Abstract
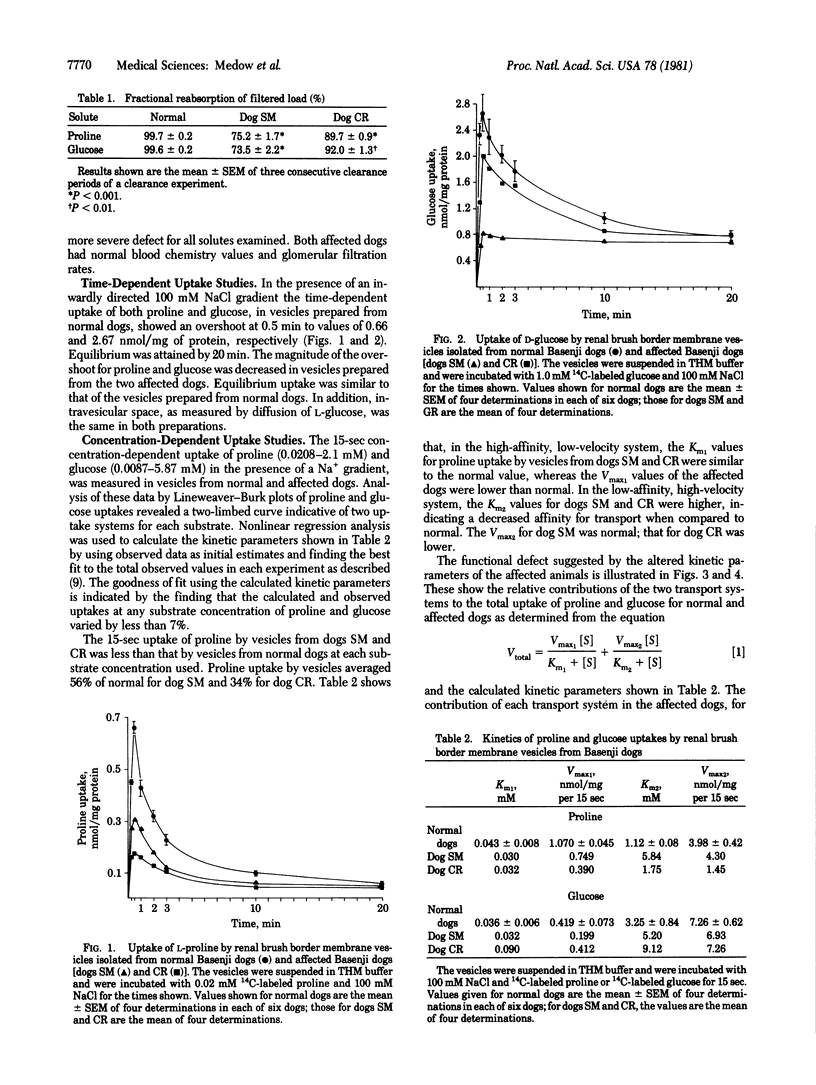
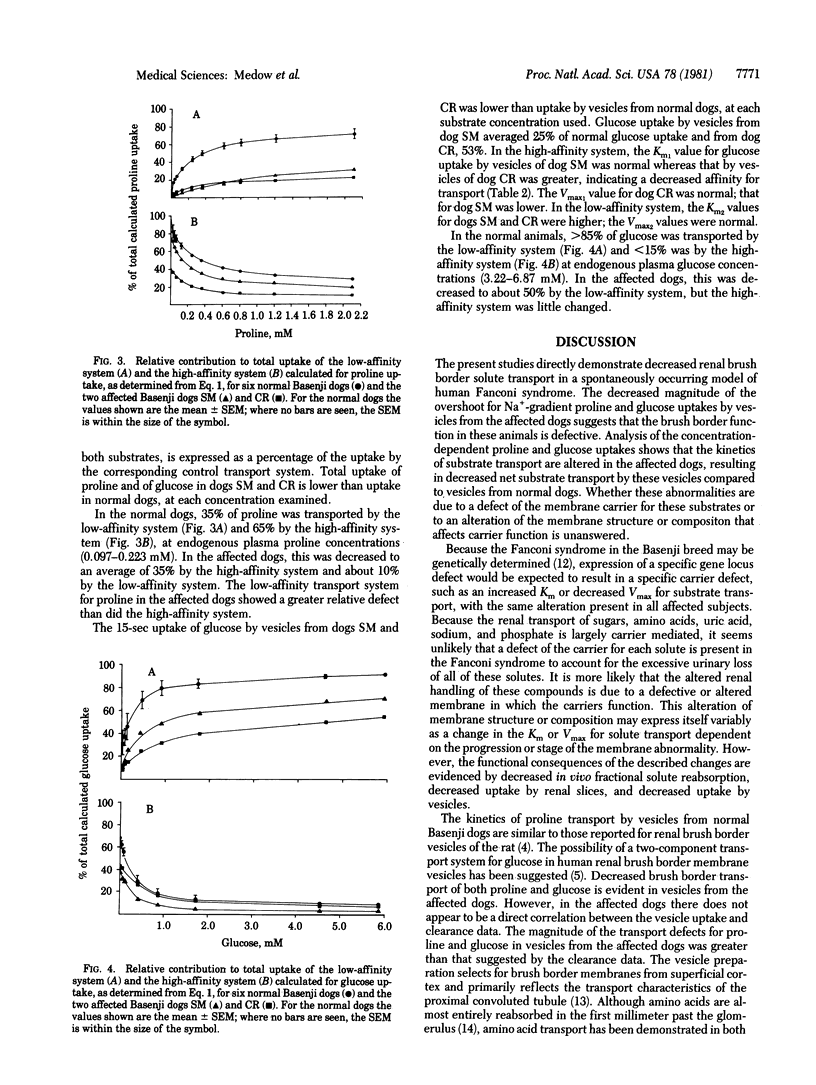
The uptake of proline and glucose by renal brush border membrane vesicles isolated from Basenji dogs exhibiting a spontaneous Fanconi syndrome was examined. The magnitude of the 1-min, Na+-gradient uptake of proline (0.02 mM) and glucose (1.0 mM) by vesicles from affected dog kidney was lower than normal. Concentration-dependent 15-sec uptake in vesicles from normal and affected dogs reveals a two-component transport system for proline and glucose. Kinetic analysis shows altered Km and Vmax values for both proline and glucose transport in the affected dogs. The data suggest that, in this model of Fanconi syndrome, the defective renal reabsorption of proline and glucose is associated with an alteration of luminal transport system.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aronson P. S., Sacktor B. The Na+ gradient-dependent transport of D-glucose in renal brush border membranes. J Biol Chem. 1975 Aug 10;250(15):6032–6039. [PubMed] [Google Scholar]
- BERLINER R. W., KENNEDY T. J., HILTON J. G. Effect of maleic acid on renal function. Proc Soc Exp Biol Med. 1950 Dec;75(3):791–794. doi: 10.3181/00379727-75-18344. [DOI] [PubMed] [Google Scholar]
- Bergeron M., Morel F. Amino acid transport in rat renal tubules. Am J Physiol. 1969 May;216(5):1139–1149. doi: 10.1152/ajplegacy.1969.216.5.1139. [DOI] [PubMed] [Google Scholar]
- Booth A. G., Kenny A. J. A rapid method for the preparation of microvilli from rabbit kidney. Biochem J. 1974 Sep;142(3):575–581. doi: 10.1042/bj1420575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bovee K. C., Joyce T., Reynolds R., Segal S. The fanconi syndrome in Basenji dogs: a new model for renal transport defects. Science. 1978 Sep 22;201(4361):1129–1131. doi: 10.1126/science.684432. [DOI] [PubMed] [Google Scholar]
- Bovée K. C., Joyce T., Blazer-Yost B., Goldschmidt M. S., Segal S. Characterization of renal defects in dogs with a syndrome similar to the Fanconi syndrome in man. J Am Vet Med Assoc. 1979 May 15;174(10):1094–1099. [PubMed] [Google Scholar]
- Bovée K. C., Joyce T., Reynolds R., Segal S. Spontaneous Fanconi syndrome in the dog. Metabolism. 1978 Jan;27(1):45–52. doi: 10.1016/0026-0495(78)90122-1. [DOI] [PubMed] [Google Scholar]
- Easley J. R., Breitschwerdt D. B. Glucosuria associated with renal tubular dysfunction in three Basenji dogs. J Am Vet Med Assoc. 1976 May 15;168(10):938–943. [PubMed] [Google Scholar]
- Eisenbach G. M., Weise M., Stolte H. Amino acid reabsorption in the rat nephron. Free flow micropuncture study. Pflugers Arch. 1975;357(1-2):63–76. doi: 10.1007/BF00584545. [DOI] [PubMed] [Google Scholar]
- HARRISON H. E., HARRISON H. C. Experimental production of renal glycosuria, phosphaturia, and aminoaciduria by injection of maleic acid. Science. 1954 Oct 15;120(3120):606–608. doi: 10.1126/science.120.3120.606. [DOI] [PubMed] [Google Scholar]
- Kramer H. J., Gonick H. C. Effect of maleic acid on sodium-linked tubular transport in experimental Fanconi syndrome. Nephron. 1973;10(5):306–319. doi: 10.1159/000180202. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- McNamara P. D., Ozegović B., Pepe L. M., Segal S. Proline and glycine uptake by renal brushborder membrane vesicles. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4521–4525. doi: 10.1073/pnas.73.12.4521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds R. A., Wald H., McNamara P. D., Segal S. An improved method for isolation of basolateral membranes from rat kidney. Biochim Biophys Acta. 1980 Sep 2;601(1):92–100. doi: 10.1016/0005-2736(80)90516-7. [DOI] [PubMed] [Google Scholar]
- Reynolds R., McNamara P. D., Segal S. On the maleic acid induced Fanconi syndrome: effects on transport by isolated rat kidney brushborder membrane vesicles. Life Sci. 1978 Jan;22(1):39–43. doi: 10.1016/0024-3205(78)90409-5. [DOI] [PubMed] [Google Scholar]
- Rosenberg L. E., Segal S. Maleic acid-induced inhibition of amino acid transport in rat kidney. Biochem J. 1964 Aug;92(2):345–352. doi: 10.1042/bj0920345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth K. S., Hwang S. M., Segal S. Effect of maleic acid on the kinetics of alpha-methyl-D-glucoside uptake by isolated rat renal tubules. Biochim Biophys Acta. 1976 Apr 5;426(4):675–687. doi: 10.1016/0005-2736(76)90132-2. [DOI] [PubMed] [Google Scholar]
- Silverman M., Huang L. Mechanism of maleic acid-induced glucosuria in dog kidney. Am J Physiol. 1976 Oct;231(4):1024–1032. doi: 10.1152/ajplegacy.1976.231.4.1024. [DOI] [PubMed] [Google Scholar]
- Szczepańska M., Angielski S. Prevention of maleate-induced tubular dysfunction by acetoacetate. Am J Physiol. 1980 Jul;239(1):F50–F56. doi: 10.1152/ajprenal.1980.239.1.F50. [DOI] [PubMed] [Google Scholar]
- Turner R. J., Silverman M. Sugar uptake into brush border vesicles from normal human kidney. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2825–2829. doi: 10.1073/pnas.74.7.2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss S. D., McNamara P. D., Pepe L. M., Segal S. Glutamine and glutamic acid uptake by rat renal brushborder membrane vesicles. J Membr Biol. 1978 Sep 29;43(1):91–105. doi: 10.1007/BF01869043. [DOI] [PubMed] [Google Scholar]
- Wen S. F. Micropuncture studies of glucose transport in the dog: mechanism of renal glycosuria. Am J Physiol. 1976 Aug;231(2):468–475. doi: 10.1152/ajplegacy.1976.231.2.468. [DOI] [PubMed] [Google Scholar]



