Abstract
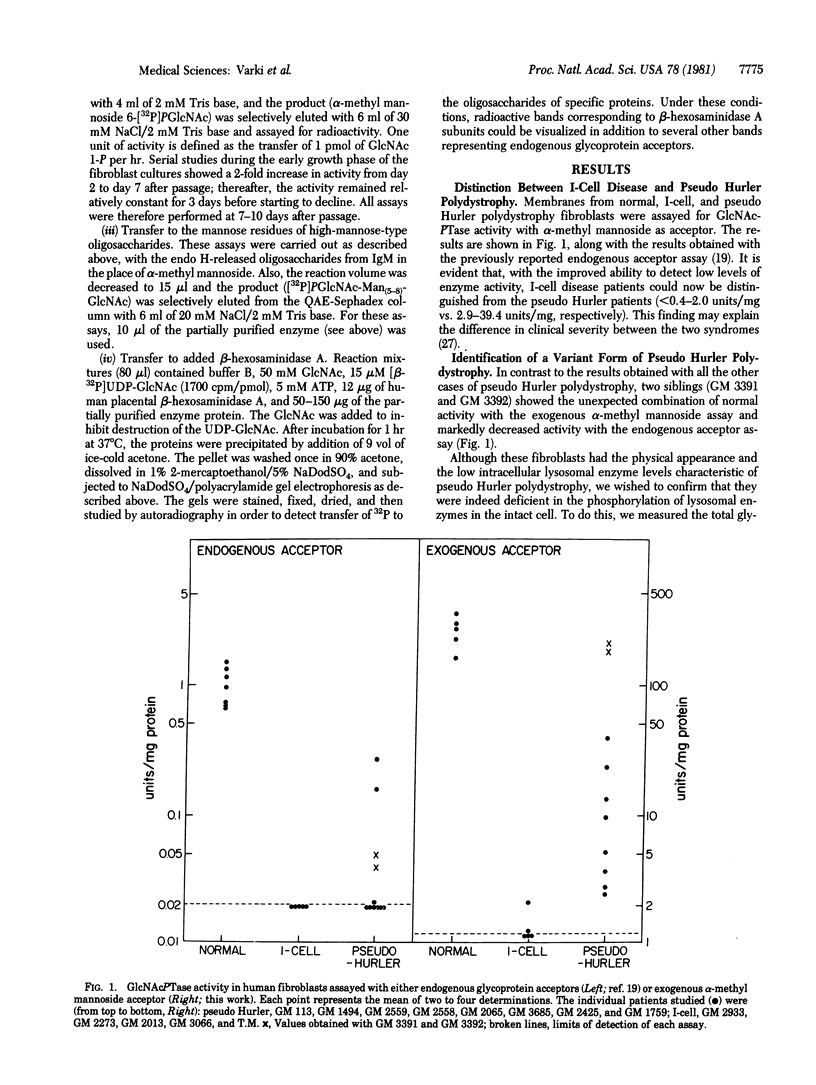
Fibroblasts from patients with I-cell disease (mucolipidosis II) or with pseudo Hurler polydystrophy (mucolipidosis III) are markedly deficient in UDP-N-acetylglucosamine:lysosomal enzyme N-acetylglucosamine-1-phosphotransferase. As a consequence, the common phosphomannosyl recognition marker of acid hydrolases is not regenerated, and these enzymes are not targeted to lysosomes. We have developed a sensitive assay for the transferase that uses alpha-methyl mannoside as an acceptor, and this has allowed us to distinguish between fibroblasts from these two types of patients. The enzyme activity is less in the former than in the latter (less than 0.4-2.0 pmol/mg per hr vs 2.9-39.4). This may provide an explanation for the difference in clinical severity between the two syndromes, However, in two siblings with pseudo Hurler polydystrophy (GM 3392), the enzyme activity was normal when assayed by using alpha-methyl mannoside as acceptor whereas it was low when assayed with endogenous glycoprotein acceptors or with human placental beta-hexosaminidase A. The apparent Km values of the mutant enzyme toward alpha-methyl mannoside, high-mannose oligosaccharides, and UDP-GlcNAc were not different from those of the normal enzyme. Mixing experiments demonstrated that the mutant fibroblasts contained endogenous acceptors and were free of inhibitors. We conclude that the N-acetylglucosaminylphosphotransferase in the mutant fibroblasts has normal catalytic activity but is defective in the ability to recognize lysosomal enzymes as specific substrates for phosphorylation. This variant form of pseudo Hurler polydystrophy demonstrates the biological importance of this recognition mechanism in the generation of the phosphomannosyl marker.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bach G., Bargal R., Cantz M. I-cell disease: deficiency of extracellular hydrolase phosphorylation. Biochem Biophys Res Commun. 1979 Dec 14;91(3):976–981. doi: 10.1016/0006-291x(79)91975-2. [DOI] [PubMed] [Google Scholar]
- Chapman A., Kornfeld R. Structure of the high mannose oligosaccharides of a human IgM myeloma protein. I. The major oligosaccharides of the two high mannose glycopeptides. J Biol Chem. 1979 Feb 10;254(3):816–823. [PubMed] [Google Scholar]
- Distler J., Hieber V., Sahagian G., Schmickel R., Jourdian G. W. Identification of mannose 6-phosphate in glycoproteins that inhibit the assimilation of beta-galactosidase by fibroblasts. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4235–4239. doi: 10.1073/pnas.76.9.4235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer H. D., Natowicz M., Sly W. S., Bretthauer R. K. Fibroblast receptor for lysosomal enzymes mediates pinocytosis of multivalent phosphomannan fragment. J Cell Biol. 1980 Jan;84(1):77–86. doi: 10.1083/jcb.84.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasilik A., Klein U., Waheed A., Strecker G., von Figura K. Phosphorylated oligosaccharides in lysosomal enzymes: identification of alpha-N-acetylglucosamine(1)phospho(6)mannose diester groups. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7074–7078. doi: 10.1073/pnas.77.12.7074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasilik A., Neufeld E. F. Biosynthesis of lysosomal enzymes in fibroblasts. Phosphorylation of mannose residues. J Biol Chem. 1980 May 25;255(10):4946–4950. [PubMed] [Google Scholar]
- Hasilik A., Neufeld E. F. Biosynthesis of lysosomal enzymes in fibroblasts. Synthesis as precursors of higher molecular weight. J Biol Chem. 1980 May 25;255(10):4937–4945. [PubMed] [Google Scholar]
- Hasilik A., Waheed A., von Figura K. Enzymatic phosphorylation of lysosomal enzymes in the presence of UDP-N-acetylglucosamine. Absence of the activity in I-cell fibroblasts. Biochem Biophys Res Commun. 1981 Feb 12;98(3):761–767. doi: 10.1016/0006-291x(81)91177-3. [DOI] [PubMed] [Google Scholar]
- Hickman S., Kulczycki A., Jr, Lynch R. G., Kornfeld S. Studies of the mechanism of tunicamycin in hibition of IgA and IgE secretion by plasma cells. J Biol Chem. 1977 Jun 25;252(12):4402–4408. [PubMed] [Google Scholar]
- Kaplan A., Achord D. T., Sly W. S. Phosphohexosyl components of a lysosomal enzyme are recognized by pinocytosis receptors on human fibroblasts. Proc Natl Acad Sci U S A. 1977 May;74(5):2026–2030. doi: 10.1073/pnas.74.5.2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan A., Fischer D., Sly W. S. Correlation of structural features of phosphomannans with their ability to inhibit pinocytosis of human beta-glucuronidase by human fibroblasts. J Biol Chem. 1978 Feb 10;253(3):647–650. [PubMed] [Google Scholar]
- Kelly T. E., Thomas G. H., Taylor H. A., Jr, McKusick V. A., Sly W. S., Glaser J. H., Robinow M., Luzzatti L., Espiritu C., Feingold M. Mucolipidosis III (pseudo-Hurler polydystrophy): Clinical and laboratory studies in a series of 12 patients. Johns Hopkins Med J. 1975 Oct;137(4):156–175. [PubMed] [Google Scholar]
- Kornfeld S., Li E., Tabas I. The synthesis of complex-type oligosaccharides. II. Characterization of the processing intermediates in the synthesis of the complex oligosaccharide units of the vesicular stomatitis virus G protein. J Biol Chem. 1978 Nov 10;253(21):7771–7778. [PubMed] [Google Scholar]
- Natowicz M. R., Chi M. M., Lowry O. H., Sly W. S. Enzymatic identification of mannose 6-phosphate on the recognition marker for receptor-mediated pinocytosis of beta-glucuronidase by human fibroblasts. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4322–4326. doi: 10.1073/pnas.76.9.4322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitman M. L., Kornfeld S. UDP-N-acetylglucosamine:glycoprotein N-acetylglucosamine-1-phosphotransferase. Proposed enzyme for the phosphorylation of the high mannose oligosaccharide units of lysosomal enzymes. J Biol Chem. 1981 May 10;256(9):4275–4281. [PubMed] [Google Scholar]
- Reitman M. L., Varki A., Kornfeld S. Fibroblasts from patients with I-cell disease and pseudo-Hurler polydystrophy are deficient in uridine 5'-diphosphate-N-acetylglucosamine: glycoprotein N-acetylglucosaminylphosphotransferase activity. J Clin Invest. 1981 May;67(5):1574–1579. doi: 10.1172/JCI110189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sando G. N., Neufeld E. F. Recognition and receptor-mediated uptake of a lysosomal enzyme, alpha-l-iduronidase, by cultured human fibroblasts. Cell. 1977 Nov;12(3):619–627. doi: 10.1016/0092-8674(77)90262-8. [DOI] [PubMed] [Google Scholar]
- Tabas I., Kornfeld S. Biosynthetic intermediates of beta-glucuronidase contain high mannose oligosaccharides with blocked phosphate residues. J Biol Chem. 1980 Jul 25;255(14):6633–6639. [PubMed] [Google Scholar]
- Varki A., Kornfeld S. Identification of a rat liver alpha-N-acetylglucosaminyl phosphodiesterase capable of removing "blocking" alpha-N-acetylglucosamine residues from phosphorylated high mannose oligosaccharides of lysosomal enzymes. J Biol Chem. 1980 Sep 25;255(18):8398–8401. [PubMed] [Google Scholar]
- Varki A., Kornfeld S. Purification and characterization of rat liver alpha-N-acetylglucosaminyl phosphodiesterase. J Biol Chem. 1981 Oct 10;256(19):9937–9943. [PubMed] [Google Scholar]
- Varki A., Kornfeld S. Structural studies of phosphorylated high mannose-type oligosaccharides. J Biol Chem. 1980 Nov 25;255(22):10847–10858. [PubMed] [Google Scholar]
- Waheed A., Hasilik A., von Figura K. Processing of the phosphorylated recognition marker in lysosomal enzymes. Characterization and partial purification of a microsomal alpha-N-acetylglucosaminyl phosphodiesterase. J Biol Chem. 1981 Jun 10;256(11):5717–5721. [PubMed] [Google Scholar]
- Waheed A., Pohlmann R., Hasilik A., von Figura K. Subcellular location of two enzymes involved in the synthesis of phosphorylated recognition markers in lysosomal enzymes. J Biol Chem. 1981 May 10;256(9):4150–4152. [PubMed] [Google Scholar]
- Wang C., Smith R. L. Lowry determination of protein in the presence of Triton X-100. Anal Biochem. 1975 Feb;63(2):414–417. doi: 10.1016/0003-2697(75)90363-2. [DOI] [PubMed] [Google Scholar]
- von Figura K., Klein U. Isolation and characterization of phosphorylated oligosaccharides from alpha-N-acetylglucosaminidase that are recognized by cell-surface receptors. Eur J Biochem. 1979 Mar;94(2):347–354. doi: 10.1111/j.1432-1033.1979.tb12900.x. [DOI] [PubMed] [Google Scholar]