Abstract
Polyethylene glycol (PEG) was genetically incorporated into a polypeptide. Stop-anticodon-containing tRNAs were acylated with PEG-containing amino acids and were then translated into polypeptides corresponding to DNA sequences containing the stop codons. The molecular weights of the PEG used were 170, 500, 700, 1000, and 2000 Da, and the translation was confirmed by mass spectrometry. The PEG incorporation ratio decreased as the molecular weight of PEG increased, and PEG with a molecular weight of 1000 Da was only slightly incorporated. Although improvement is required to increase the efficiency of the process, this study demonstrates the possibility of genetic PEGylation.
Introduction
Synthetic polymer–protein hybrids have been developed for use as therapeutic proteins or bioreactor enzymes [1]–[10]. Polyethylene glycol (PEG), which is nontoxic, nonimmunogenic, highly soluble in water, and approved by the U.S. Food and Drug Administration (FDA), is very useful in the preparation of therapeutic proteins [11], [12]. Some proteins trigger immune reactions, and proteases and other compounds inside the body can rapidly degrade them and remove them from the body. Many PEGylated protein drugs have been developed since the pioneering work of Abuchowsky et al. on the PEGylation of proteins [13], [14]. Although protein PEGylation has proven very valuable, many of the first-generation PEGylation products suffered from a severe loss in bioactivity [5]. This reduction in activity was mainly attributed to the chain lengths of the attached polymers and the site on the protein to which they are coupled. To overcome this difficulty, a more specific modification was achieved by exploiting the difference in the pKa value of the amine in the lysine side chain or the replacement of lysine residues with other amino acids [15]–[18]. The thiol groups in cysteine residues, the phenol groups of tyrosines, the amide groups of glutamines, or the incorporated His tag have also been used for this specific modification [19]–[29]. The site-specific polymer attachment has also been achieved with the complete synthetic construction of an erythropoietic protein [30], [31], consisting of an α-amino acid polypeptide chain of 166 residues by using native chemical ligation. Recent development of ligation methods has significantly expanded the possibility of bioorthogonal chemistry [32]–[36].
A genetic-encoding approach was first reported in 1989 as an alternative method for the site-specific incorporation of nonnatural amino acids into peptides or proteins [37], [38], and various amino acids have been incorporated in this way [39]–[43]. The method utilizes the UAG codon (the amber nonsense stop codon), which normally directs the termination of protein synthesis, to encode instead a nonnatural amino acid that is loaded onto the complementary tRNA. Deiters et al. [44] made 20 different versions of human growth factor, each of which had a nonnatural amino acid, p-acetylphenylalanine, inserted at a different site using the misacylated tRNA method. They linked a single PEG molecule to each keto group as a posttranslational modification. Johnson et al. [45] enhanced the efficiency of the ribosomal incorporation of unnatural amino acids at multiple sites by RF1 knockout in a live cell. However, it is difficult to precisely insert more than two PEG chains of different lengths or two different unnatural amino acids into each desirable position in one protein molecule using this posttranslational modification method.
Sisido and co-workers developed a frameshift-suppression method, in which nonnatural amino acids are incorporated into proteins using four-base codon–anticodon pairs instead of the stop codon [46]. Using the four-base codon method, Shozen et al. [47] recently attempted to incorporate a short polyethylene glycol chain into a polypeptide, although its production was not directly confirmed by mass spectrometry, and the incorporation of a long PEG chain was not attempted. Therefore, in this study, we attempted to add tRNAs that recognize a stop codon, to which PEG of various molecular weights was attached, into a translation system, as shown in Figure 1. Here, the incorporation of PEG with a molecular weight of 1000 Da using the in vitro translation system was directly confirmed by mass spectrometry.
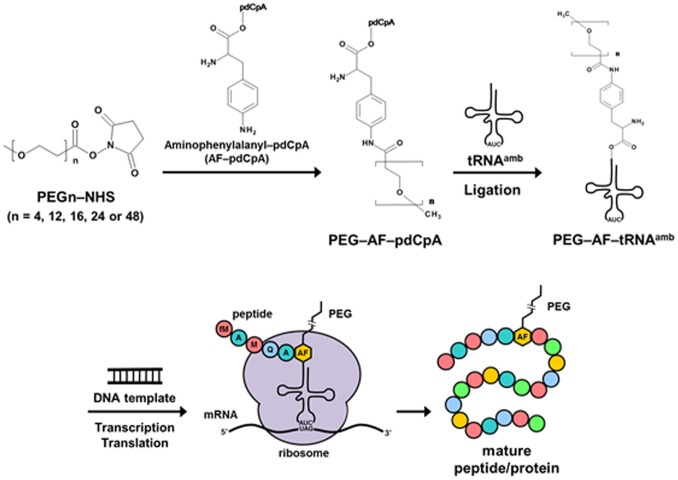
Figure 1. Illustration of the synthesis of polyethylene-glycol-carrying tRNA and the incorporation of PEG into a polypeptide by in vitro translation.
Materials and Methods
Materials
Ethylene glycol tetramer (PEG4, Mw 170 Da)- or ethylene glycol dodecamer (PEG12, Mw 500 Da)-conjugated aminophenylalanyl tRNAs containing a CUA anticodon were purchased from Protein Express (Chiba, Japan). The single-molecular-weight PEG compounds m-dPEG®16–N-hydroxysuccinimide (NHS) ester (PEG16–NHS, Mw 700 Da) and m-dPEG®24–NHS ester (PEG24–NHS, Mw 1000 Da) were purchased from Quanta Biodesign (Powell, OH, USA). Methoxypoly(ethylene glycol)–NHS ester of molecular weight 2000 Da, corresponding to PEG48–NHS, was purchased from NOF (Tokyo, Japan). PURESYSTEM classic II for in vitro translation and the PURExpress In Vitro Protein Synthesis Kit were purchased from BioComber (Tokyo, Japan) and New England Biolabs (Ipswich, MA, USA), respectively. Monoclonal anti-FLAG M2 antibody was purchased from Sigma-Aldrich (St Louis, MO, USA).
Preparation of PEGylated tRNA
The acylated tRNAs were prepared as previously reported [48]. Aqueous solutions of PEG16, 24, and 48 (25 µM, 40 µL) were mixed with aminophenylalanyl–pdCpA (5 µM, 40 µL) in aqueous pyridine-HCl (5 M, pH 5.0, 80 µL). The product is referred to as PEG–AF–pdCpA. After incubation at 37°C for 3 h, PEG–AF–pdCpA was purified by reversed-phase HPLC (Waters XTerra C18; 2.5 µm, 4.6 mm×20 mm) at a flow rate of 1.5 mL/min, with a linear gradient of 0%–100% acetonitrile in 0.1% trifluoroacetic acid (TFA) for 10 min. The PEG–AF–pdCpA products were confirmed by matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI–TOF–MS; Voyager DE-PRO, Applied Biosystems, Foster City, CA, USA): PEG16–AF–pdCpA, calculated 1543.61 for (M–H)–, found 1544.09; PEG24–AF–pdCpA, calculated 1895.82 for (M–H)–, found 1896.41. PEG–AF–pdCpA was ligated to a yeast phenylalanine tRNA that contained a CUA anticodon and lacked the 3′-terminal dinucleotide. The ligation reaction mixture (20 µL) contained tRNA lacking the 3′ dinucleotide (0.5 nmol), PEG–AF–pdCpA in water (4.4 nmol, 2 µL), HEPES-Na (55 mM, pH 7.5), ATP (1 mM), MgCl2 (15 mM), DTT (3.3 mM), BSA (20 µg/mL), and T4 RNA ligase (30 U). After incubation at 4°C for 2 h, potassium acetate (pH 4.5) was added to a final concentration of 0.3 M. PEG4– and PEG12–AF–tRNA were purified with the RNeasy Mini Kit (Qiagen, Hilden, Germany). PEG16–, PEG24–, and PEG48–AF–tRNA were purified by reversed-phase HPLC (Applied Biosystems POROS R2/10; 10 µm, 4.6 mm×100 mm) at a flow rate of 1.0 mL/min, with a linear gradient of 0%–100% acetonitrile in 0.1% triethylammonium acetate for 20 min. The purified PEG–AF–tRNAs were lyophilized and stored at –80°C. For the experiments, the PEG–AF–tRNAs were dissolved in potassium acetate (1 mM, pH 4.5) and stored at –80°C.
DNA Templates
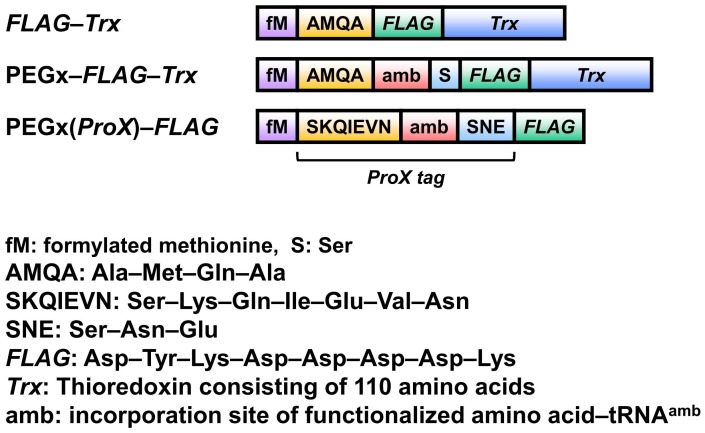
The DNA templates for the cell-free translation of mRNAs that encode the peptide or proteins shown in Figure 2 were constructed by ligating complementary oligonucleotides with the appropriate single-stranded overhangs into pCR2.1 (Invitrogen), previously cut with NotI and HindIII. The plasmid DNA was prepared from ampicillin-resistant transformants and used as the template in PCR reactions with primers corresponding to the T7 RNA polymerase promoter and terminator sequences. The PCR products were purified using the QIAquick PCR Purification Kit (Qiagen) and used as the templates for cell-free translation reactions.
Figure 2. Prepared DNA templates. ProX tag sequence (SKQIEVN–amber-SNE) was contained in PEGx(ProX)–FLAG.
Cell-free Translation
Cell-free translation was performed using PURESYSTEM classic II (BioComber) or the PURExpress In Vitro Protein Synthesis Kit (New England Biolabs) according to each manufacturer’s protocol. For the incorporation of PEG4, PEG12, PEG16, PEG24, or PEG48, the translation reaction was performed in the presence of 8, 8, 20, 40, or 60 µM, respectively, of each PEG–AF–tRNA. The reaction mixture was incubated at 37°C for 2.5 h, unless otherwise stated.
Mass Spectra Measurements
The samples were prepared for mass spectrometry as previously reported [39]. The translated peptides were purified from 50 µL reactions using prewashed FLAG M2 agarose (Sigma). After two washes with buffer (50 mM Tris-HCl, 300 mM NaCl, pH 8.0), the peptides were eluted from the matrix with 0.2% TFA. For the mass analysis, the peptides were desalted using ZipTip μ-C18 (Millipore) and mixed with 2,5-dihydroxybenzoic acid or 3-hydroxy-2-pyridinecarboxylic acid as the matrix. The samples were subjected to MALDI–TOF–MS analysis on an Ultraflex spectrometer (Bruker Daltonics) in linear or reflector mode.
Western Blotting
The reaction mixtures of the translated products (6 µL) were mixed with 3× loading buffer and boiled at 95°C for 3 min, before they were subjected to SDS–PAGE (16.5% acrylamide separation gel and 4% acrylamide stacking gel). The products were detected after western blotting by analysis with horseradish-peroxidase-conjugated anti-FLAG M2 monoclonal antibody (Sigma).
Results and Discussion
Preparation of PEGylated Phenylalanyl–tRNAs
To prepare the nonnatural amino acids carrying PEG chains, PEG was conjugated to aminophenylalanyl–pdCpA. The nonnatural amino acid p-aminophenylalanine has been found to be a good substrate for the in vitro translation system [48]–[51]. The PEG–aminophenylalanyl–pdCpA conjugate was connected to the truncated tRNA with ligase. The synthesis was confirmed by mass analysis, as shown in Figure 3.
Figure 3. Mass spectra of polyethylene-glycol-carrying tRNAs.
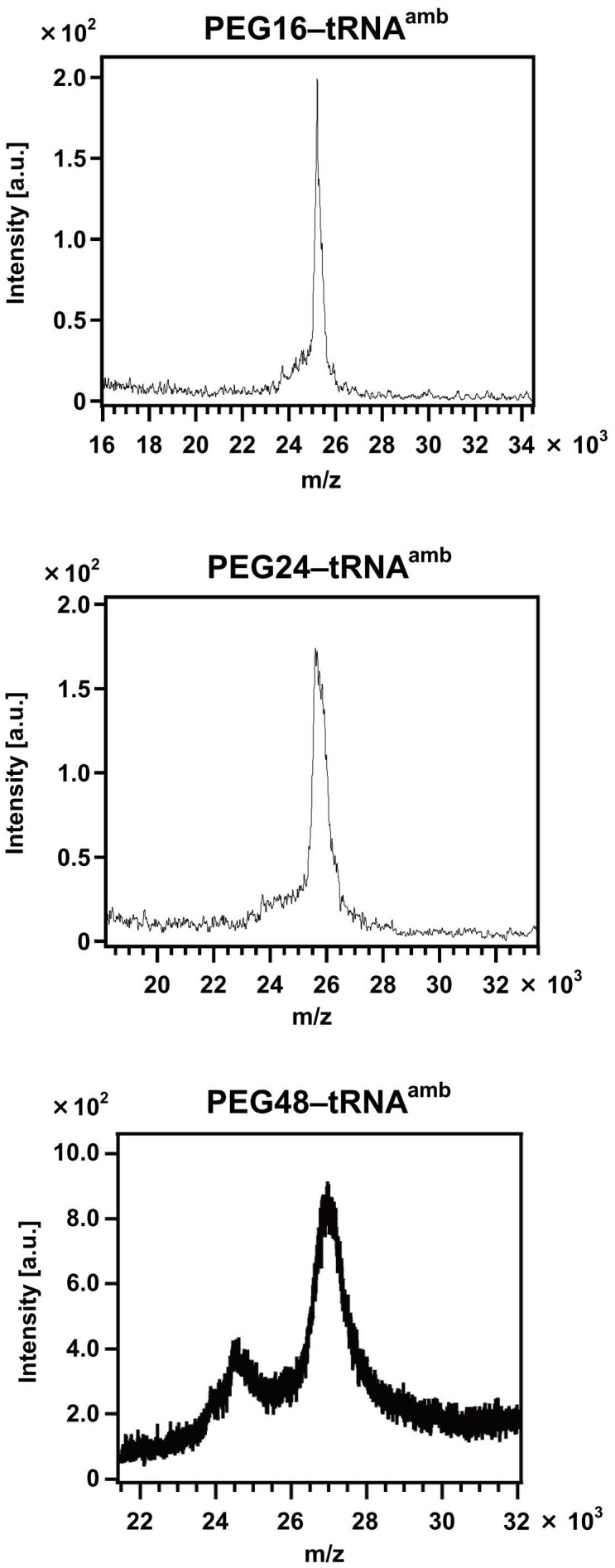
The molecular weights of PEG16–tRNA, PEG24–tRNA, and PEG24–tRNA were calculated to be 24485.69, 24838.10, and 25983.45, respectively.
PEGylation Conditions
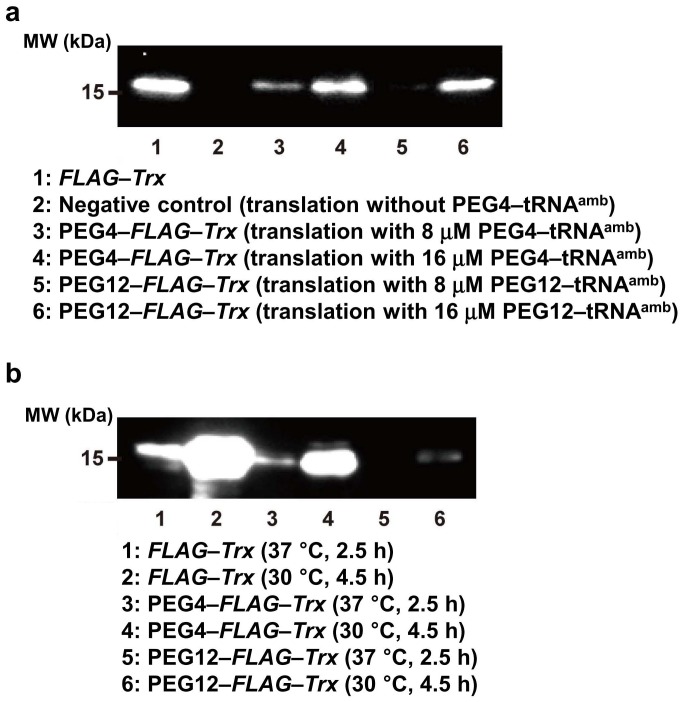
Thioredoxin (Trx) was synthesized by in vitro translation using PEG–AF–tRNA (Figure 4). The synthesis of Trx using PEG4 and PEG12 was confirmed by western blotting. The amount of PEGylated Trx produced increased with the addition of increasing amounts of PEG–AF–tRNA (Figure 4a). In the presence of 8 µM PEG–AF–tRNA, more PEGylated product was produced at 30°C for 4.5 h than that at 37°C for 2.5 h (Figure 4b). The yield of Trx protein that contained no amber codons was also increased in the translation reaction at 30°C for 4.5 h. Thus, the efficiency of the cell-free translation was greater at the lower temperature, so the yield of PEGylated protein increased. Bundy and Swartz reported similar results in that the protein yield of a cell-free translation reaction was higher at 30°C for a longer reaction period than at 37°C [52]. They attributed the difference in the protein yields to the rapid reduction of the translation rate at 37°C after 1 h from the beginning of the translation reaction, whereas the reduction in the translation rate at 30°C was relatively slow. The influence of temperature change on cell-free translation and the incorporation of unnatural amino acids requires further investigation.
Figure 4. Western blot analysis using anti-FLAG tag antibody of the in vitro translation of thioredoxin (Trx) with an incorporated PEG chain.
a. Dependence of protein synthesis on PEG–AF–tRNA concentration. b. PEG-incorporated protein synthesis under different conditions.
PEGylation with Long Chains
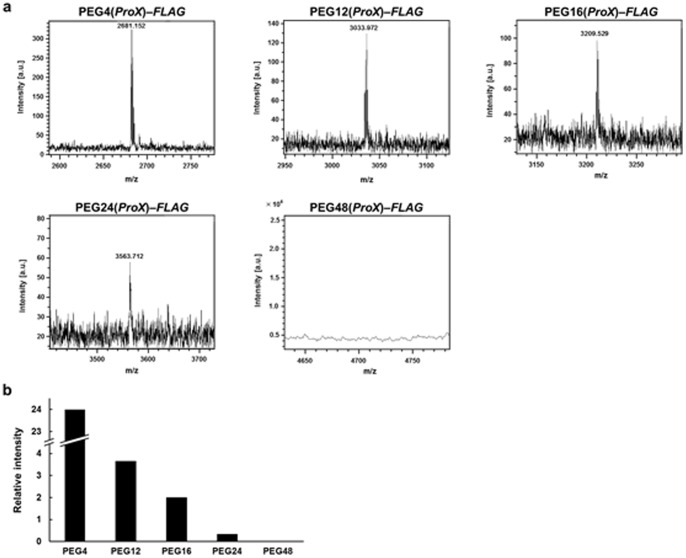
Under the conditions described above, FLAG-labeled peptide was prepared by an in vitro translation reaction using PEG4, PEG12, PEG16, PEG24, and PEG48 (Figure 5). To enhance the efficiency of PEG chain incorporation, a peptide sequence containing the ProX tag was used (Figure 2). The sequence of the ProX tag is optimized for the effective incorporation of a nonnatural amino acid into a protein, with minimum irregular product [49]. The translation reactions were performed at 30°C for 7 h and the products were confirmed by mass analysis (Figure 5a). PEG4 or PEG12 was successfully incorporated in the presence of 8 µM PEG–AF–tRNA. However, PEG chains longer than PEG24 were not incorporated. Therefore, the amount of PEG–AF–tRNA in the translation system was increased. PEG16 or PEG24 was incorporated in the presence of 20 µM or 40 µM PEG–AF–tRNA, respectively. However, no incorporation of PEG with a molecular weight of >2000 Da was observed, even in the presence of 60 µM PEG–AF–tRNA.
Figure 5. Mass spectrometry analysis of poly(ethylene glycol)-incorporated polypeptide.
a. Mass spectra of polypeptide incorporated with poly(ethylene glycol) chain. PEG4–FLAG, calculated 2681.177 for (M+H)+, found 2681.152; PEG12–FLAG, calculated 3033.386 for (M+H)+, found 3033.972; PEG16–FLAG, calculated 3209.492 for (M+H)+, found 3209.529; PEG24–FLAG, calculated 3563.890 for (M+H)+, found 3563.712; PEG48–FLAG, calculated 4709.244 for (M+H)+, not found. b. Molecular weight dependence of coefficient of incorporation of poly(ethylene glycol) into a polypeptide by in vitro translation.
The dependence of the translational incorporation of PEG on its molecular weight was roughly estimated by mass analysis. Each product peak was compared with a standard peak at a molecular weight of 4364 Da, attributed to ribosomal protein L36 in the cell-free translation system, and the corresponding value was plotted, as shown in Figure 5b. The higher the molecular weight, the less translation product was produced. We inferred that the longer PEG chains sterically hindered the processing of the polypeptide because the tunnel space in the ribosome is narrow. It is also possible that the PEG groups hinder the binding of EF-Tu to the acylated tRNA.
Site-specific PEGylation will be important for the preparation of defined protein drugs. In the future, some modifications to the ribosome should be investigated that enlarge the entrance and exit spaces or increase the binding of EF-Tu to allow the incorporation of longer PEG chains.
Acknowledgments
The authors thank Dr R. Abe at Protein Express Company for his technical support and useful discussions. The western blotting experiments were performed by Ms H. Shirai and Dr A. Wada.
Funding Statement
These authors have no support or funding to report.
References
- 1. Ito Y, Sugimura N, Kwon OH, Imanishi Y (1999) Enzyme modification by polymers with solubilities that change in response to photoirradiation in organic media. Nature Biotechnol 17: 73–75. [DOI] [PubMed] [Google Scholar]
- 2. Kwon OH, Imanishi Y, Ito Y (1999) Catalytic activity and conformation of chemically modified subtilisin Carlsberg in organic media. Biotechnol Bioeng 66: 265–270. [DOI] [PubMed] [Google Scholar]
- 3. Pennadam S, Firman K, Alexander C, Gorecki D (2004) Protein–polymer nano-machines. Towards synthetic control of biological processes. J Nanotechnol 2: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Elvira C, Gallardo A, Roman JS, Cifuentes A (2005) Covalent polymer–drug conjugates. Molecules 10: 114–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hest JCM (2007) Biosynthetic–synthetic polymer conjugates. J Macromol Sci Part C Polym Rev 47: 63–92. [Google Scholar]
- 6. Broyer RM, Grover GN, Maynard HD (2011) Emerging synthetic approaches for protein-polymer conjugations, Chem Commun. 47: 2212–2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Klok H-A (2009) Peptide/protein–polymer conjugates: Quo Vadis. Macromolecules 42: 7990–8000. [Google Scholar]
- 8. De Graaf AJ, Kooijiman M, Hennink WE, Mastrobattista E (2009) Nonnatural amino acids for site-specific protein conjugation. Bioconjug Chem 20: 1281–1295. [DOI] [PubMed] [Google Scholar]
- 9. Krishna OD, Kiick KL (2010) Protein- and peptide-modified synthetic polymeric biomaterials. Biopolymers (Pep Sci) 94: 32–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Velonia K (2010) Protein–polymer amphiphilic chimeras: recent advances and future challenges. Polym Chem 1: 944–952. [Google Scholar]
- 11. Veronese FM, Mero A (2008) The impact of PEGylation on biological therapies. Biodrugs 22: 315–329. [DOI] [PubMed] [Google Scholar]
- 12. Joralemon MJ, McRae S, Emrick T (2010) PEGylated polymers for medicine: from conjugation to self-assembled systems. Chem Commun 46: 1377–1393. [DOI] [PubMed] [Google Scholar]
- 13. Abuchowsky A, Van Es T, Palczuk NC, Davis FF (1977) Alteration of immunological properties of bovine serum by covalent attachment of polyethylene glycol. J Biol Chem 251: 3578–3581. [PubMed] [Google Scholar]
- 14. Abuchowsky A, McCoy TJR, Palczuk NC, Van Es T, Davis FF (1977) Effect of covalent attachment of polyethylene glycol on immunogenicity and circulating life of bovine liver catalase. J Biol Chem 251: 3578–3581. [PubMed] [Google Scholar]
- 15. Harris JM, Chess RB (2003) Effect of PEGylation on pharmaceuticals. Nat Rev Drug Discovery 2: 214–221. [DOI] [PubMed] [Google Scholar]
- 16. Catillo B, Sola RJ, Ferrer A, Barletta G, Griebenow K (2007) Effect of PEG modification on subtilisin Carsberg activity, enantioselectivity, and structural dynamics in 1,4-dioxane. Biotechnol Bioeng 99: 9–17. [DOI] [PubMed] [Google Scholar]
- 17. Kinstler O, Molinewx G, Treuheit M, Ladd D, Gegg C (2002) Mono-N-terminal poly(ethylene glycol)–protein conjugates. Adv Drug Del Rev 54: 477–485. [DOI] [PubMed] [Google Scholar]
- 18. Yamamoto Y, Tsutsumi Y, Yoshioka Y, Nishibata T, Kobayashi K, et al. (2003) Site-specific PEGylation of a lysine-deficient TNF-α with full bioactivity. Nat Biotechnol 21: 546–552. [DOI] [PubMed] [Google Scholar]
- 19. Wang JH, Tam SC, Huang H, Ouyang DY, Wang YY, et al. (2004) Site-directed PEGylation of trichostanthin retained its anti-HIV activity with reduced potency in vitro. Biochim Biophys Res Comm 3317: 965–971. [DOI] [PubMed] [Google Scholar]
- 20. Doherty DH, Rosendahl MS, Smith DJ, Hughes JM, Chlipala EA, et al. (2005) Site-specific PEGylation of engineered cysteine analogues of recombinant human granulocyte-macrophage colony-stimulating factor. Bioconjug Chem 16: 1291–1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sato H (2002) Enzymatic procedure for site-specific pegylation of proteins. Adv Drug Delivery 54: 487–504. [DOI] [PubMed] [Google Scholar]
- 22. Kostiainen MA, Szilvay GR, Lehtinen J, Smith DK, Linder MB, et al. (2007) Precisely defined protein-polymer conjugates: Construction of synthetic DNA binding domains on proteins by using multivalent dendrons. ACS Nano 1: 103–113. [DOI] [PubMed] [Google Scholar]
- 23. Wang H, He L, Lensch M, Gabius H-J, Fee CJ, et al. (2008) Single-site cys-substituting mutation of human lectin galectin-2: Modulating solubility in recombinant production, reducing long-term aggregation, and enabling site-specific monoPEGylation. Biomacromolecules. 9: 3223–3230. [DOI] [PubMed] [Google Scholar]
- 24. Hu J, Duppatla V, Harth S, Schmitz W, Sebald W (2010) Site-specific PEGylation of bone morphogenetic protein-2 cysteine analogues. Bioconjug Chem 21: 1762–1772. [DOI] [PubMed] [Google Scholar]
- 25. Schumacher FF, Nobles M, Ryan CP, Smith MEB, Tinker A, et al. (2011) In situ maleimide bridging of disulfides and a new approach to protein PEGylation. Bioconjug Chem 22: 132–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Joshi NS, Whitaker LR, Francis MB (2004) A three-component Mannich-type reaction for selective tyrosine bioconjugation. J Am Chem Soc 126: 15942–15943. [DOI] [PubMed] [Google Scholar]
- 27. Tilley SD, Francis MB (2006) Tyrosine-selective protein alkylation using p-palladium complexes. J Am Chem Soc 128: 1080–1081. [DOI] [PubMed] [Google Scholar]
- 28. Cazalis CS, Haller CA, Sease-Cargo L, Chaikof EL (2004) C-Terminal site-specific PEGylation of a truncated thrombomodulin mutant with retention of full bioactivity. Bioconjug Chem 15: 1005–1009. [DOI] [PubMed] [Google Scholar]
- 29. Cong Y, Pawlisz E, Bryant P, Balan S, Laurine E, et al. (2012) Site-specific PEGylation at histidine tags. Bioconjug Chem 23: 248–263. [DOI] [PubMed] [Google Scholar]
- 30. Kochendoerfer GG, Chen SY, Mao F, Cressman S, Traviglia S, et al. (2003) Design and chemical synthesis of homogeneous polymer-modified erythropoiesis protein. Science 299: 884–887. [DOI] [PubMed] [Google Scholar]
- 31. Chen SY, Cressman S, Mao F, Shao H, Low DW, et al. (2005) Synthetic erythropoietic proteins: tuning biological performance by site-specific polymer attachment. Chem Biol 12: 371–383. [DOI] [PubMed] [Google Scholar]
- 32. Sletten EM, Bertozzi CR (2009) Bioorthogonal chemistry: fishing for selectivity in a sea of functionality. Angew Chem Int Ed Engl 48: 6974–6998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kalia J, Raines T (2010) Advances in bioconjugation. Curr Org Chem 14: 138–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Boyce M, Bertozzi CR (2011) Bridging chemistry to life. Nat Meth 8: 638–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Berzozzi CR (2011) Guest Editorial A decade of bioorthogonal chemistry. Acc Chem Res 44: 651–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Stephanopoulos N, Francis MB (2011) Choosing an effective protein bioconjugation strategy. Nat Chem Biol 7: 876–884. [DOI] [PubMed] [Google Scholar]
- 37. Noren CJ, Anthony-Cahill SJ, Griffith MC, Schultz PG (1989) A general method for site-specific incorporation of unnatural amino acids into proteins. Science 244: 182–188. [DOI] [PubMed] [Google Scholar]
- 38. Bain JD, Glabe CG, Dix TA, Chamberlin AR, Diala ES (1989) Biosynthetic site-specific incorporation of a non-natural amino acid into a polypeptide. J Am Chem Soc 111: 8013–8014. [Google Scholar]
- 39. Josephson K, Hartman MC, Szostak JW (2005) Ribosomal synthesis of unnatural peptides. J Am Chem Soc 127: 11727–11735. [DOI] [PubMed] [Google Scholar]
- 40. Wang L, Xie J, Schultz PG (2006) Expanding the genetic code. Annu Rev Biophys Biomol Struct 35: 225–249. [DOI] [PubMed] [Google Scholar]
- 41. Liu CC, Schultz PG (2010) Adding new chemistries to the genetic code. Annu Rev Biochem 79: 413–444. [DOI] [PubMed] [Google Scholar]
- 42. Young TS, Schults PG (2010) Beyoud the canonical 20 amino acids: Expanding the genetic lexicon. J Biol Chem 285: 11039–11044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wang F, Robbins S, Guo J, Shen W, Schults PG (2010) Genetic incorporation of unnatural amino acids into proteins in Mycobacterium tuberculosis. PLoS ONE 5: e9354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Deiters A, Cropp TA, Summerer D, Mukherji M, Schultz PG (2004) Site-specific PEGylation of proteins containing unnatural amino acids. Bioorg Med Chem Lett 14: 5743–5745. [DOI] [PubMed] [Google Scholar]
- 45. Johnson DB, Xu J, Shen Z, Takimoto JK, Schultz MD, et al. (2011) RF1 knockout allows ribosomal incorporation of unnatural amino acids at multiple sites. Nat Chem Biol 7: 779–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hohsaka T, Ashizuka Y, Murakami H, Sisido M (1996) Incorporation of non-natural amino acids into streptavidin through in vitro frame-shift suppression. J Am Chem Soc 118: 9778–9779. [Google Scholar]
- 47. Shozen N, Iijima I, Hohsaka T (2009) Site-specific incorporation of PEGylated amino acids into proteins using nonnatural amino acid mutagenesis. Bioorg Med Chem Lett 19: 4909–4911. [DOI] [PubMed] [Google Scholar]
- 48. Hohsaka T, Kajihara D, Ashizuka Y, Murakami H, Sisido M (1999) Efficient incorporation of nonnatural amino acids with large aromatic groups into streptavidin in in vitro protein synthesizing systems. J Am Chem Soc 121: 34–40. [Google Scholar]
- 49. Kajihara D, Abe R, Iijima I, Komiyama C, Sisido M, et al. (2006) FRET analysis of protein conformational change through position-specific incorporation of fluorescent amino acids. Nat Methods 3: 923–929. [DOI] [PubMed] [Google Scholar]
- 50. Watanabe T, Muranaka N, Iijima I, Hohsaka T (2007) Position-specific incorporation of biotinylated non-natural amino acids into a protein in a cell-free translation system. Biochem Biophys Res Commun 361: 794–799. [DOI] [PubMed] [Google Scholar]
- 51. Abe R, Shiraga K, Ebisu S, Takagi H, Hohsaka T (2010) Incorporation of fluorescent non-natural amino acids into N-terminal tag of proteins in cell-free translation and dependence of position and neighboring codons. J Biosci Bioeng 110: 32–38. [DOI] [PubMed] [Google Scholar]
- 52. Bundy BC, Swartz JR (2010) Site-specific incorporation of p-propargyloxyphenylalanine in a cell-free environment for direct protein–protein click conjugation. Bioconjug Chem 21: 255–263. [DOI] [PubMed] [Google Scholar]