Thereafter, the control group of db/db mice were fed a standard semisynthetic diet (AIN-76) [15], [16], while another group of db/db mice were fed a standard semisynthetic diet with the powered PL (5 g/100 g diet, w/w) for 5 weeks. The mice had free access to food and water ad libitum. At the end of the experimental period, the mice were anesthetized with ketamine after withholding food for 12 hours, and blood samples were taken from the inferior vena cava to determine the plasma biomarkers. In addition, the liver was removed after the blood was collected, then rinsed with a physiological saline solution, and immediately stored at −70°C. All procedures were approved by the animal ethics committee of Kyungpook National University (Approval No. KNU-2011-28).
Fasting Blood Glucose Level and Homeostatic Index of Insulin Resistance (HOMA-IR)
Every week after 12 hours of fasting, the blood glucose concentration was monitored in the venous blood from the tail vein using a glucometer (Arkary, Japan). HOMA-IR was calculated according to the homeostasis of the assessment as follows (Eq. 1) [17]:
.
Plasma Analyses
The blood was collected in a heparin-coated tube and centrifuged at 1,000×g for 15 min at 4°C. The plasma insulin and adiponectin levels were determined using an insulin RIA kit (Diagnostic Systems Laboratories, USA) and a sandwich ELISA kit (R&D system, USA), respectively. The plasma free fatty acid concentration was measured using an enzymatic non-esterified fatty acid kit (Wako, Osaka, Japan). Meanwhile, the plasma triglyceride, total cholesterol, and high-density lipoprotein (HDL)-cholesterol concentrations were measured spectrophotometrically using a commercial kit (Sigma Chemical Co.). The paraoxonase (PON) activity was assayed spectrophotometrically using the method described by Mackness et al. [18], which measured the increase in absorbance for 90 s at 405 nm and 25°C.
Hepatic and Fecal Lipids and Hepatic Glycogen, Hydrogen Peroxide and Lipid Peroxidation
The feces from each group were collected daily for one week, and the hepatic and fecal lipids were extracted using the procedure developed by Folch et al. [19]. The levels of cholesterol and triglyceride in the liver and feces were analyzed with the same commercial kit as used in the plasma analysis.
The hepatic glycogen content was determined as previously described by Seifter et al. [20] with modification. Briefly, the liver tissue was homogenized in 5 volumes of an 30% (w/v) KOH solution and dissolved at 100°C for 30 min. The glycogen was determined by treatment with an anthrone reagent (2 g anthrone/1 L of 95% (v/v) H2SO4) and measuring the absorbance at 620 nm.
The hydrogen peroxide levels in liver were measured by Wolff’s method [21]. FOX 1 (Ferrous Oxidation with Xylenol orange) reagent was prepared as the following mixture: 100 µM xylenol orange, 250 µM ammonium ferrous sulfate, 100 mM sorbitol, and 25 mM H2SO4. Fifty microliters of test sample was added to 950 µL FOX 1 reagent, vortexed, and incubated at room temperature for a minimum of 30 min at which time color development is virtually complete. The absorbance was read at 560 nm and the standard was linear in the 0∼5 µM concentration range. The hepatic thiobarbituric acid-reactive substances (TBARS) concentration, as a marker of lipid peroxide production, was measured spectrophotometrically by the method of Ohkawa et al. [22].
Hepatic Morphology
The livers were removed from the mice and fixed in a buffer solution of 10% formalin. Fixed tissues were processed routinely for paraffin embedding, and 4-µm sections were prepared and stained with hematoxylin eosin (H&E); stained areas were viewed using an optical microscope with a magnifying power of ×200.
Liver Enzyme Analyses
The hepatic cytosolic, mitochondrial and microsomal preparations were performed according to Hulcher and Oleson [23] with a slight modification, and the protein concentration was determined using Bradford’s method [24].
The glucokinase (GK) activity was determined using a spectrophotometric continuous assay as described by Davidson and Arion [25] and Newgard et al. [26] with a slight modification, in which the formation of glucose-6-phosphate was coupled to its oxidation by glucose-6-phosphate dehydrogenase and NAD+ at 37°C. The glucose-6-phosphatase (G6Pase) activity was determined using the method of Alegre et al. [27] with a slight modification. The reaction mixture contained 40 mmol/L sodium Hepes (pH 6.5), 14 mmol/L glucose-6-phosphate, 18 mmol/L EDTA, both previously adjusted to pH 6.5, 2 mmol/L NADP+, 0.6 IU/mL mutarotase, and 0.6 IU/mL glucose dehydrogenase. The phosphoenolpyruvate carboxykinase (PEPCK) activity was monitored in the direction of oxaloacetate synthesis using the spectrophotometric assay developed by Bentle and Lardy [28] with a slight modification. A 1 mL final volume of the purified enzyme was pipetted with a reaction mixture (pH 7.0) containing 77 mmol/L sodium Hepes, 1 mmol/L IDP, 1 mmol/L MnCl2, 1 mmol/L dithiothreitol, 0.25 mmol/L NADH, 2 mmol/L phosphoenolpyruvate, 50 mmol/L NaHCO3, and 7.2 units of malic dehydrogenase into a Eppendorf tube. The enzyme activity was then measured for 2 min at 25°C based on a decrease in the absorbance at 340 nm. The fatty acid synthase (FAS) activity was measured according to the method of Carl et al. [29] by monitoring the malonyl-CoA-dependent oxidation of NADPH at 340 nm, where the activity was represented by the oxidized NADPH nmol/min/mg protein. The phosphatidate phosphohydrolase (PAP) activity was determined using the method of Walton and Possmayer [30]. The carnitine palmitoyl transferase (CPT) activity was determined according to the method of Markwell et al. [31] and the results were expressed as nmol/min/mg protein. The fatty acid β-oxidation was determined using the method of Lazarow [32] by monitoring the reduction of NAD to NADH at 340 nm, where the activity was expressed as the reduced NAD nmol/min/mg protein. The 3-hydroxy-3-methylglutaryl-coenzyme reductase (HMGR) activity was measured in the microsomes with [14C]-HMG-CoA as the substrate based on a modification of the method of Shapiro et al. [33], where the activity was expressed as the synthesized mevalonate pmol/min/mg protein. The acyl CoA: cholesterol acyltransferase (ACAT) activity in the microsomes was determined by the rate of incorporation of [14C]-Oleoyl CoA into cholesterol ester fractions, as described by Erickson et al. [34], where the activity was expressed as the synthesized cholesteryl oleate pmol/min/mg protein. Superoxide dismutase (SOD) activity was spectrophotometrically measured by the inhibition of pyrogallol autoxidation at 420 nm for 10 min according to the method of Marklund and Marklund [35]. One unit was determined as the amount of enzyme that inhibited the oxidation of pyrogallol by 50%. Catalase (CAT) activity was measured using Aebi’s [36] method with a slight modification, in which the disappearance of hydrogen peroxide was monitored at 240 nm for 5 min using a spectrophotometer. A molar extinction coefficient of 0.041 mM−1cm−1 was used to determine CAT activity. Glutathione peroxidase (GPX) activity was measured using the spectrophotometric assay at 25°C, as described previously by Paglia and Valentine’s [37] method with a slight modification. The reaction mixture contained 2.525 mL of a 0.1 M of Tris-HCl (pH 7.2) buffer, 75 µL of 30 mM glutathione, 100 µL of 6 mM NADPH, and 100 µL of glutathione reductase (0.24 unit). One hundred microliters of the solution was added to 2.8 mL of the reaction mixture and incubated at 25°C for 5 min. The reaction was initiated by adding 100 µL of 30 mM H2O2 and the absorbance measured at 340 nm for 5 min. A molar extinction coefficient of 6.22 mM−1cm−1 was used to determine GPX activity.
RNA Isolation and mRNA Expression Analysis
Total RNA was isolated from the livers by the guanidine thiocyanate-phenol method of Chomzynski and Sacchi [38]. For Northern blotting, the total RNA (20 µg) was separated on a 0.9% agarose gel containing 2.2 M formaldehyde and transferred to Nytran-Plus membranes (Schleicher & Schuell, Dassel, Germany). The membranes were then hybridized with a [32P]-labeled cDNA probe, washed at room temperature with 2× sodium chloride sodium citrate (SSC) containing 0.1% SDS followed by two washes at 65°C with 0.2× SSC containing 0.1% SDS, and exposed to X-ray film with an intensifying screen at −70°C. Thereafter, DNA probes were prepared from the mouse liver RNA using RT-PCR with the following primers: for GK, 5'-TTCACCTTCTCCTTCCCTGTAAGGC-3' and 5'-TACCAGCTTGAGCAGCACAAGTCG-3'; for G6Pase, 5'-AAGACTCCCAGGACTGGTTCATCC-3' and 5'-TAGCAGGTAGAATCCAAGCG CG-3'; for PEPCK, 5'-TGCTGATCCTGGGCATAACTAACC-3' and 5'-TGGGTACTCCTTCTGGAGATTCCC-3'; for Cu/Zn SOD, 5′-AGGATTAACTGAAGGCGAGCAT-3′ and 5′-TCTACAGTTAGCAGGCCAGCA G-3′; for CAT, 5′-ACGAGATGGCACACTTTGACAG-3′ and 5′-TGGGTTTCTCTTCTGGCTATGG-3′; for GPX, 5′-AAGGTGCTGCTCATTGAGAATG-3′ and 5′-CGTCTGGACCTACCAGGAACTT-3′; and for glyceraldehyde-3-phosphate dehydrogenase (GAPDH), 5'-TTGAAGGGTGGAGCCAAACG-3' and 5'-AGTGGGAGTTGCTGTTGAAGTCG-3'. The intensities of the mRNA bands were quantified using a Bio Image Whole Band Analyzer (50S, B.I. System Co., USA) and subsequently normalized based on the intensity of the respective GAPDH mRNA bands. For real-time PCR, complementary DNA was synthesized using Moloney murine leukemia virus reverse transcriptase (Fermentase, Burlington, ON, Canada), random hexamers, deoxyribonucleoside triphosphates, and 5 µg of total RNA. After first-strand complementary DNA synthesis, the RNA expression was quantified by a real-time quantitative PCR using SYBR green PCR reagnets (Applied Biosynstems, Foster City, CA) and the SDS7000 sequence-detection system (Applied Biosystems). Transcripts of the housekeeping gene GAPDH in the same incubations were used for normalization. The primer sequences were as follows: for ATP-citrate lyase (ACL), 5′–CAGCAAGCACTGTCAGAATA–3′ and 5′–TTAAAACTTGCATTCCCTTC–3′; for CPT, 5′-ATCTTCTAATCCCACCCAGT-3′ and 5′-AAAGCACCCATTACTTGAGA-3′; for diacylglycerol transferase (DGAT), 5′-ACTCCATCATGTTCCTCAAG-3′ and 5′-CTCTGCTTGTGAGAAGGAAC-3′; for PAP, 5′-GGGTTCTACTGTGGAGATGA-3′ and 5′-TGACAGTAGCTGTGATGATGA-3′; for peroxisome proliferator activated receptorγ (PPARγ), 5′–AGAGTCTGCTGATCTGCGAGC–3′ and 5′–TTCCTGTCAAGATCGCCCTC–3′; for stearoyl-CoA desaturase-1 (SCD-1), 5′–TAAGCTCAGTCTCACTCCTT–3′ and 5′–AAAAGATTTCTGCAAACCAA–3′.
Statistical Analysis
All data were presented as the mean ± S.E. Statistical analyses were performed using the statistical package for the social science software (SPSS) program. Student’s t-test was used to assess the differences between the groups. Statistical significance was considered at p<0.05.
Results
PL Ameliorates Hyperglycemia and Insulin Resistance and Increased Plasma Adiponectin Level
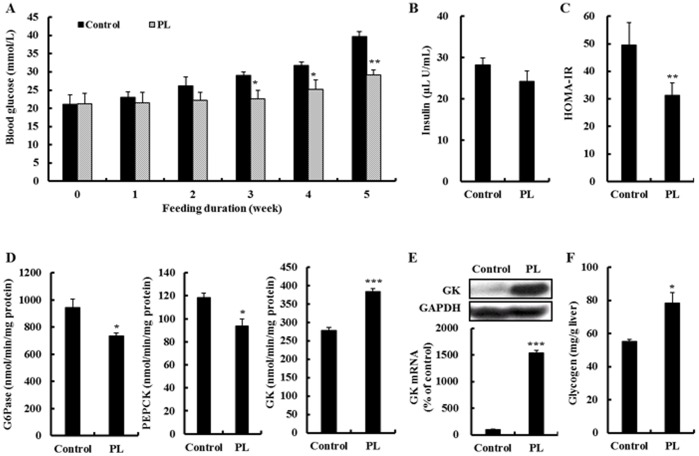
First, we examined the effect of PL on hyperglycemia and insulin resistance in type 2 diabetic db/db mice. All db/db mice were diabetic when the experiment began, as indicated by the fasting blood glucose level (≥21.06 mmol/L) (Fig. 1A). The supplementation of PL suppressed the increase of the fasting blood glucose level from the 3rd week to the 5th week in the db/db mice. The plasma insulin level was not affected by PL supplementation, while PL improved HOMA-IR in the db/db mice (Fig. 1B&C). Furthermore, PL did not alter the plasma leptin level, yet it significantly increased the plasma adiponectin level (Table 1). There was no significant difference in food intake, body weight and body fat mass between the groups (data not shown).
Figure 1. Effect of supplementation with powdered persimmon leaf on the glucose regulation in C57BL/KsJ-db/db mice.
A; fasting blood glucose level. B&C plasma insulin level and HOMA-IR. D&E activity and/or mRNA expression of enzymes for hepatic gluconeogenesis and glucose utilization. The mRNA levels were analyzed by Northern blot analysis using GK probes and normalized to an internal control (GAPDH). Three independent analyses were performed. F; hepatic glycogen content. Values are the mean ± S.E., n = 10. *p<0.05, **p<0.01, ***p<0.001. GAPDH; glyceraldehyde-3-phosphate dehydrogenase, G6Pase; glucose-6-phosphatase, GK; glucokinase, HOMA-IR; homeostatic index of insulin resistance, PEPCK; phosphoenolpyruvate carboxykinase, PL; powdered persimmon leaf.
Table 1. Effects of supplementation with persimmon leaf on the levels of lipids and adipokines and on the activity of paraoxonase in plasma of C57BL/KsJ-db/db mice.
| Control | PL | |
| Free fatty acid (mmol/L) | 1.54±0.03 | 1.56±0.07 |
| Triglyceride (mmol/L) | 3.32±0.19 | 2.09±0.25* |
| Total cholesterol (mmol/L) | 5.62±0.12 | 4.73±0.2** |
| HDL-cholesterol (mmol/L) | 1.06±0.07 | 1.44±0.06* |
| HTR (%) | 18.86±0.93 | 30.35±1.17*** |
| Atherogenic index | 4.31±0.02 | 2.29±0.13*** |
| Adiponectin (µg/mL) | 2.26±0.22 | 3.72±0.14** |
| Leptin (ng/mL) | 49.1±3.16 | 50.5±4.07 |
| PON (µmol/min/mL) | 1.30±0.02 | 1.62±0.05* |
Data are Mean ± S.E. (n = 10).
p<0.05, ** p<0.01, *** p<0.001 vs. control group as determined by student’s t-test.
HTR (%); [(HDL-cholesterol)/(Total cholesterol)]×100, Atherogenic index; [(Total cholesterol)– (HDL-cholesterol)]/(HDL-cholesterol), PON; paraoxonase.
PL Increases Glycogen Content and GK Activity and its Gene Expression and Decreases G6Pase and PEPCK Activity in the Liver
To examine how PL ameliorated hyperglycemia, we determined the activity of the hepatic glucose-regulating enzymes, their gene expression and glycogen content (Fig. 1D–F). The GK activity and mRNA expression as well as glycogen content were significantly higher in the liver of the PL-supplemented db/db mice compared to the control db/db mice. In contrast, the supplementation of PL significantly lowered the activity of hepatic gluconeogenic enzymes, G6Pase and PEPCK. However, no difference was observed in the mRNA expression of hepatic G6Pase and PEPCK between the groups (data not shown).
PL Attenuates Dyslipidemia and Hepatic Steatosis
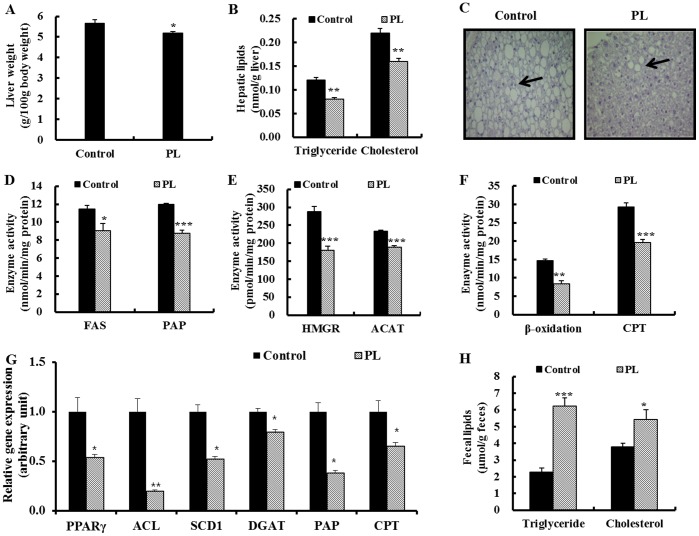
Next, we evaluated the effect of PL on dyslipidemia and hepatic steatosis in the db/db mice. The plasma triglyceride and total cholesterol concentrations were significantly lower in the PL group than in the control group, whereas the plasma HDL-cholesterol concentration was significantly higher in the PL group (Table 1). Thus, an increased HTR and decreased atherogenic index were observed in the PL group. Furthermore, PL supplementation significantly lowered hepatic triglyceride and cholesterol contents compared to the control db/db mice, and liver weight was significantly lower in the PL group (Fig. 2A&B). Histological analysis of the liver also revealed lower numbers and size of hepatic lipid droplets in the PL-supplemented db/db mice compared to the control db/db mice (Fig. 2C).
Figure 2. Effect of supplementation with powdered persimmon leaf on the lipid regulation in C57BL/KsJ-db/db mice.
A; liver weight. B; hepatic triglyceride and cholesterol contents. C; hepatic morphology. Arrows indicate hepatic lipid droplets. H&E staining. Original magnification ×200. D; activity of the enzymes for hepatic fatty acid synthesis and esterification. E; activity of the enzymes for hepatic cholesterol synthesis and esterification. F; activity of the enzymes for hepatic fatty acid oxidation and uptake. G; mRNA expression of lipogenic transcription factor and its target gene. The mRNA levels were analyzed by real-time PCR and normalized to an internal control (GAPDH). H; fecal lipids content. Values are the mean ± S.E., n = 10. *p<0.05, **p<0.01, ***p<0.001. ACAT; acyl CoA: cholesterol acyltransferase, ACL; ATP-citrate lyase, CPT; carnitine palmitoyl transferase, DGAT; glyceraldehyde-3-phosphate dehydrogenase, FAS; fatty acid synthase, HMGR; 3-hydroxy-3-methylglutaryl-coenzyme, PAP; phosphatidate phosphohydrolase, PL; powdered persimmon leaf, PPARγ; peroxisome proliferator activated receptor γ, SCD1; steraroyl-CoA desaturase-1.
PL Inhibits Hepatic Lipogenic Gene Expression and Enzymes Activity as well as Transcriptional Factor PPARγ mRNA Expression and Increases Fecal Lipids
To clarify the mechanism in which PL decreased the plasma and hepatic lipids levels, we examined the hepatic lipid-regulating enzyme activity and gene expression along with fecal lipids levels. The activity of the hepatic lipogenic enzymes, FAS, PAP, HMGR and ACAT, were significantly lower in the PL-supplemented db/db mice compared to the control db/db mice (Fig. 2D&E). PL also down-regulated mRNA expression of lipogenic transcription factor (PPARγ) and its target genes (ACL, SCD1, DGAT, PAP) in the liver (Fig. 2G). Moreover, the supplementation of PL significantly increased the fecal triglyceride and cholesterol content (Fig. 2H). The fatty acid oxidation as well as CPT activity and mRNA level were markedly lower in the PL group compared to the control group (Fig. 2F&G).
PL Improves Plasma and Hepatic Oxidative Stress
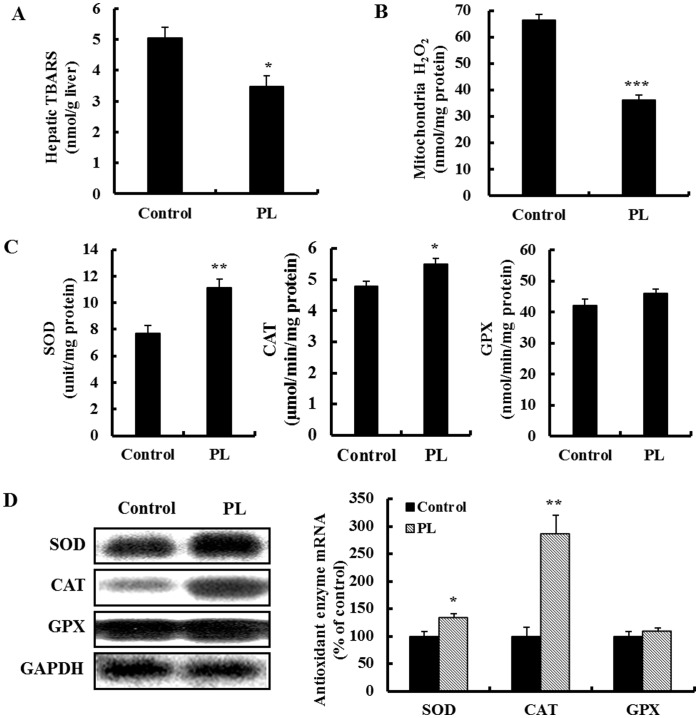
We next examined the effect of PL on hepatic lipid peroxidation, hydrogen peroxide levels, and antioxidant enzymes activities and their mRNA expression, which influence the regulation of glucose and lipid metabolism in the db/db mice. The lipid peroxidation and mitochondrial hydrogen peroxide levels were significantly lower in the liver of the PL-supplemented db/db mice compared to the control db/db mice (Fig. 3A&B). Hepatic GPX activity and mRNA expression was not different between the groups; however, SOD and CAT activities and their mRNA expression were significantly increased by PL supplementation (Fig. 3C&D). Furthermore, PL significantly increased the activity of PON in plasma (Table 1).
Figure 3. Effect of supplementation with powdered persimmon leaf on hepatic oxidative stress in C57BL/KsJ-db/db mice.
A; hepatic TBARS level. B; hepatic mitochondria hydrogen peroxide content. C; hepatic antioxidant enzyme activity. D; hepatic antioxidant gene expression. The mRNA levels were analyzed by Northern blot analysis using SOD, CAT and GPX probes and normalized to an internal control (GAPDH). Three independent analyses were performed. Values are the mean ± S.E., n = 10. *p<0.05, **p<0.01, ***p<0.001. CAT; catalase, GPX; glutathione peroxidase, GAPDH; glyceraldehyde-3-phosphate dehydrogenase, PL; powdered persimmon leaf, SOD; superoxide dismutase.
Discussion
Type 2 diabetes is a metabolic syndrome with diverse pathological manifestations and is often associated with abnormal glucose and lipid metabolism. Previously, we reported that the supplementation of PL that is rich in fiber and phenolic compounds suppressed the body weight gain and lowered plasma and hepatic lipid levels in rats fed a high-fat diet [10]. Furthermore, several in vitro studies showed the possible beneficial effect of PL on glucose regulation. For example, PL inhibited enzymes involved in the digestion of carbohydrates (α-amylase and α-glucosidase) and the regulation of insulin signaling (protein tyrosine phosphatase 1B) [5]–[7]. However, no report has been published on the in vivo anti-diabetic effects of PL in type 2 diabetes. In this study, we firstly demonstrated that dietary supplementation of PL for 5 weeks ameliorated hyperglycemia, dyslipidemia and hepatic steatosis in type 2 diabetic mice, at least in part, by regulation of hepatic glucose and lipid metabolism and antioxidant status.
db/db mice are a widely used genetic model of type 2 diabetes since they exhibit most of the human characteristics of type 2 diabetes, including hyperglycemia, dyslipidemia and insulin resistance [11]. A recent study suggests that defects in hepatic insulin signaling contribute to the development of diabetes in C57BL/KsJ-db/db mice [39]. The liver is a major insulin-sensitive organ responsible for maintaining glucose and lipid homeostasis. A failure of insulin to increase hepatic glucose utilization and to suppress hepatic endogenous glucose production is a major factor contributing to hyperglycemia in diabetes [40]. The key enzyme responsible for the regulation of glucose utilization is GK that catalyzes glucose phosphorylation as the first step of storage of glucose as glycogen and glucose disposal by glycolysis [41]. Conversely, PEPCK and G6Pase are rate-controlling enzymes of gluconeogenesis in the liver [42]. We found that in the db/db mice, the supplementation of PL significantly decreased the fasting blood glucose level and HOMA-IR index, which primarily reflects hepatic insulin resistance [43]. These changes were accompanied by decreases in hepatic G6Pase and PEPCK activity and increases in hepatic GK activity along with glycogen content. Thus, these observations suggest that PL can promote hepatic insulin sensitivity and thus effectively regulate the activity of enzymes involved in hepatic glucose homeostasis, leading to lower blood glucose level in db/db mice.
The regulation of GK activity is primarily due to changes in the transcription of its gene [44]. We also found that the change in GK activity by PL was accompanied by its increased transcriptional level. In contrast, the gene expression of hepatic G6Pase and PEPCK was not affected. Similarly, a lack of regulation of G6Pase and PEPCK gene expression was reported following treatment with some phenolic compounds in rat hepatocytes, despite a significant reduction in glucose production and enhanced hepatic GK mRNA expression [45]. In addition, it is known that the suppression of hepatic glucose production by metformin results from the inhibition of G6Pase activity along with an increase in glycogen stores with minimal effects on the gluconeogenic gene expression in the livers of rats [46], [47]. Thus, we think that PL may act mainly by suppressing the activity of hepatic gluconeogenic enzymes independent of the transcriptional repression of gluconeogenic genes. Since elevated GK expression led to a reduced endogenous glucose production in the liver [48], it is possible that the observed decrease in activity of gluconeogenic enzymes in PL-supplemented db/db mice is related to the inhibition of the substrate flux through GK activation.
On the other hand, some studies have raised concerns about the manipulation of GK activator for diabetes treatment since a decline in glucose level in response to hepatic GK overexpression is accompanied by an increase in circulating lipids and hepatic lipogenesis [49], [50]. However, several lines of evidence suggest that hepatic GK activation does not alter plasma and hepatic lipid metabolism in normal and high-fat fed animals [49], [51]. The present results also showed that PL induced a marked decrease in triglyceride and cholesterol accumulation in the liver, together with the inhibition of activity of hepatic lipogenic enzymes involved in the synthesis and esterification of fatty acid (FAS, PAP) or cholesterol (HMGR, ACAT), which may subsequently reduce the formation of lipid droplets within hepatocytes and the secretion of triglycerides and cholesterol into the blood. Simultaneously, this effect could be related to the down-regulated expression of several lipogenic genes (ACL, SCD1, PAP, DGAT) as well as key transcription factor (PPARγ) in the liver. Normally, PPARγ is expressed at very low levels in the liver, but its expression is dramatically increased in animal model with insulin resistance and hepatic steatosis such as db/db mice [52]. The genetic deletion of hepatic PPARγ protected against hepatic steatosis in high fat diet-induced obese mice [53]. We also found that PL appeared to facilitate fecal excretion of triglycerides as well as cholesterol in the db/db mice, in accordance with our previous data on high-fat fed rats [10]. Accordingly, PL seemed to lower plasma and hepatic lipid accumulation by decreasing hepatic lipogenesis and increasing fecal lipids. Since inhibition of FAS induces an increase in hepatic malonyl-CoA which is a potent inhibitor of CPT [54], [55], a key enzyme involved in mitochondrial fatty acids uptake for oxidation [56], the reduced activity of hepatic fatty acid oxidation and CPT could be a secondary consequence of the decrease in hepatic FAS activity. Also, the reduced fatty acid oxidation might be associated with activated glucose utilization and a reduction in glucose production in the liver [57].
In addition to its role in regulating lipogenesis, it is known that PPARγ is required for transcription of the PEPCK gene in adipocytes [58]. PPARγ agonists such as pioglitazone and rosiglitazone were potent inducer of PEPCK gene transcription and enzymatic activity in adipose tissue of obese Zucker rats [59], [60]. Moreover, hepatocyte specific PPARγ-knockout mice showed reduced serum glucose level and PEPCK mRNA expression [53]. However, liver-specific disruption of PPARγ in leptin-deficient mice dramatically increased basal endogenous glucose production [61]. Also, PPARγ agonist, troglitazone, inhibited the expression of PEPCK gene by a PPARγ-independent, antioxidant-related mechanism, and other PPARγ agonists, including rosiglitazone and ciglitazone, had little effect on PEPCK gene expression in hepatocyte [62], suggesting that the regulation of PEPCK by PPARγ is cell-specific [63]. We also observed that PEPCK mRNA expression was not altered by PL supplementation, although the PL down-regulated hepatic PPARγ and its target lipogenic genes expression. In fact, the regulation of PEPCK gene transcription coordinated by the action of a number of transcriptional factors and various hormones, including insulin, glucocorticoids, retinoic acid, thyroid hormone, and cyclic AMP [64]–[66]. Thus, it is possible that the PEPCK gene expression in PL-supplemented db/db mice was controlled by cooperative interaction of multiple transcription factors and hormones involved in PEPCK gene regulation.
Oxidative stress has been implicated in the pathogenesis of diabetes and other metabolic syndrome, including fatty liver disease and cardiovascular disease. In particular, the mitochondria is a major source of reactive oxygen species (ROS), and the ROS generated from the mitochondria damages proteins, DNA, and lipids in the membrane components, which results in mitochondrial dysfunction [67], [68]. Under normal physiological conditions, ROS are continuously produced, and oxidative damage induced by ROS can be prevented by antioxidant enzymes, where superoxide anion is rapidly converted by SOD into hydrogen peroxide, which is eliminated by CAT and GPX. However, an imbalance between ROS production and antioxidant capacity can induce cell damage associated with diabetes. Increased ROS levels were observed in the liver of db/db mice [69] and the expression of the antioxidant gene was down-regulated in the liver of type 2 diabetic rats [70]. In contrast, overexpression of SOD or CAT protected against hepatic oxidative injury in the livers of db/db mice [68] or HepG2 cells [71]. We found that PL up-regulated the mRNA expression of SOD and CAT in the liver. In parallel with the enhanced antioxidant gene expression, hepatic SOD and CAT activity was increased in the PL-supplemented db/db mice, suggesting that the elevated SOD and CAT expression may be regulated at the transcriptional level. Thus, the decreased level of mitochondria hydrogen peroxide in the liver of PL-supplemented mice may be attributed to the improved hepatic antioxidant capacity, which may contribute to the decreased hepatic lipid peroxidation and provide protection against hepatic oxidative stress in type 2 diabetes.
We also found that PL significantly increased the plasma PON activity as well as the plasma HDL-cholesterol and adiponectin levels. Plasma PON is another antioxidant enzyme contained in plasma HDL, which protects LDL and HDL from oxidation by ROS, and possesses other multiple anti-atherogenic activities [72]. Serum PON activity is low in patients with diabetes and it has potential as a marker for atherosclerosis in diabetes [73]. Along with this enzyme activity, HDL-cholesterol is an independent predictor of atherosclerotic cardiovascular complications. Furthermore, the adiponectin concentration is positively correlated with HDL cholesterol and negatively associated with HOMA-IR, independent of age and BMI, in type 2 diabetic subjects [74]. Taken together, our findings suggest the potential protective effects of PL on atherosclerotic cardiovascular complications in type 2 diabetes.
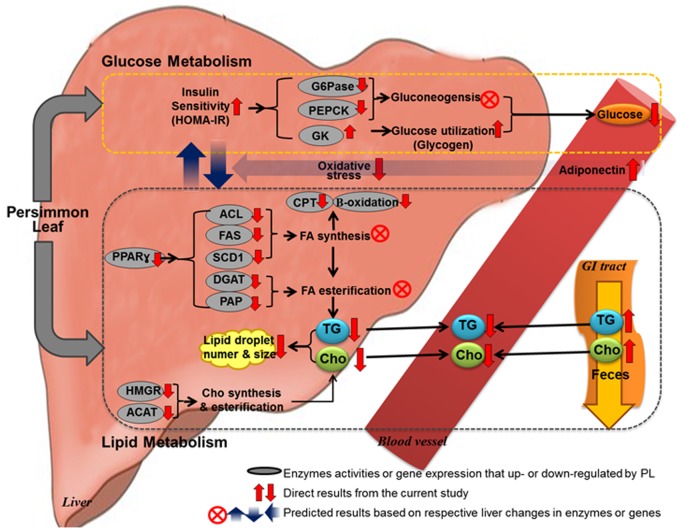
In conclusion, our results show that dietary PL improved hyperglycemia by alterations in activity and/or mRNA expression of hepatic enzymes involved in glucose utilization and glucose production (Fig. 4). Furthermore, PL ameliorated dyslipidemia and hepatic steatosis through a combined decrease in hepatic lipogenesis and an increase in the excretion of fecal lipids, which seemed to be related to the enhanced responsiveness of the liver to insulin (Fig. 4). The beneficial metabolic effects were also related to decreased plasma and hepatic oxidative stress as well as increased adiponectin secretion (Fig. 4). Thus, we believe that PL is a promising anti-diabetic compound that will be helpful for improving type 2 diabetes, although further study is required to identify its active components that mediate the hypoglycemic, hypolipidemic and hepatoprotective effects of PL.
Figure 4. Proposed mechanism of PL on the glucose and lipid lowering action in C57BL/KsJ-db/db mice.
PL improved HOMA-IR, which may activate glucokinase activity and its mRNA expression and inhibit gluconeogenic enzymes activity in the liver, resulting in lowered blood glucose level. The enhanced hepatic insulin sensitivity may be related to the improved hepatic steatosis and dyslipidemia, since PL led to lower plasma and hepatic lipid levels via reduction of transcription factor PPARγ, lipogenic gene expression and enzyme activity with a simultaneous increase in fecal lipids excretion. Furthermore, PL ameliorated oxidative stress and increased adiponectin secretion, which may be also associated with improved insulin sensitivity, hepatic steatosis and dyslipidemia. ACAT; acyl CoA: cholesterol acyltransferase, ACL; ATP-citrate lyase, Cho; cholesterol, CPT; carnitine palmitoyl transferase, DGAT; glyceraldehyde-3-phosphate dehydrogenase, FA; fatty acid, FAS; fatty acid synthase, G6Pase; glucose-6-phosphatase, GK; glucokinase, GI tract; gastrointestinal tract, HMGR; 3-hydroxy-3-methylglutaryl-coenzyme, HOMA-IR; homeostatic index of insulin resistance, PAP; phosphatidate phosphohydrolase, PL; powdered persimmon leaf, PEPCK; phosphoenolpyruvate carboxykinase, PPARγ; peroxisome proliferator activated receptor γ, SCD1; steraroyl-CoA desaturase-1, TG; triglyceride.
Funding Statement
This work was supported by grants from the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (MEST) (No. 2011-0000920 and No. 2011-0022387). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Sun L, Zhang J, Lu X, Zhang L, Zhang Y (2011) Evaluation to the antioxidant activity of total flavonoids extract from persimmon (Diospyros kaki L.) leaves. Food Chem Toxicol 49: 2689–2696. [DOI] [PubMed] [Google Scholar]
- 2. Kameda K, Takaku T, Okuda H, Kimura Y, Okuda T, et al. (1987) Inhibitory effects of various flavonoids isolated from leaves of persimmon on angiotensin-converting enzyme activity. J Nat Prod 50: 680–683. [DOI] [PubMed] [Google Scholar]
- 3. Kotani M, Matsumoto M, Fujita A, Higa S, Wang W, et al. (2000) Persimmon leaf extract and astragalin inhibit development of dermatitis and IgE elevation in NC/Nga mice. J Allergy Clin Immunol 106: 159–166. [DOI] [PubMed] [Google Scholar]
- 4. Kawakami K, Aketa S, Sakai H, Watanabe Y, Nishida H, et al. (2011) Antihypertensive and vasorelaxant effects of water-soluble proanthocyanidins from persimmon leaf tea in spontaneously hypertensive rats. Biosci Biotechnol Biochem. 75: 1435–1439. [DOI] [PubMed] [Google Scholar]
- 5. Kawakami K, Aketa S, Nakanami M, Iizuka S, Hirayama M (2010) Major water-soluble polyphenols, proanthocyanidins, in leaves of persimmon (Diospyros kaki) and their alpha-amylase inhibitory activity. Biosci Biotechnol Biochem 74: 1380–1385. [DOI] [PubMed] [Google Scholar]
- 6. Wang L, Xu ML, Rasmussen SK, Wang MH (2011) Vomifoliol 9-O-α-arabinofuranosyl (1→6)-β-D-glucopyranoside from the leaves of Diospyros Kaki stimulates the glucose uptake in HepG2 and 3T3-L1 cells. Carbohydr Res 346: 1212–1216. [DOI] [PubMed] [Google Scholar]
- 7. Thuong PT, Lee CH, Dao TT, Nguyen PH, Kim WG, et al. (2008) Triterpenoids from the leaves of Diospyros kaki (persimmon) and their inhibitory effects on protein tyrosine phosphatase 1B. J Nat Prod 71: 1775–1778. [DOI] [PubMed] [Google Scholar]
- 8. Postic C, Girard J (2008) Contribution of de novo fatty acid synthesis to hepatic steatosis and insulin resistance: lessons from genetically engineered mice. J Clin Invest 118: 829–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bonnard C, Durand A, Peyrol S, Chanseaume E, Chauvin MA, et al. (2008) Mitochondrial dysfunction results from oxidative stress in the skeletal muscle of diet-induced insulin-resistant mice. J Clin Invest 118: 789–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lee JS, Lee MK, Ha TY, Bok SH, Park HM, et al. (2006) Supplementation of whole persimmon leaf improves lipid profiles and suppresses body weight gain in rats fed high-fat diet. Food Chem Toxicol 44: 1875–1883. [DOI] [PubMed] [Google Scholar]
- 11. Shafrir E (1992) Animal models of non-insulin dependent diabetes. Diabetes Metab Rev 8: 179–208. [DOI] [PubMed] [Google Scholar]
- 12. Lee SC, Prosky L, DeVries JW (1992) Determination of total, soluble, and insoluble dietary fiber in foods enzymatic-gravimetric method, MES-TRIS buffer: collaborative study. J Assoc Off Anal Chem Int 75: 395–416. [Google Scholar]
- 13. Singleton VL, Orthofer R, Lamuela-Raventos RM (1999) Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymol 299: 152–178. [Google Scholar]
- 14. Moreno MI, Isla MI, Sampietro AR, Vattuone MA (2000) Comparison of the free radical-scavenging activity of propolis from several regions of Argentina. J Ethnopharmacol. 71: 109–114. [DOI] [PubMed] [Google Scholar]
- 15. American Institute of Nutrition (1980) Report of ad hoc Committee on Standards for Nutritional Studies. J Nutr 110: 1717–1726. [DOI] [PubMed] [Google Scholar]
- 16. American Institute of Nutrition (1977) Report of the American Institute of Nutrition ad hoc Committee on Standards for Nutritional Studies. J Nutr 107: 1340–1348. [DOI] [PubMed] [Google Scholar]
- 17. Haffner SM, Miettinen H, Stern MP (1997) The homeostasis model in the San Antonio Heart Study. Diabetes Care 20: 1087–1092. [DOI] [PubMed] [Google Scholar]
- 18. Mackness MI, Arrol S, Durrington PN (1991) Paraoxonase prevents accumulation of lipoperoxides in low-density lipoprotein. FEBS Lett. 286: 152–154. [DOI] [PubMed] [Google Scholar]
- 19. Folch J, Lees M, Sloan-Stanley GH (1956) A simple method for isolation and purification of total lipids from animal tissues. J Biol Chem 226: 497–509. [PubMed] [Google Scholar]
- 20. Seifter S, Dayton S, Novic B, Muntwyler E (1950) The estimation of glycogen with the anthrone reagent. Arch Biochem Biophys 50: 191–200. [PubMed] [Google Scholar]
- 21. Wolff SP (1994) Ferrous ion oxidation in presence of ferric ion indicator xylenol orange for measurement of hydroperoxide. Methods Enzymol 233: 182–189. [Google Scholar]
- 22. Ohkawa H, Ohishi N, Yake K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95: 351–358. [DOI] [PubMed] [Google Scholar]
- 23. Hulcher FH, Oleson WH (1973) Simplified spectrophotometric assay for microsomal 3-hydroxy-3-methylglutaryl CoA reductase by measurement of coenzyme A. J Lipid Res. 14: 625–631. [PubMed] [Google Scholar]
- 24. Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248–254. [DOI] [PubMed] [Google Scholar]
- 25. Davidson AL, Arion WJ (1987) Factors underlying significant underestimations of glucokinase activity in crude liver extracts: Physiological implications of higher cellular activity. Arch Biochem Bophys 253: 156–167. [DOI] [PubMed] [Google Scholar]
- 26. Newgard CB, Hirsch LJ, Foster DW, McGarry DJ (1995) Studies on the mechanism by which exogenous glucose is converted into liver glycogen in the rat. J Biol Chem 258: 8046–8052. [PubMed] [Google Scholar]
- 27. Alegre M, Ciudad CJ, Fillat C, Guinovart JJ (1988) Determination of glucose-6-phosphatase activity using the glucose dehydrogenase-coupled reaction. Anal Biochem 173: 185–189. [DOI] [PubMed] [Google Scholar]
- 28. Bentle LA, Lardy HA (1976) Interaction of anions and divalent metal ions with phosphopyruvate carboxykinase. J Biol Chem 251: 2916–2921. [PubMed] [Google Scholar]
- 29. Carl MN, Lakshmanan MR, Porter JW (1975) Fatty acid synthase from rat liver. Methods in Enzymology 35: 37–44. [DOI] [PubMed] [Google Scholar]
- 30. Walton PA, Possmayer F (1984) The role of Mg2+-dependent phosphatidate phosphohydrolase in pulmonary glycerolipid biosynthesis. Biochim Biophys Acta 796: 346–372. [DOI] [PubMed] [Google Scholar]
- 31. Markwell MAK, McGroarty EJ, Bieber LL, Tolbert NE (1973) The subcellular distribution of carnitine acyltransferases in mammalian liver and kidney. J Biol Chem 248: 3426–3432. [PubMed] [Google Scholar]
- 32. Lazarow PB (1981) Assay of peroxisomal β-oxidation of fatty acids. Methods in Enzymology 72: 315–319. [DOI] [PubMed] [Google Scholar]
- 33. Shapiro DJ, Nordstrom JL, Mitschelen JJ, Rodwell VW, Schimke RT (1974) Micro assay for 3-hydroxy-3-methylglutaryl-CoA reductase in rat liver and in L-cell fibroblasts. Biochim Biophys Acta 370: 369–377. [DOI] [PubMed] [Google Scholar]
- 34. Erickson SK, Schrewsbery MA, Brooks C, Meyer DJ (1980) Rat liver acyl-coenzyme A:cholesterol acyltransferase: its regulation in vivo and some of properties in vitro. J Lipid Res 21: 930–941. [PubMed] [Google Scholar]
- 35. Marklund S, Marklund G (1974) Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem 47: 469–474. [DOI] [PubMed] [Google Scholar]
- 36. Aebi H (1974) Catalase in method of enzymatic analysis. New York, Academic Press 2: 673–684. [Google Scholar]
- 37. Paglia ED, Valentine WN (1967) Studies on the quantitative and qualitative characterization of erythrocytes glutathione peroxidase. J Lab Clin Med 70: 158–169. [PubMed] [Google Scholar]
- 38. Chomzynski P, Sacchi N (1987) Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem 162: 156–159. [DOI] [PubMed] [Google Scholar]
- 39. Davis RC, Castellani LW, Hosseini M, Ben-Zeev O, Mao HZ, et al. (2010) Early hepatic insulin resistance precedes the onset of diabetes in obese C57BLKS-db/db mice. Diabetes 59: 1616–1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Michael MD, Kulkarni RN, Postic C, Previs SF, Shulman GI, et al. (2000) Loss of insulin signaling in hepatocytes leads to severe insulin resistance and progressive hepatic dysfunction. Mol Cell 6: 87–97. [PubMed] [Google Scholar]
- 41. Tahrani AA, Bailey CJ, Del Prato S, Barnett AH (2011) Management of type 2 diabetes: new and future developments in treatment. Lancet 378: 182–197. [DOI] [PubMed] [Google Scholar]
- 42. Barthel A, Schmoll D (2003) Novel concepts in insulin regulation of hepatic gluconeogenesis. Am J Physiol Endocrinol Metab 285: E685–E692. [DOI] [PubMed] [Google Scholar]
- 43. Abdul-Ghani MA, Tripathy D, DeFronzo RA (2006) Contributions of beta-cell dysfunction and insulin resistance to the pathogenesis of impaired glucose tolerance and impaired fasting glucose. Diabetes Care 29: 1130–1139. [DOI] [PubMed] [Google Scholar]
- 44. Pal M (2009) Recent advances in glucokinase activators for the treatment of type 2 diabetes. Drug Discov Today 14: 784–792. [DOI] [PubMed] [Google Scholar]
- 45. Valentová K, Truong NT, Moncion A, de Waziers I, Ulrichová J (2007) Induction of glucokinase mRNA by dietary phenolic compounds in rat liver cells in vitro. J Agric Food Chem 55: 7726–7731. [DOI] [PubMed] [Google Scholar]
- 46. Cleasby ME, Dzamko N, Hegarty BD, Cooney GJ, Kraegen EW, et al. (2004) Metformin prevents the development of acute lipid-induced insulin resistance in the rat through altered hepatic signaling mechanisms. Diabetes 53: 3258–3266. [DOI] [PubMed] [Google Scholar]
- 47. Mithieux G, Guignot L, Bordet J C, Wiernsperger N (2002) Intrahepatic mechanisms underlying the effect of metformin in decreasing basal glucose production in rats fed a high-fat diet. Diabetes 51: 139–143. [DOI] [PubMed] [Google Scholar]
- 48. Torres TP, Catlin RL, Chan R, Fujimoto Y, Sasaki N, et al. (2009) Restoration of hepatic glucokinase expression corrects hepatic glucose flux and normalizes plasma glucose in Zucker diabetic fatty rats. Diabetes 58: 78–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. O'Doherty RM, Lehman DL, Télémaque-Potts S, Newgard CB (1999) Metabolic impact of glucokinase overexpression in liver: lowering of blood glucose in fed rats is accompanied by hyperlipidemia. Diabetes 48: 2022–2027. [DOI] [PubMed] [Google Scholar]
- 50. Ferre T, Riu E, Franckhauser S, Agudo J, Bosch F (2003) Long-term overexpression of glucokinase in the liver of transgenic mice leads to insulin resistance. Diabetologia 46: 1662–1668. [DOI] [PubMed] [Google Scholar]
- 51. Nakamura A, Shimazaki H, Ohyama S, Eiki J, Terauchi Y (2011) Effect of long-term treatment with a small-molecule glucokinase activator on glucose metabolism, lipid profiles and hepatic function. J Diabetes Invest 2: 276–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Memon RA, Tecott LH, Nonogaki K, Beigneux A, Moser AH, et al. (2000) Up-regulation of peroxisome proliferator-activated receptors (PPAR-alpha) and PPAR-gamma messenger ribonucleic acid expression in the liver in murine obesity: troglitazone induces expression of PPAR-gamma-responsive adipose tissue-specific genes in the liver of obese diabetic mice. Endocrinology 141: 4021–4031. [DOI] [PubMed] [Google Scholar]
- 53. Morán-Salvador E, López-Parra M, García-Alonso V, Titos E, Martínez-Clemente M, et al. (2011) Role for PPARγ in obesity-induced hepatic steatosis as determined by hepatocyte- and macrophage-specific conditional knockouts. FASEB J. 25: 2538–2550. [DOI] [PubMed] [Google Scholar]
- 54. Chakravarthy MV, Pan Z, Zhu Y, Tordjman K, Schneider JG, et al. (2011) “New” hepatic fat activates PPARalpha to maintain glucose, lipid, and cholesterol homeostasis. Proc Natl Acad Sci U S A 108: 5378–5383. [DOI] [PubMed] [Google Scholar]
- 55. Wu M, Singh SB, Wang J, Chung CC, Salituro G, et al. (2011) Antidiabetic and antisteatotic effects of the selective fatty acid synthase (FAS) inhibitor platensimycin in mouse models of diabetes. Proc Natl Acad Sci U S A 108: 5378–5383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Foster DW (2004) The role of the carnitine system in human metabolism. Ann N Y Acad Sci 1033: 1–16. [DOI] [PubMed] [Google Scholar]
- 57. Randle PJ (1998) Regulatory interactions between lipids and carbohydrates: The glucose fatty acid cycle after 35 years. Diabetes Metab Rev 14: 263–283. [DOI] [PubMed] [Google Scholar]
- 58. Devine JH, Eubank DW, Clouthier DE, Tontonoz P, Spiegelman BM, et al. (1999) Adipose expression of the phosphoenolpyruvate carboxykinase promoter requires peroxisome proliferator-activated receptor gamma and 9-cis-retinoic acid receptor binding to an adipocyte-specific enhancer in vivo. J Biol Chem 274: 13604–13612. [DOI] [PubMed] [Google Scholar]
- 59. Hallakou S, Doaré L, Foufelle F, Kergoat M, Guerre-Millo M, et al. (1997) Pioglitazone induces in vivo adipocyte differentiation in the obese Zucker fa/fa rat. Diabetes. 46: 1393–1399. [DOI] [PubMed] [Google Scholar]
- 60. Cadoudal T, Blouin JM, Collinet M, Fouque F, Tan GD, et al. (2007) Acute and selective regulation of glyceroneogenesis and cytosolic phosphoenolpyruvate carboxykinase in adipose tissue by thiazolidinediones in type 2 diabetes. Diabetologia. 50: 666–675. [DOI] [PubMed] [Google Scholar]
- 61. Matsusue K, Haluzik M, Lambert G, Yim SH, Gavrilova O, et al. (2003) Liver-specific disruption of PPARgamma in leptin-deficient mice improves fatty liver but aggravates diabetic phenotypes. J Clin Invest 111: 737–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Davies GF, Khandelwal RL, Wu L, Juurlink BH, Roesler WJ (2001) Inhibition of phosphoenolpyruvate carboxykinase (PEPCK) gene expression by troglitazone: a peroxisome proliferator-activated receptor-gamma (PPARgamma)-independent, antioxidant-related mechanism. Biochem Pharmacol 62: 1071–1079. [DOI] [PubMed] [Google Scholar]
- 63. Glorian M, Duplus E, Beale EG, Scott DK, Granner DK, et al. (2001) A single element in the phosphoenolpyruvate carboxykinase gene mediates thiazolidinedione action specifically in adipocytes. Biochimie 83: 933–943. [DOI] [PubMed] [Google Scholar]
- 64. Giralt M, Park EA, Gurney AL, Liu JS, Hakimi P, et al. (1991) Identification of a thyroid hormone response element in the phosphoenolpyruvate carboxykinase (GTP) gene. Evidence for synergistic interaction between thyroid hormone and cAMP cis-regulatory elements. J Biol Chem 266: 21991–21996. [PubMed] [Google Scholar]
- 65. Lucas PC, O'Brien RM, Mitchell JA, Davis CM, Imai E, et al. (1991) A retinoic acid response element is part of a pleiotropic domain in the phosphoenolpyruvate carboxykinase gene. Proc Natl Acad Sci U S A 88: 2184–2188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Sasaki K, Cripe TP, Koch SR, Andreone TL, Petersen DD, et al. (1984) Multihormonal regulation of phosphoenolpyruvate carboxykinase gene transcription. The dominant role of insulin. J Biol Chem 259: 15242–15251. [PubMed] [Google Scholar]
- 67. Nakamura S, Takamura T, Matsuzawa-Nagata N, Takayama H, Misu H, et al. (2009) Palmitate induces insulin resistance in H4IIEC3 hepatocytes through reactive oxygen species produced by mitochondria. J Biol Chem 284: 14809–14818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Green K, Brand MD, Murphy MP (2004) Prevention of mitochondrial oxidative damage as a therapeutic strategy in diabetes. Diabetes 53: S110–S118. [DOI] [PubMed] [Google Scholar]
- 69. Kumashiro N, Tamura Y, Uchida T, Ogihara T, Fujitani Y, et al. (2008) Impact of oxidative stress and peroxisome proliferator-activated receptor gamma coactivator-1alpha in hepatic insulin resistance. Diabetes 57: 2083–2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Suh Y H, Kim Y, Bang JH, Choi KS, Lee JW, et al. (2005) Analysis of gene expression profiles in insulin-sensitive tissues from pre-diabetic and diabetic Zucker diabetic fatty rats. J Mol Endocrinol 34: 299–315. [DOI] [PubMed] [Google Scholar]
- 71. Bai J, Rodriguez AM, Melendez JA, Cederbaum AI (1999) Overexpression of catalase in cytosolic or mitochondrial compartment protects HepG2 cells against oxidative injury. J Biol Chem 274: 26217–26224. [DOI] [PubMed] [Google Scholar]
- 72. Rosenblat M, Aviram M (2009) Paraoxonases role in the prevention of cardiovascular diseases. Biofactors 35: 98–104. [DOI] [PubMed] [Google Scholar]
- 73. Abbott CA, Mackness MI, Kumar S, Boulton AJ, Durrington PN (1995) Serum paraoxonase activity, concentration, and phenotype distribution in diabetes mellitus and its relationship to serum lipids and lipoproteins. Arterioscler Thromb Vasc Biol 15: 1812–1818. [DOI] [PubMed] [Google Scholar]
- 74. Daimon M, Oizumi T, Saitoh T, Kameda W, Hirata A, et al. (2003) Decreased serum levels of adiponectin are a risk factor for the progression to type 2 diabetes in the Japanese population. Diabetes Care 26: 2015–2020. [DOI] [PubMed] [Google Scholar]