Abstract
Lysophosphatidic acid (LPA) mediates diverse cellular responses through the activation of at least six LPA receptors – LPA1–6, but the interacting proteins and signaling pathways that mediate the specificity of these receptors are largely unknown. We noticed that LPA1 contains a PDZ binding motif (SVV) identical to that present in two other proteins that interact with the PDZ protein GIPC. GIPC is involved in endocytic trafficking of several receptors including TrkA, VEGFR2, lutropin and dopamine D2 receptors. Here we show that GIPC binds directly to the PDZ binding motif of LPA1 but not that of other LPA receptors. LPA1 colocalizes and coimmunoprecipitates with GIPC and its binding partner APPL, an activator of Akt signaling found on APPL signaling endosomes. GIPC depletion by siRNA disturbed trafficking of LPA1 to EEA1 early endosomes and promoted LPA1 mediated Akt signaling, cell proliferation, and cell motility. We propose that GIPC binds LPA1 and promotes its trafficking from APPL-containing signaling endosomes to EEA1 early endosomes and thus attenuates LPA-mediated Akt signaling from APPL endosomes.
Introduction
Lysophosphatidic acid (LPA) mediates diverse biological effects including cell migration, differentiation, proliferation and survival [1], [2]. LPA induces these effects by binding to, and activating at least six different G protein coupled receptors (GPCRs), termed LPA1 through LPA6 [1]–[3], which are differentially expressed in different tissues and have distinct effects in animal models [1], [2]. These receptors are coupled to three classes of heterotrimeric G proteins, Gq/11, Gi/o and G12/13, which mediate cellular responses to LPA [1], [2].
LPA receptors 1–3 are the most studied and share high sequence homology (∼55% overall sequence identity) except for their carboxy-terminus (CT) [3], [4]. LPA1 and LPA2 but not LPA3 contain the Class I PDZ binding motif sequence X-(S/T)-X-(V/I/L)-COOH (where X is any amino acid) at the extreme CT [3]. LPA2 CT, but not LPA1 or LPA3, interacts with the PDZ domain proteins NHERF2 and MAGI-3 which couple LPA2 to PLC-β3, RhoA and Erk signaling [3], demonstrating that the CT can couple LPA receptors to specific signaling pathways and thereby confer the specificity of the responses to each receptor [3], [4].
We noticed that LPA1 has a PDZ binding motif (SVV) identical to that present in two other proteins, semaphorin family member SemF and the melanosomal membrane protein GP75 [5], [6], which interact with the PDZ protein GIPC [7]. Like LPA1, GIPC plays a key role in cell motility as GIPC (a.k.a. Synectin) knock out mice have defects in endothelial cell migration and angiogenesis [8], [9]. We therefore wondered if GIPC might interact with the PDZ binding motif of LPA1 to regulate its activity.
GIPC (GAIP-interacting protein, C terminus) was originally identified based on its ability to bind to the RGS (regulator of G protein signaling) protein GAIP (RGS19), a GTPase activating protein (GAP) for heterotrimeric G proteins [7]. We subsequently found that GIPC binds to the TrkA nerve growth factor receptor [10]–[11] and is required for efficient endocytosis and trafficking of TrkA from peripheral (APPL) signaling endosomes to juxtanuclear (EEA1) endosomes [11]. GIPC accomplishes this in part by binding to the actin based molecular motor myosin VI (Myo6) [12] and in part by binding to APPL [11], [13], a Rab5 effector protein found on a subpopulation of peripheral endosomes. APPL is required for recruitment of GIPC to endosomes, and regulates key events in signal transduction from endosomes [14]–[16]. Additional studies demonstrated that GIPC also binds to the receptor tyrosine kinase VEGFR2 [17] as well as to G protein coupled receptors (GPCRs) such as the lutropin (hLHR) [18] and dopamine D2 (D2R) receptors [19] and promotes their endocytic trafficking. Previous studies of LPA1 trafficking indicate that LPA1 is taken up by endocytosis in clathrin coated pits, traffics through Rab5 endosomes, and recycles back to the cell surface [20]–[22]. Thus, we reasoned that interaction between GIPC and LPA1 might also affect trafficking of LPA1.
Here we show that GIPC directly binds to the PDZ binding motif of LPA1, forms a complex with LPA1 and APPL, and promotes LPA1 trafficking from APPL signaling endosomes to early endosomes, resulting in downregulation of LPA1 induced Akt signaling and cell proliferation.
Experimental Procedures
Vectors
GIPC1 and APPL1 constructs were as previously described [10], [11]. GST-fusion proteins were cloned into the pGEX4T3 vector (GE Healthcare). LPA1 and LPA2 cDNAs cloned into pFLAG-CMV1 expression vector were obtained from Dr. Jerold Chun (Scripps Research Institute) [23] and subcloned into pIres-Puro3 vector (Clontech, Mountain View, CA).
Antibodies
Rabbit anti-GIPC serum was affinity purified on GST-GIPC immobilized on PVDF membranes as described [11]. Rabbit anti-APPL serum was characterized previously [24]. Anti-MAP kinase (Erk1/2) mAb was purchased from Zymed Laboratories (San Francisco, CA), and anti-clathrin heavy chain (X22) mAb was from Affinity Bioreagents (Thermo Scientific, Rockford, IL). Rabbit antibodies against pERK (phospho-p44/p42) MAP kinase (Thr202/Tyr204), and pAkt (Ser473) were purchased from Cell Signaling Technology (Beverly, MA). Rabbit anti-FLAG and mouse anti-actin, anti-FLAG (M2), anti-PKBα/Akt, and anti-EEA1 IgG were obtained from Transduction Laboratories, BD Biosciences (San Diego, CA). Affinity purified mouse anti-HA (HA.11) IgG was from Covance (Berkeley, CA).
Cell Culture and Transfection
HEK-293T cells were from Thermo Scientific (Rockford, IL), and HeLa cells were from the American Type Culture Collection (ATCC, CCL2). HEK-293T and HeLa cells were maintained in DMEM containing 10% FBS with 30 U/ml penicillin, 30 µg/ml streptomycin, 2 mM L-glutamine (GIBCO Invitrogen, Grand Island, NY). Clones stably expressing FLAG-tagged LPA1 receptor (HEK-LPA1) or controls (HEK-pIRES) were generated by transfecting pIres-Puro3- LPA1 or pIres-Puro3 empty vector, into cells using Lipofectamine 2000 (Invitrogen Corp., Carlsbad, CA) and selected by resistance to puromycin (2 µg/ml). HEK-293 and HeLa cells were transfected using Lipofectamine 2000 according to manufacturer’s instructions.
Immunoprecipitation
Cells were lysed on ice for 30 min in lysis buffer (1% NP-40, 50 mM Tris, pH 8.0, 150 mM NaCl, 5 mM NaF, 2 mM sodium orthovanadate, and protease inhibitor cocktail (Sigma-Aldrich, St. Louis, MO). Insoluble material was removed by centrifugation (10,000×g for 30 min at 4°C), and the protein concentration of the supernatant was determined by Bradford assay (Bio-Rad Laboratories, Hercules, CA). Cell lysates (3–4 mg protein) were incubated at 4°C with mouse anti-FLAG IgG overnight followed by incubation with protein G-Sepharose beads (Sigma-Aldrich) for 1 h. Beads were then washed extensively with lysis buffer and resuspended in Laemmli sample buffer (50 mM Tris-HCl, pH 6.8, 2% SDS, 10% glycerol, 5% β-mercaptoethanol and 0.01% bromophenol blue) for SDS-PAGE.
Immunoblotting
Proteins separated by SDS-PAGE were transferred to PVDF membranes (Millipore, Billerica, MA). After blocking with PBS containing 5% nonfat milk, membranes were incubated with primary antibodies at room temperature (1 h) or at 4°C (overnight), followed by incubation (1 h) at room temperature with goat anti-rabbit Alexa Fluor 680 F(ab’)2 (Molecular Probes) and goat anti-mouse IRDye 800 F(ab’)2 (Rockland). Infrared imaging with two-color detection and quantification of Western blots was performed according to the manufacturer’s protocols using the Odyssey Infrared Imaging System (LiCor Biosciences, Lincoln, NE).
RNA Interference
Knockdown in HEK cells was achieved using a duplex siRNA targeting human GIPC1 (sense sequence 5-AGAGGUGGAAGUAUUCA-AGdT-dT) purchased from Dharmacon Inc., (Chicago, IL). A negative control siRNA (Silencer #1) was purchased from Ambion (Austin, TX). Transfection of HEK-293 cells was performed using Oligofectamine according to the manufacturer’s protocol (Invitrogen) with 50 nM siRNA, 0.8 µg/µl siRNA to lipid ratio, and a cell density of ∼ 100 cells/mm2 surface area.
Protein Purification and In Vitro Binding Assays
GST, GST-GIPC, GST-mouse LPA1 tail (aa 311–364), GST-mouse LPA2 tail (aa 305–348) and mutants were expressed in E. coli and purified on glutathione Sepharose 4B (Amersham). For the in vitro binding assay 10 µg GST or GST fusion protein prebound to glutathione Sepharose beads were incubated with [35S]Met (GE Healthcare)-labeled GIPC-PDZ domain prepared using the TnT Quick Coupled Transcription/Translation System (Promega, Madison WI) in 300 µl binding buffer (50 mM Tris HCl, pH 7.4, 100 mM NaCl, 0.5%NP-40) overnight at 4°C. For experiments involving cell lysates, 3 µg GST or GST-GIPC were incubated with 500 µl cell lysate. Beads were sedimented and washed extensively in binding buffer and boiled in Laemmli sample buffer. Bead-bound proteins were separated by SDS-PAGE.
Endocytosis Assay for LPA1
This assay was performed essentially as described previously [11]. HEK cells stably expressing LPA1 were grown on cover slips pre-coated with fibronectin (BD Biosciences, Bedford, MA). Cells were serum starved in DMEM at 37°C for 4 h, incubated on ice with anti-FLAG IgG (1∶1,000) for 0.5 h, washed with ice-cold PBS (3X), and shifted to fresh medium containing 1–10 µM LPA at 37°C for various times prior to fixation and processing for immunofluorescence.
Immunofluorescence
HEK cells were fixed with 3% paraformaldehyde in 100 mM phosphate buffer, pH 7.4, for 30 min, permeabilized with 0.1% Triton X-100 in 1% BSA for 10 min, and incubated with primary antibodies for 1 h followed by goat anti-rabbit Alexa-594 and/or anti-mouse Alexa-488 F(ab')2 (Molecular Probes) for 1 h. Fluorescence images were taken with either an AxioImager M1 (Carl Zeiss, Thornwood, NY) equipped with a digital ORCA-ER camera (Hamamatsu), a PerkinElmer UltraView Vox Spinning Disk Confocal unit connected to an Olympus IX81 inverted microscope and a EMCCD camera (Hamamatsu), or an inverted Olympus FluoView 1000 confocal microscope equipped with a CH350 CCD camera (Hamamatsu). Images were processed with Adobe Photoshop 5.0 (Adobe Systems, Mountain View, CA). Fluorescence images of double-labeled samples were evaluated using the colocalization analysis features of the Volocity software (PerkinElmer, Waltham, MA).
Deglycosylation Assay
Glycosylation assays (PNGase F treatments) were performed using the N-Glycanase-PLUS kit (ProZyme, San Leandro, CA) according to the manufacturer’s protocol. Briefly, HEK cells stably expressing FLAG-tagged LPA1 or empty vector were lysed in 0.1% SDS, 50 mM Tris HCl, pH 7.5, and 50 mM β-Mercapto-ethanol supplemented with protease inhibitors, and protein concentration was determined by the Bradford assay. Proteins (40 µg) were diluted in 45 µl of the above lysis buffer, and NP40 was added to a final concentration of 0.75%. 1 µl N-Glycanase-PLUS (Activity ≥10 U/ml) was added to half the samples, and the mixtures were incubated at 37°C for 3.5 h. Laemmli SDS sample buffer was added, proteins were resolved by SDS-PAGE, transferred to PVDF membranes, and analyzed by Western blotting using rabbit anti-FLAG IgG.
Statistical Methods
Data in graphs are presented as the mean ± standard error of the mean (S.E.M) for n trials. Statistical analysis was carried out by Student's t-test, as appropriate, using 95% confidence limits. Specifics are detailed in the figure legends.
Cell Migration Assay
Migration assays were performed as described by Klemke et. al. [25]. Briefly, Boyden chambers containing polycarbonate membranes (tissue culture-treated, 6.5 mm diameter, 10 µm thickness, 8 µm pores, Transwell®; Costar Corp., Cambridge, MA) were coated on both sides with human fibronectin for 2 h at 37°C. Cells were transfected with control or GIPC siRNA, and after 24 h they were incubated in serum free DMEM for an additional 24 h. 1×105 cells in 100 µl serum free DMEM containing 1 mM sodium pyruvate and 0.25% fatty acid free BSA were added to the top of each well; the bottom of each well contained the same medium with or without 1 µM LPA. Cells were allowed to migrate for 3 h at 37°C and subsequently stained with crystal violet (Sigma). Cells that migrated to the bottom of the filter in each well were counted under the microscope to assess cell migration.
Cell Proliferation Assay
Cell proliferation was assessed using a previously described crystal violet staining method [26]. Briefly, HEK cells stably expressing LPA1 or empty vector were transfected with control siRNA or GIPC siRNA in 12 well plates using lipofectamine 2000. 24 h after transfection cells were trypsinized, and 2×104 cells were transferred to each well of a 96 well plate and cultured at 37°C. At specific time points (0–72 h) cells were fixed with 3.7% paraformaldehyde for 5 min, and stained with 0.05% crystal violet for 30 min. To determine cell numbers, the crystal violet in the wells was solubilized in methanol and absorbance (OD 540 nm) determined directly using a plate reader.
Results
GIPC Specifically Interacts with the PDZ Binding Motif of LPA1
To determine if GIPC can interact with LPA1 we transiently co-expressed GIPC-GFP and N-terminally tagged FLAG- LPA1 in HEK293 cells and immunoprecipitated LPA1 with anti-FLAG IgG. We found that GIPC-GFP co-immunoprecipitated with FLAG-LPA1 (Fig. 1A), suggesting that GIPC and LPA1 are present in the same protein complexes.
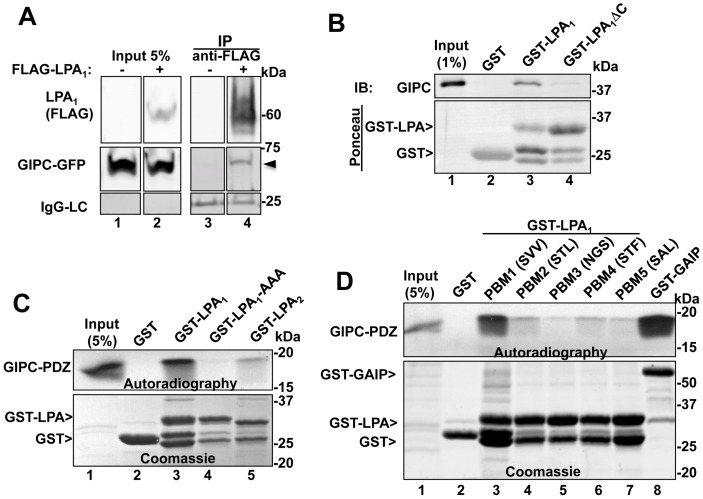
Figure 1. GIPC directly interacts with the C-terminal PDZ binding motif of LPA1 but not with other LPA receptors.
A, Endogenous GIPC and GIPC-GFP co-immunoprecipitate with FLAG- LPA1 from HEK cells expressing FLAG- LPA1 (arrowhead, lane 4) but not control HEK cells (lane 3). C-terminally tagged GIPC-GFP and N-terminally tagged FLAG-LPA1 were transiently coexpressed in HEK293 cells, and immunoprecipitation was carried out on cell lysates with mouse anti-FLAG IgG followed by immunoblotting with mouse anti-FLAG (LPA1) and rabbit anti-GIPC IgG. Lanes were cropped from a single exposure of a continuous membrane. The lower panel shows the amount of IgG light-chain (IgG-LC) in each IP. Lanes 1–2: Input showing the amounts of LPA1 and GIPC present in the lysates used for the IP. B, Upper panel: GIPC binds GST-LPA1 (GST fused to the cytoplasmic tail of mouse LPA1 (aa 311–364), lane 3) but not to GST alone (lane 2) or GST-LPA1ΔC (lacking the last three C-terminal amino acids, lane 4). Immobilized recombinant GST, GST-LPA1 and GST- LPA1ΔC were incubated 4–15 h with lysates from HEK293 cells transiently transfected with FLAG-GIPC. Proteins bound to immobilized fusion proteins were eluted with 2X sample buffer for SDS-PAGE and immunoblotted with anti-GIPC IgG. Lane 1: input, showing the amount of GIPC in 1% of the lysate used for the assay. Lower panel : Ponceau staining demonstrating the amount of GST proteins used in each assay. C, Upper panel: Autoradiography showing that in vitro translated, [35S]GIPC PDZ domain binds to GST-LPA1 (lane 3) but not to GST alone (lane 2), GST- LPA1AAA (last three amino acids mutated to alanine, lane 4), or GST-LPA2 (lane 5). GST fusion proteins were immobilized on glutathione-agarose beads as in “B” and incubated with in vitro translated [35S]Met-labeled, GIPC PDZ domain (aa 125–225). Bound proteins were separated by SDS-PAGE and detected by autoradiography. Lane 1: 5% of the in vitro translated protein. Lower panel : Coomassie Blue staining showing the GST proteins used for the assay. D, Upper panel: Autoradiography showing that in vitro translated, [35S] GIPC-PDZ interacts with the C-terminal PDZ binding motif of LPA1 (SVV, lane 3) and with GST-GAIP (lane 8, used as a positive control [7] but shows little or no interaction with GST alone (lane 2) or GST-LPA1 mutants in which the three C-terminal amino acids were modified to those of LPA2 (STL, lane 4), LPA3 (NGS, lane 5), LPA4 (STF, lane 6) or LPA5 (SAL, lane 7). Immobilized GST fusion proteins were incubated with in vitro translated [35S]Met-labeled GIPC PDZ and analyzed as in C. Lower panel: Coomassie Blue staining showing the amounts of GST proteins used.
To determine if GIPC interacts with the PDZ binding motif of LPA1 we carried out GST pull-down assays with GST-LPA1 (aa 311–364) on cell lysates from HEK293 cells transiently transfected with FLAG-GIPC. We found that GIPC bound to GST-LPA1 (Fig. 1B, lane 3), but did not bind to GST-LPA1ΔC, lacking the PDZ binding motif (-SVV) (Fig. 1B, lane 4). To find out if the interaction between GIPC and LPA1 is direct and whether the PDZ domain of GIPC is sufficient for the interaction we performed pull down assays using GST-fusion proteins and [35S] Met-labeled, in vitro translated, GIPC-PDZ (aa 125–225). GIPC-PDZ bound to GST-LPA1 (Fig. 1C, lane 3) but not to GST-LPA1-AAA, a mutated version of GST- LPA1 in which the last three amino acids were mutated to alanine (Fig. 1C, lane 4). Interaction with the cytoplasmic tail of LPA2 was much weaker (Fig. 1C, lane 5) even though it also has a class-I PDZ binding motif. To verify the specificity of GIPC’s interaction with the PDZ binding motif of LPA1 we mutated the last three amino acids of LPA1 cytoplasmic tail (-SVV) to resemble the C-terminal sequence of LPA receptor subtypes 2 (-STL), 3 (-NGS), 4 (-STF) and 5 (-SAL). In vitro translated GIPC-PDZ bound to the PDZ binding motif of LPA1 whereas interactions with other PDZ binding motifs were much weaker (Fig. 1D), suggesting that GIPC interacts specifically with LPA1 and can distinguish the PDZ binding motif of LPA1 from closely related PDZ binding motifs of other members of the LPA receptor family. Taken together these results demonstrate that GIPC directly binds to the PDZ binding motif of LPA1, that this interaction is specific for LPA1, and that it is mediated via the PDZ domain of GIPC and the C-terminal PDZ binding motif of LPA1.
LPA1 and GIPC Traffic Together to APPL Endosomes
We have previously shown [11] that GIPC binds to the receptor tyrosine kinase TrkA and regulates its trafficking and signaling through interaction with APPL, a Rab5 effector that serves as a marker for APPL signaling endosomes [14]–[16]. To investigate if GIPC similarly regulates trafficking and signaling of LPA1 we prepared HEK293 cell lines stably expressing FLAG- LPA1 (HEK-LPA1) or empty vector (HEK-pIRES) (Fig. S1). We chose HEK293 cells because they were previously shown to express LPA1 but not LPA2 or LPA3, [27], and therefore any response to LPA observed is likely to be via activation of LPA1 and its downstream signaling network. First we followed the trafficking of LPA1 and its association with GIPC and APPL in these cells. In serum starved HEK-LPA1 cells stably expressing LPA1, LPA1 colocalized with GIPC along the PM (Figs. 2A, and S3 upper panel). Similar results were also obtained in HeLa cells transiently expressing LPA1 (Fig. S2). By 2–5 min after stimulation with LPA, LPA1 had been partially internalized and accumulated on peripheral vesicles located just beneath the plasma membrane that colocalize with both GIPC and APPL (Fig. 2A, middle panels and S4 upper panel). Beginning at 15 min (see Figs. 3A and S4) and especially by 30 min after ligand stimulation (Figs. 2A and S4, lower panels) LPA1 colocalized with the early endosome marker EEA1 in the juxtanuclear region and no longer colocalized with either GIPC (Fig. S3) or APPL (Fig. S4). Thus our results suggest that, like TrkA [11], after agonist stimulation LPA1 is internalized and passes first through APPL endosomes located at the cell periphery and then to EEA1 early endosomes located in the juxtanuclear region.
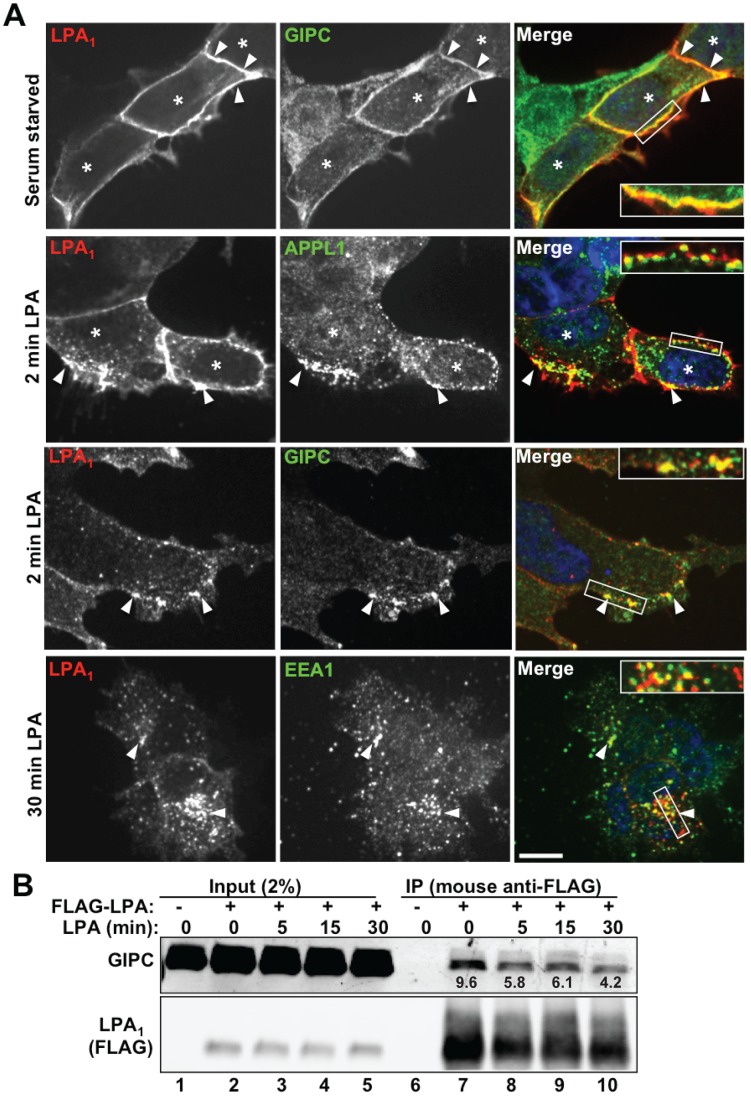
Figure 2. Trafficking of LPA1 and GIPC to Endosomes.
A, Upper panel: In serum starved cells stably expressing LPA1 (asterisks), GIPC is concentrated at the plasma membrane (arrowheads) where it colocalizes with LPA1. Middle panels : 2 min following stimulation with LPA, LPA1 colocalizes with APPL and GIPC (arrowheads) in endocytic vesicles at the cell periphery. Lower panel : 30 min following stimulation, LPA1 colocalizes with EEA1 in early endosomes concentrated in the juxtanuclear region (arrowheads). Boxed regions are enlarged (2.2×) in the insets. Bar = 10 µm. HEK- LPA1 cells were serum starved for 4 h, incubated on ice with mouse anti-FLAG IgG to label FLAG-LPA1 at the cell surface, washed in PBS and shifted to fresh medium containing LPA (10 µM) 2 or 5 min before fixation. Cells were processed for immunofluorescence using affinity purified rabbit anti-GIPC, anti-APPL1 or anti-EEA1 IgG, followed by goat anti-rabbit Alexa-488 and goat-anti-mouse Alexa-594 F(ab’)2 (the latter to detect FLAG-LPA1). Images were taken with a PerkinElmer UltraView Vox Spinning Disk Confocal unit connected to an Olympus IX81 inverted microscope and a EMCCD camera (Hamamatsu) using a 60X oil immersion lens (1.42 NA). B, GIPC co-immunoprecipitates with FLAG-LPA1 from serum-starved cells (lane 7) at all time points after LPA stimulation, but the interaction gradually decreases after LPA stimulation (lanes 8–10). The relative abundance of GIPC that coprecipitated with FLAG-LPA1 is indicated beneath each band. As expected, both LPA1 and GIPC are absent from immunoprecipitates of cells transiently transfected with empty vector instead of FLAG- LPA1 (lane 6, vector control). HEK293 cells were transiently co-transfected with full-length GIPC and FLAG-LPA1 (lanes 2–5 and 7–10) or GIPC alone (lanes 1 and 6). Cells were serum starved overnight (lanes 1, 2, 6 and 7) or starved and stimulated with 10 µM LPA for 5 (lanes 3 and 8), 15 (lanes 4 and 9) or 30 min (lanes 5 and 10) before lysis. IP was carried out on cell lysates using mouse anti-FLAG IgG and immunoblotted as is Fig. 1A. The abundance of GIPC and LPA1 in each immunoprecipitation reaction was quantified using the LICOR imaging system, and the GIPC abundance relative to LPA1 was calculated for each reaction. Similar results were obtained in 2 additional experiments. Input (lanes 1–5): Lysates (2%) are shown to verify comparable expression levels.
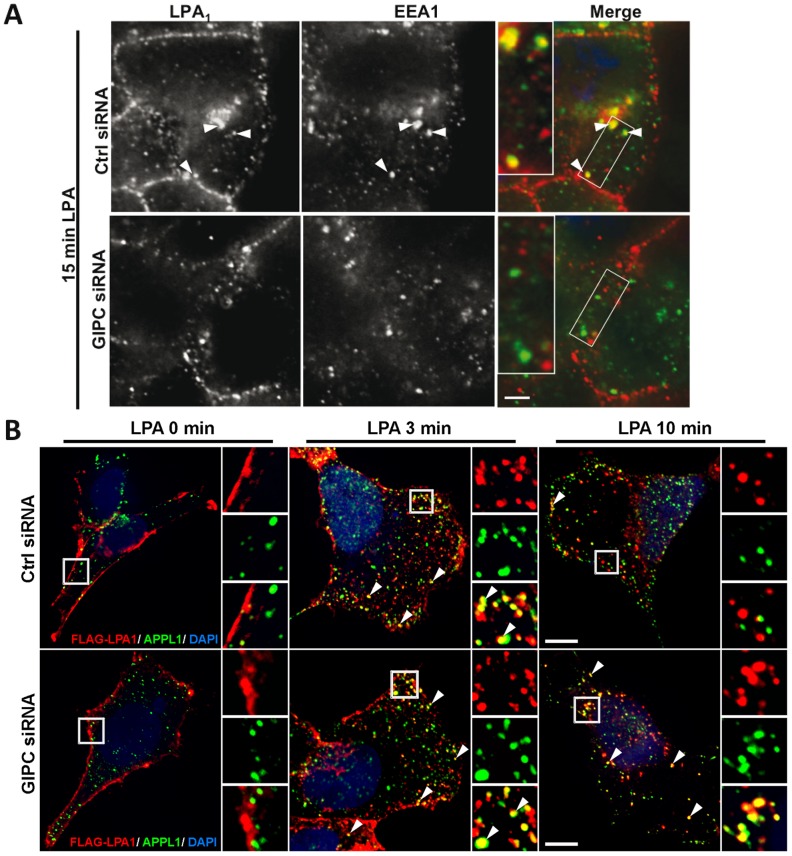
Figure 3. GIPC depletion delays trafficking of LPA1 from APPL1 to early EEA1 endosomes.
A, GIPC depletion inhibits internalization of LPA1 and its trafficking to early endosomes after stimulation with LPA. Upper panel: In HEK-LPA1 cells transfected with control siRNA and stimulated with LPA for 15 min, LPA1 is found in cytoplasmic vesicles where it colocalizes with EEA1 (arrowheads). Lower Panel: In cells transfected with GIPC siRNA fewer vesicles containing LPA1 are present 15 min after LPA stimulation, and less colocalization is seen between LPA1 and EEA1 (compare yellow in right panels). Boxed regions are enlarged (2.2×) in the insets. Images were acquired with a Zeiss AxioImager M1 microscope, and overlap in staining between LPA1 and EEA1 was evaluated using Volocity software. Statistical significance (p value) was determined by t-test. B, Trafficking of LPA1 is delayed in APPL1 endosomes after depletion of GIPC. Left panel: In both GIPC-depleted (GIPC siRNA) and controls (Ctrl siRNA), LPA1 is localized along the plasma membrane after serum starvation (0 min) whereas APPL1 is found in peripheral cytoplasmic vesicles. Middle Panel: In both GIPC depleted and control cells stimulated with LPA for 3 min, LPA1 colocalizes with APPL1 in cytoplasmic vesicles (arrowheads). Right Panel: In controls stimulated with LPA for 10 min, very few LPA1 receptors remain in APPL endosomes (yellow, arrowhead) whereas in GIPC-depleted cells the majority of the receptors are retained in APPL endosomes (yellow, arrowhead). Boxed regions are enlarged (3×) in the insets. HEK-LPA1 cells grown on coverslips were transfected with GIPC or control siRNA. 72 h after transfection cells were serum starved for 4–6 h and subsequently incubated on ice with rabbit (A) or mouse (C) anti-FLAG IgG, shifted to fresh medium containing LPA for the indicated times, then fixed and processed for immunofluorescence using mouse anti-EEA1 IgG (A) or rabbit-anti-APPL1 IgG (C) as in Fig. 2A. Images in “A” were acquired with a Zeiss AxioImage M1 microscope, and those in “C” were acquired with an Ultra View Vox Spinning Disk Confocal. Bar = 10 µm.
To determine if LPA1 and GIPC are internalized via clathrin mediated endocytosis we performed double labeling for clathrin and GIPC or LPA1 (Fig. S5). We found that 2–3 minutes following addition of LPA, both LPA1 and GIPC colocalized with clathrin in punctate structures at or just beneath the plasma membrane indicating that following LPA stimulation, GIPC and LPA1 are internalized into clathrin coated pits which pinch off the plasma membrane to form clathrin coated vesicles.
To find out if ligand stimulation affects the association between LPA1 and GIPC we immunoprecipitated LPA1 from HEK293 cells transiently expressing FLAG-LPA1 before and after stimulation with LPA (5–30 min). GIPC co-immunoprecipitated with LPA1 at all time points, but the amount of GIPC that co-immunoprecipitated with LPA1 gradually declined after ligand stimulation (Fig. 2B). Collectively the immunofluorescence and biochemical results suggest that, as for TrkA [11], GIPC associates with LPA1 at the plasma membrane, GIPC and LPA1 travel together to APPL endosomes (2–5 min), and they dissociate from one another before LPA1 reaches early (EEA1) endosomes (30 min).
GIPC Depletion Disrupts LPA1 Trafficking
Next we investigated the effects of GIPC depletion on LPA1 trafficking at 0 and 15 min after LPA stimulation. In serum starved cells LPA1 was present largely at the plasma membrane in both GIPC-depleted cells and controls (not shown). At 15 min after addition of LPA, in controls LPA1 appeared both at the PM and in vesicles inside the cell where it partially colocalized with the early endosome marker EEA1 (Fig. 3A, upper panel). By contrast in GIPC-depleted cells fewer vesicles with LPA1 were seen in the cytoplasm, and colocalization between LPA1 and EEA1 was markedly reduced (Fig. 3A, middle panel). Quantification of the overlap between LPA1 and EEA1 (Fig. 3B) using Volocity software revealed a 32% decrease in the average overlap coefficient (OC) in GIPC depleted cells (OC = 0.45) compared to controls (OC = 0.66). The decreased localization of LPA1 in EEA1 early endosomes at 15 min after LPA addition suggests that in GIPC depleted cells there is a delay in trafficking of LPA1 from the plasma membrane or peripheral vesicles to early endosomes.
To test if following GIPC depletion, LPA1 accumulates in peripheral (APPL) signaling endosomes we carried out double labeling for LPA1 and APPL1 0–10 min after LPA stimulation (Fig. 3C). We found that in control cells, colocalization between APPL1 and LPA1 in APPL endosomes peaked at 3 min and was barely detected at 10 min after LPA stimulation. In contrast, in GIPC depleted cells, colocalization between APPL1 and LPA1 increased 3 min after LPA stimulation but remained high even after 10 min. Taken together, these results suggest that GIPC promotes trafficking of LPA1 from peripheral APPL signaling endosomes to early endosomes after internalization of the receptor from the plasma membrane.
GIPC Depletion Enhances LPA1 Signaling
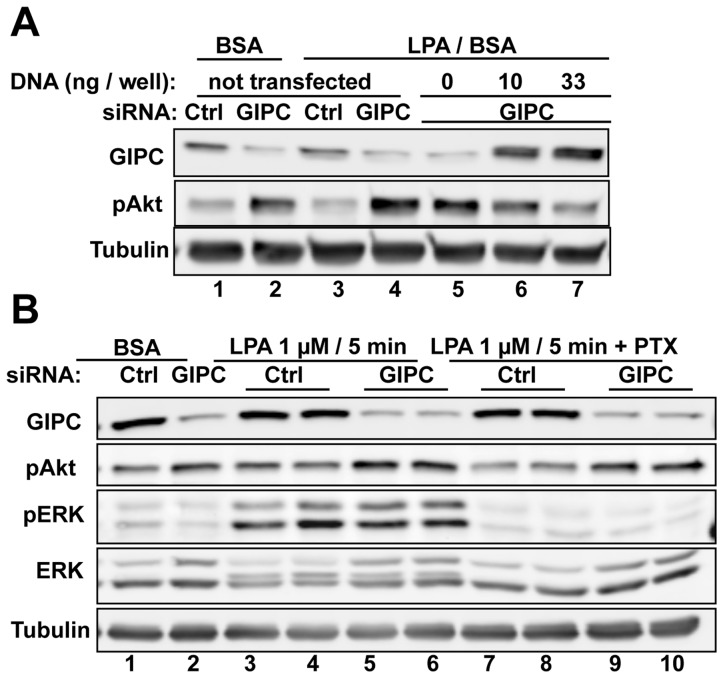
To investigate the effects of GIPC depletion on LPA1 signaling we stimulated HEK-LPA1 cells with LPA and assessed activation (phosphorylation) of Erk and Akt–two signaling pathways that mediate cell survival, proliferation and motility. We found that at both 5 and 20 min after LPA stimulation GIPC depletion (∼70%) enhanced Akt activation by ∼2-fold (Fig. 4A and B) but had no effect on pErk levels (Fig. 4A and C). Similar findings were obtained using different clones of HEK-LPA1 cells. Transfection of siRNA resistant GIPC into GIPC depleted cells reversed the effect of GIPC siRNA on Akt phosphorylation in a dose dependent manner (Fig. 5A, lanes 5–7) verifying that the effects of GIPC expression on Akt phosphorylation are not due to off target effects of the siRNA.
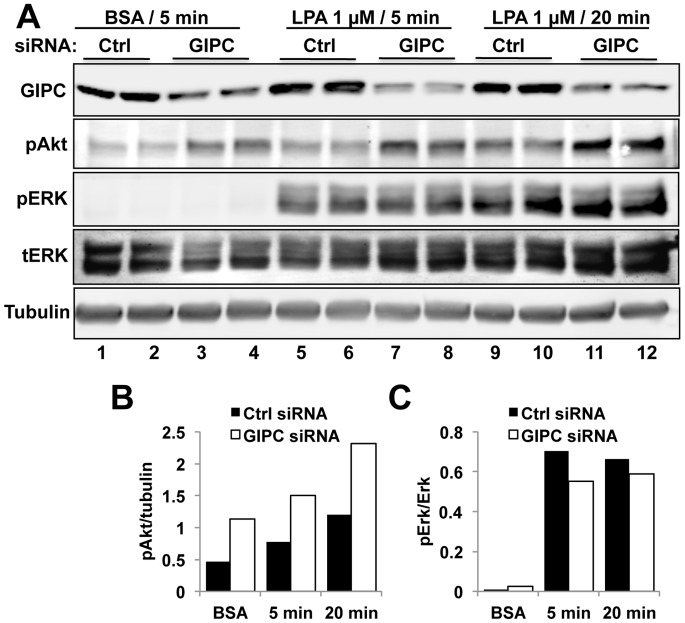
Figure 4. Akt phosphorylation is increased in GIPC depleted HEK-LPA cells.
A, In HEK- LPA1 cells transfected with control siRNA both Akt and ERK1/2 activation are enhanced after stimulation with LPA for 5 (lanes 5–6) or 20 min (lanes 9–10). GIPC depletion (GIPC siRNA) enhanced Akt phosphorylation (pAkt) at 5 min (lanes 7–8) and 20 min (lanes 11–12) after LPA stimulation. GIPC depletion also enhanced Akt signaling in the absence of ligand (lanes 1–4), possibly due to enhanced basal activity of LPA1. Erk phosphorylation (pERK) was not affected by GIPC depletion. HEK-LPA1 cells were transfected with control or GIPC siRNA, serum starved overnight, stimulated with 1 µM LPA or incubated with BSA alone for 5 or 20 min, lysed in RIPA buffer and analyzed by immunoblotting using phospho-Erk (pErk), total Erk (tErk), phospho-Akt (pAkt) and α-tubulin IgG. Each treatment was done in duplicate. α-tubulin was used as a loading control. In cells transfected with GIPC siRNA (lanes 3–4, 7–8, 11–12), GIPC expression is reduced 70–80% in cells transfected with control siRNA (Ctrl, lanes 1–2, 5–6, 9–10). B–C, Densitometric analysis of the immunoblots in A demonstrating that GIPC depletion (siRNA) leads to a two-fold increase in Akt phosphorylation (B) at both 5 and 20 min after LPA stimulation (B, P<0.05) but does not significantly affect Erk phosphorylation (C) compared to controls (Ctrl siRNA).
Figure 5. Enhancement of Akt activation following GIPC depletion is reversed by GIPC expression and is independent of Gαi signaling.
A, GIPC depleted HEK-LPA1 cells show reduced Akt phosphorylation after transfection of siRNA resistant GIPC DNA (lanes 6–7, middle panel) verifying that GIPC is responsible for the enhanced Akt phosphorylation seen after GIPC depletion. HEK-LPA1 cells were transfected with GIPC or control siRNA, and 12 h later they were transfected with siRNA-resistant GIPC DNA (0, 10, or 33 ng). After 24 h cells were serum starved overnight, stimulated with 1 µM LPA for 5 min, and cell lysates were immunoblotted for GIPC, pAkt and tubulin (used as loading control). B, Activation of Gαi is not required for the enhanced Akt phosphorylation seen after GIPC depletion. In GIPC-depleted cells PTX treatment (lanes 7–10) prevented LPA induced Erk phosphorylation (pErk) (which is Gαi dependent) but did not affect Akt phosphorylation (pAkt) compared with controls (lanes 3–6). 36 h after siRNA transfection, HEK-LPA1 cells were cultured for another 12 h in serum-free media in the presence or absence of PTX and then stimulated for 5 min with 1 µM LPA (in 0.1% BSA, lanes 3–10) or incubated in BSA alone (0.1%) for 5 min (lanes 1–2).
We showed previously that GIPC recruits GAIP (RGS19), a GAP for Gαi proteins [28], and inhibits Gi signaling [10]. To determine if Gαi activity is required for LPA1 mediated Akt phosphorylation we pre-treated GIPC depleted and control cells with pertussis toxin (PTX), an inhibitor of Gαi/GPCR coupling, before LPA stimulation. PTX abolished LPA induced Erk activation but did not affect activation of Akt (Fig. 5B). Notably, PTX did not inhibit the increased Akt phosphorylation seen in GIPC depleted cells (Fig. 5B), indicating that the effect of GIPC on Akt phosphorylation is most likely not mediated through Gαi subunits.
APPL is Present in LPA1 Complexes and APPL Depletion Inhibits Akt Activation
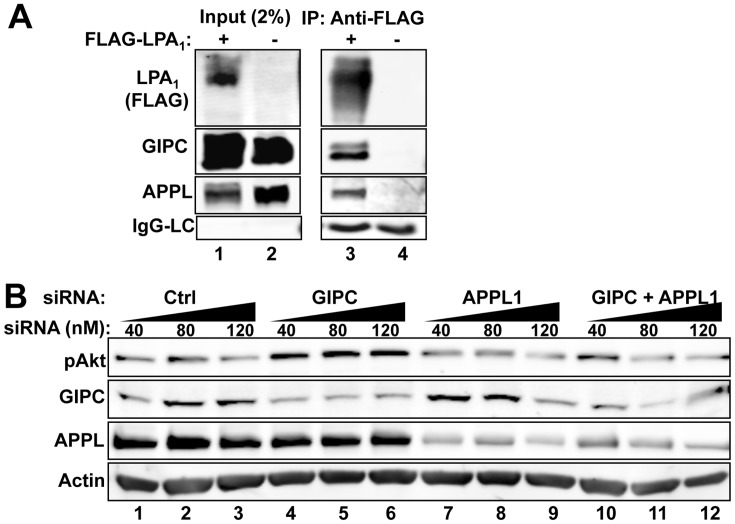
APPL directly binds GIPC as well as the TrkA receptor [11], [13] and promotes Akt signaling and cell survival [14]. To determine whether LPA1 forms a complex with APPL and GIPC we immunoprecipitated FLAG-LPA1 from HEK-LPA1 cells at steady-state (10% FBS) and immunoblotted for APPL and GIPC. We found that APPL and GIPC co-immunoprecipitated with LPA1 (Fig. 6A, lane 3), indicating that LPA1 is present in the same protein complexes as GIPC and APPL.
Figure 6. APPL interacts with LPA1 and affects LPA1 mediated Akt signaling.
A, APPL and GIPC co-immunoprecipitate with FLAG- LPA1 (lane 3). HEK cells were co-transfected with HA-APPL and GIPC together with FLAG-LPA1 (lanes 1 and 3) or empty vector (lanes 2 and 4), and cultured in the presence of 10% PBS for 48 h before lysis. IP was carried out as in Fig 1A. Aliquots of cell lysates (input, 2%) were loaded to verify comparable expression levels. B, APPL depletion inhibits Akt activation in HEK-LPA1 cells stimulated with LPA. Depletion of GIPC (lanes 4–6) leads to increased Akt signaling (top panel) compared with controls (lanes 1–3). In contrast, depletion of APPL1 alone (lanes 7–9) or double knockdown of GIPC and APPL (lanes 10–12) results in reduced Akt signaling. HEK-LPA1 cells were transfected with increasing amounts of control (lanes 1–3), GIPC (lanes 4–6) or APPL siRNA (lanes 7–9) or GIPC and APPL siRNA combined (lanes 10–12). Cells were serum starved overnight, stimulated with 5 µM LPA for 15 min, lysed and analyzed by immunoblotting as in Fig. 3D.
To determine if APPL is required for enhancing Akt phosphorylation following GIPC depletion we treated HEK- LPA1 cells with control siRNA (Fig. 6B, lanes 1–3), GIPC siRNA alone (Fig. 6B, lanes 4–6), APPL siRNA alone (Fig. 6B, lanes 7–9) or both GIPC and APPL siRNA (Fig. 6B, lanes 10–12) and stimulated the cells with LPA. Depletion of GIPC led to enhanced Akt signaling as before, whereas depletion of APPL or double knockdown of GIPC and APPL reduced Akt signaling. The reversal of Akt enhancement in the double knockdown suggests that APPL is required for the enhancement of Akt signaling. Taken together, these results suggest that following ligand stimulation APPL associates with LPA1 protein complexes and mediates Akt activation downstream of LPA1.
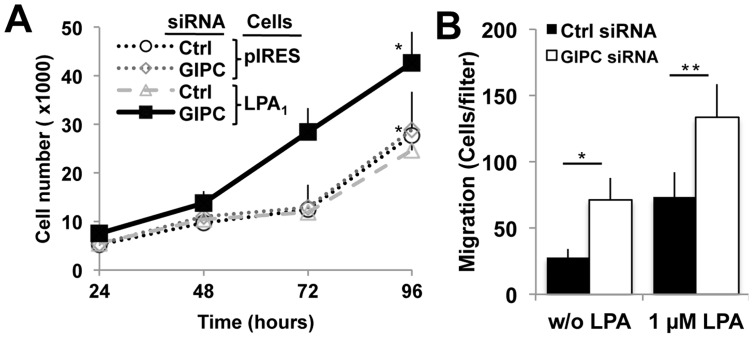
GIPC Depletion Promotes LPA1 Mediated Cell Proliferation and Cell Motility
Next we investigated if GIPC depletion can affect cell growth in the presence of LPA. GIPC was depleted from HEK-LPA1 cells, and the growth of GIPC depleted vs control HEK- LPA1 cells was followed for 96 h after siRNA transfection. GIPC depletion resulted in a 72% increase in the number of cells per well (43,000+/−6,000 vs 25,000+/−8,000 cells/well) (Fig. 7A). GIPC depletion did not significantly affect growth of HEK-pIres controls that do not express FLAG- LPA1 (Fig. 7A), indicating that enhancement of cell growth is mediated through LPA1. In addition, the number of HEK-LPA1 cells that incorporated BrdU was increased from 18% in controls to 23% in GIPC depleted cells (data not shown), suggesting a slightly faster cell cycle. These results are consistent with a role for GIPC in down-regulating LPA1 mediated cell growth or cell survival.
Figure 7. Depletion of GIPC in cells overexpressing LPA1 promotes cell proliferation and cell motility.
A, GIPC-depleted HEK-LPA1 cells (solid line) grow faster than those transfected with control (Ctrl) siRNA (*, p<0.05). In contrast, GIPC depletion had little effect on the growth rate of HEK-pIRES cells which do not overexpress LPA1. HEK-LPA1 and HEK-pIRES cells were transfected with GIPC (siRNA) or control siRNA (Ctrl) and transferred to 96 well plates 24 h post-transfection. Cells were cultured for up to 72 h in medium containing 10% serum without puromycin. Cell number was determined as described in “Material and Methods”. Data are the mean ± s.e.m (n = 16 wells). B, Depletion of GIPC promotes HEK-LPA1 cell motility. Serum starved HEK-LPA1 cells were transfected with either GIPC (white bars) or control (black bars) siRNAs and allowed to migrate for 3 h on fibronectin-coated filters. LPA (1 µM) was added to the bottom chambers in half the wells. The number of migrating cells was determined by counting cells on the underside of the filters as described in “Material and Methods”. Each bar represents the mean ± s.e.m. of triplicate wells in three independent experiments.
Because LPA1 is also known to trigger cell motility we next examined the effect of GIPC depletion on cell migration by analyzing movement of HEK-LPA1 cells across a porous membrane in a Boyden chamber in the presence of concentration gradient of LPA [25]. GIPC depletion enhanced motility of HEK-LPA1 cells in that increased numbers of cells migrated across the membrane both in the presence and absence of a concentration gradient (Fig. 7B). These results demonstrate that GIPC inhibits cell motility in cells expressing LPA1. Previously, LPA1 was shown to possess intrinsic basal activity even in the absence of ligand binding [29]. Thus the inhibitory effect of GIPC on cell motility in the absence of ligand is most likely due to inhibition of the basal activity of LPA1.
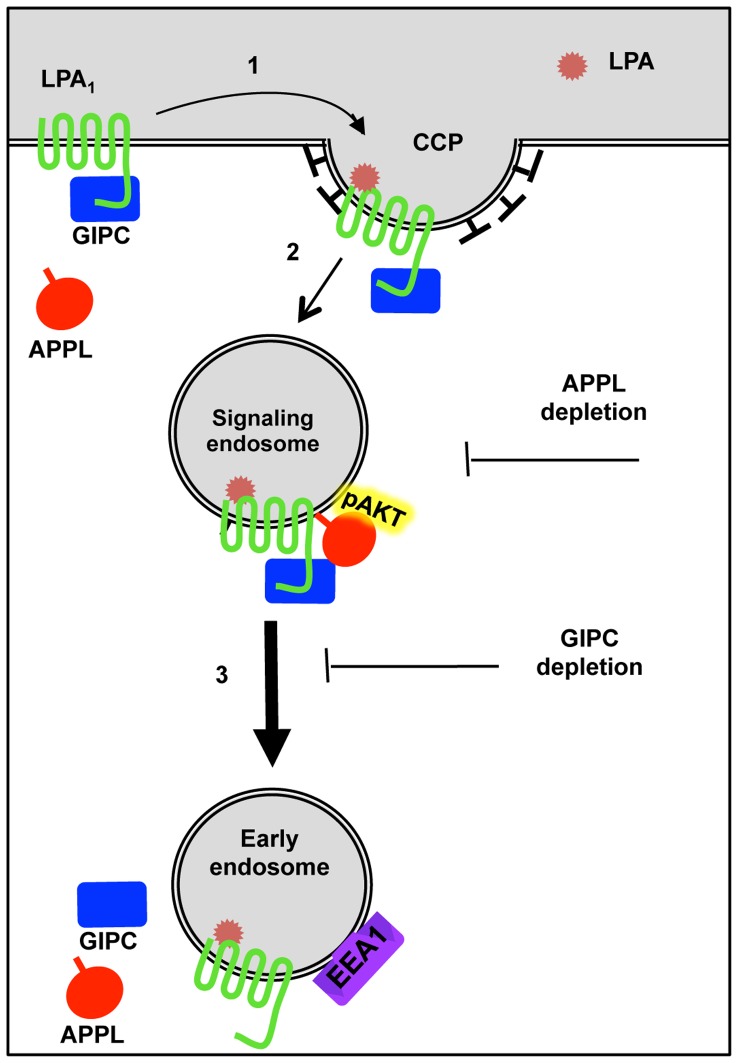
Based on our results we propose a working model (Fig. 8) in which GIPC associates with LPA1 at the PM in a ligand independent manner, and following ligand stimulation the receptor and GIPC are internalized in clathrin-coated vesicles and associate with APPL which activates Akt signaling. Subsequently, GIPC promotes LPA1 trafficking to early (EEA1) endosomes and thus terminates APPL/Akt signaling. Depletion of GIPC delays trafficking of LPA1, prolongs its stay in APPL signaling endosomes, and enhances Akt signaling leading to increased cell motility and cell proliferation.
Figure 8. Working model depicting LPA1 endocytic trafficking and signaling and its interactions with GIPC and APPL.
In the absence of ligand LPA1 is found at the plasma membrane in a complex with GIPC. When LPA is added, LPA1 and GIPC move into clathrin-coated pits (1). Clathrin-coated vesicles containing LPA1-GIPC complexes pinch off the cell membrane and uncoat and APPL is recruited (2). APPL binds pAkt to form peripheral signaling endosomes. GIPC, by binding to APPL and the motor protein myosin VI, facilitates movement of these endosomes to the juxtanuclear region (3). In juxtanuclear early endosomes, GIPC and APPL are released into the cytoplasm thus terminating APPL-pAkt signaling. Depletion of GIPC inhibits LPA1 trafficking to EEA1 endosomes and prolongs LPA1 signaling from APPL endosomes whereas depletion of APPL inhibits Akt signaling.
Discussion
We demonstrate here that GIPC binds LPA1 and that binding is direct and is mediated through the PDZ domain of GIPC and the C-terminal PDZ binding motif of LPA1. siRNA depletion of GIPC delayed trafficking of LPA1 to early endosomes and resulted in enhanced LPA1-mediated Akt signaling and enhanced cell proliferation and cell motility. APPL, a marker APPL/GIPC signaling endosomes, was present in LPA1 complexes and necessary for LPA1 mediated Akt signaling. Taken together, these results support a model in which GIPC promotes trafficking of LPA1 from APPL signaling endosomes to early (EEA1) endosomes thus attenuating LPA1 mediated signaling and cellular responses (see Figure 8).
Both LPA1 and GIPC have been implicated in cell migration [8], [17], [30]–[32], neuronal cell activity [33], [34] and cell proliferation [9], [35], [36]. GIPC has been shown to inhibit endothelial cell migration through interaction with Endoglin [37] or syndecan-4 [38], but it promotes migration of primary arterial endothelial cells [8]. LPA1 has been shown to promote migration and proliferation of many cell types [30]–[32], [35]. Our results showing that GIPC binds LPA1 and regulates its activity suggest a novel mechanism by which GIPC affects cell migration. We also observed an apparent increase in cell proliferation following GIPC depletion in cells expressing LPA1. The effects of LPA1 on cell proliferation are most likely indirect and are believed to reflect a combination of the secondary release of growth factors following initial LPA stimulation combined with anti-apoptotic actions [39]–[41]. The increase in cell number following GIPC depletion coincides with enhanced LPA1 activity and presumably stems from primary effects on cell survival coupled with secondary effects on cell proliferation.
GIPC was previously shown to define the signaling specificity of β-adrenergic receptor subtypes [42]. Our finding that GIPC interacts with LPA1 but shows much weaker or no interaction with other LPA receptor subtypes may similarly explain the differential effect of LPA1 and LPA2 on cell migration and proliferation [35]. In the case of LPA receptors, binding to PDZ domain proteins has recently been shown to influence the signaling outcomes of different LPA receptors [43]–[45]. The PDZ proteins NHERF2 and MAGI-3 were shown to couple LPA2 to PLC-β3, RhoA and Erk signaling [43], [44], and two additional PDZ proteins, PDZ-RhoGEF and LARG, have been shown to interact with both LPA1 and LPA2 [45]. Because the latter proteins bind to both LPA1 and LPA2, these interactions can’t explain the different effects of LPA1 and LPA2 on cell behavior [30], [35].
Shano et. al. [46] recently reported that a point mutation in the LPA1 PDZ binding motif led to increased Akt signaling and cell proliferation. Our findings that GIPC binds to the PDZ binding motif of LPA1 and depletion of GIPC has similar effects suggests that the findings of Shano et al can be explained by loss of interaction of LPA1 with GIPC. In contrast, loss of interaction between LPA1 and the PDZ proteins PDZ-RhoGEF and LARG [45] had different consequences suggesting that these proteins do not mediate the effects on Akt and cell proliferation. Previously it was shown that deletion of the LPA1 PDZ binding motif enhances Akt signaling [46] but did not affect inositol phosphate production [21]. These observations suggest that Akt enhancement is mediated by PLC- and inositol phosphate-independent mechanisms [13].
We previously discovered that in PC12 cells, GIPC binds to APPL on peripheral endosomes and that depletion of GIPC slows down endocytosis and trafficking of TrkA and the Rab5-effector APPL to early EEA1 endosomes [11]. Here we show that GIPC depletion similarly delays trafficking of LPA1 to early EEA1 endosomes and prolongs the residence of LPA1 receptor on APPL1 signaling endosomes. Despite the fact that GIPC depletion has similar effects on the trafficking of TrkA and LPA1, their signaling outcomes differ: GIPC depletion reduced TrkA mediated Akt and Erk signaling but enhanced LPA1 mediated Akt signaling [11]. This illustrates that signaling outcomes can be widely divergent among different receptors. Signaling depends on protein-protein interaction networks, and each receptor has a distinctive set of binding partners. TrkA and LPA1 are representatives of two diverse families, the receptor tyrosine kinases (RTKs) and G protein coupled receptors (GPCR), respectively, which have very different modes of signaling. As discussed earlier, even closely related receptors, such as LPA1, LPA2 and LPA3, form distinct protein-protein interactions with distinct signaling outcomes. Thus the molecular mechanisms underlying the different effects of GIPC depletion on TrkA and LPA1 signaling will be fully understood only when their specific binding partners and protein interaction networks are established.
Urs et al reported that deletion of the PDZ binding motif of LPA1 did not affect inositol phosphate signaling or the amount of LPA1 receptor that accumulated at the surface of HeLa cells 30 min after ligand stimulation [22]. The lack of effect on receptor accumulation suggests that the PDZ binding motif is not required for internalization of receptor from the surface. Indeed, we and others have previously shown that binding of GIPC to the PDZ motif does not promote internalization of receptors from the surface but rather promotes trafficking of receptors from peripheral signaling endosomes to early endosomes [11], [12], [17]. The association between LPA1 and APPL and the effects of GIPC on LPA1 trafficking further expand the role of GIPC and APPL to regulation of the activity of G-protein coupled receptors.
We found here that following ligand stimulation, LPA1 internalizes and traffics through APPL peripheral endosomes on its way to EEA1 early endosomes. Our results are in keeping with previous findings showing that ligand induced endocytosis of LPA1 is dependent on dynamin2 and Rab5 and that internalized LPA1 traverses the same endocytic pathway as the transferrin receptor in that it passes through sorting endosomes, early (EEA1) endosomes and juxtanuclear recycling endosomes [33]. GIPC is believed to affect receptor trafficking in part by binding to the Rab5 effector APPL [16], [47] and in part by binding to the actin based motor protein myosin VI [12], [48], [49]. We demonstrated that APPL associates with LPA1 complexes and colocalizes with LPA1 in peripheral endosomes. We also found that APPL depletion inhibits Akt signaling in cells expressing LPA1. This is in keeping with previous reports that APPL is required for activation of Akt on endosomes and for cell survival [11], [14]–[16], [50]. It appears that GIPC depletion prolongs LPA1 association with APPL signaling endosomes by delaying LPA1 trafficking to early (EEA1) endosomes, leading to increased Akt signaling and promoting cell proliferation and motility.
Our finding that interaction between GIPC and LPA1 leads to downregulation of Akt signaling has important pathophysiological implications, given 1) that LPA1 has been shown to promote the development of various carcinomas, 2) that mutations in the PDZ binding motif of LPA1 induces oncogenic transformation [1], [42]–[44], [46], [51], [52], and 3) that GIPC plays a tumor suppressor role in breast cancer progression [51], [53]. Whether and how the interaction of these two proteins is abrogated during cancer progression remains unknown.
In summary, the identification of signaling pathways involving GIPC and APPL downstream of LPA1 extend the role of these proteins as regulators of GPCRs and opens exciting directions for investigation. The ability of GIPC to bind LPA1, APPL and myosin VI in a ligand dependent manner positions GIPC as a key target for regulation of LPA1 activities. GIPC was previously shown to interact with additional GPCRs, including the dopamine D2 receptor and the lutropin receptor, but it is not known if APPL also associates with these receptors. Future studies will reveal if GIPC and APPL regulate signaling and trafficking of these and other GPCRs.
Supporting Information
Characterization of HEK-LPA1 cell lines stably expressing FLAG-LPA1. A, Immunoblot of LPA1 from HEK-LPA1 cell lysates demonstrating receptor expression and glycosylation. A prominent broad band at ∼60 kD is seen in HEK-LPA1 cells (Lane 1) but not in HEK-pIRES controls stably expressing empty vector (lane 2). The electrophoretic mobility of FLAG-LPA1 shifts to the predicted theoretical molecular mass (38 kD) following treatment with PNGase-F (Lane 3) which removes N-glycans. The broad mobility and fuzziness of the 38 kD band most likely is due to remaining O-glycans. Lysates from HEK-LPA1 and HEK-pIRES cells were treated with PNGase (lanes 3–4) or sham treated (lanes 1–2), and proteins were immunoblotted with anti-FLAG IgG. B, LPA (0.01–1 µM) induces phosphorylation of Erk and Akt in HEK-LPA1 cells (lanes 2, 4, 6 and 8) but not in HEK-pIRES cells (lanes 1, 3, 5 and 7). HEK-LPA1 and HEK-pIRES cells were serum starved overnight, stimulated with the indicated amounts of LPA in 0.1% fatty acid free BSA for 5 min, lysed and analyzed by immunoblotting for LPA1 (FLAG), pErk, tErk, and pAkt. C, Phase contrast microscopy of HEK-pIRES and HEK-LPA1 cells showing that stable expression of LPA1 induces morphological changes in HEK293 cells. HEK-pIRES controls exhibit elongated processes (arrowheads, left panel) and overall morphology similar to the parental HEK293 cell line whereas HEK-LPA1 cells are flatter, more spread out and have shorter cell processes (right panel).
(TIF)
FLAG-LPA1 and GIPC colocalize at the plasma membrane in HeLa cells. A, Endogenous GIPC (red, in merged image) is widely distributed throughout the cytoplasm and is also concentrated along the plasma membrane whereas LPA1-FLAG (green) is mainly localized at the plasma membrane where it partially colocalizes with GIPC as demonstrated by yellow overlapping pixels (arrowheads, right lower panel). HeLa cells were transfected with FLAG-LPA1 and subsequently serum starved and processed for immunofluorescence using affinity purified rabbit anti-GIPC and mouse anti-FLAG IgG followed by goat anti-rabbit Alexa-593 and goat-anti-mouse Alexa-488 F(ab’)2 and examined with an Olympus FluoView 1000 confocal microscope using a 60X objective.
(TIF)
LPA1 receptor trafficking and its colocalization with GIPC at the PM. Upper panel: In serum starved cells GIPC (green) is concentrated at the plasma membrane were it colocalizes (yellow pixels, arrowheads) with LPA1 (red). Lower panel : At 30 min following stimulation, colocalization of LPA1 with GIPC is greatly diminished. Boxed regions are enlarged (3.2×) in the insets. HEK-LPA1 cells were stimulated with 10 µM LPA, processed for immunofluorescence, and images acquired exactly as for Fig. 2. Bar = 10 µm.
(TIF)
LPA1 traffics through APPL positive endosomes enroute to EEA1 positive early endosomes. Upper panel : 2 min following stimulation with LPA (10 µM), LPA1 (red) colocalizes (arrowheads) with APPL (green) in endocytic vesicles at the cell periphery. 30 min following LPA stimulation, LPA1 appears in internal vesicles and does not colocalize with APPL. Lower panel : 15 and 30 min following stimulation with LPA (10 µM), LPA1 (red) partially colocalizes (arrowheads) with EEA1 (green). Boxed regions are enlarged (3.2×) in the insets. HEK-LPA1 cells were stimulated, processed for immunofluorescence and images acquired as described for Fig. 2. Bar = 10 µm.
(TIF)
LPA1 and GIPC are internalized into clathrin coated vesicles. Upper panels : 3 min after stimulation with LPA (10 µM), LPA1 receptors (red) colocalize (arrowheads) with clathrin (green) on punctate structures at the plasma membrane and in endocytic vesicles immediately below the plasma membrane. Lower panels : GIPC (red) colocalizes (arrowheads) with clathrin (green) at the plasma membrane and on endocytic vesicles at 3 min after LPA stimulation. Boxed regions are enlarged (2.3×) in the insets. HEK-LPA1 cells were stimulated, processed for immunofluorescence and images acquired exactly as described for Fig. 2. Bar = 10 µm.
(TIF)
Acknowledgments
We thank Dr. Jerold Chun for the FLAG-LPA1 construct, Dr. Richard Klemke for assistance with the cell migration assay, and Drs. Gordon Gill and Pradipta Ghosh for helpful suggestions. We also thank Karen Sykes for assistance with cell culture and the UCSD School of Medicine Light Microscopy Facility and its director, Jennifer Meerloo for help with the confocal microscopy.
Funding Statement
This work was supported by National Institutes of Health (NIH) grants CA100768 and DK17780 to M.G.F. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Liu S, Murph M, Panupinthu N, Mills GB (2009) ATX-LPA receptor axis in inflammation and cancer. Cell Cycle 8: 3695–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Noguchi K, Herr D, Mutoh T, Chun J (2009) Lysophosphatidic acid (LPA) and its receptors. Curr Opin Pharmacol 9: 15–23. [DOI] [PubMed] [Google Scholar]
- 3. Lin FT, Lai YJ (2008) Regulation of the LPA2 receptor signaling through the carboxyl-terminal tail-mediated protein-protein interactions. Biochim Biophys Acta 1781: 558–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ishii S, Noguchi K, Yanagida K (2009) Non-Edg family lysophosphatidic acid (LPA) receptors. Prostaglandins Other Lipid Mediat 89: 57–65. [DOI] [PubMed] [Google Scholar]
- 5. Liu TF, Kandala G, Setaluri V (2001) PDZ domain protein GIPC interacts with the cytoplasmic tail of melanosomal membrane protein gp75 (tyrosinase-related protein-1). J Biol Chem 276: 35768–77. [DOI] [PubMed] [Google Scholar]
- 6. Wang LH, Kalb RG, Strittmatter SM (1999) A PDZ protein regulates the distribution of the transmembrane semaphorin, M-SemF. J Biol Chem 274: 14137–46. [DOI] [PubMed] [Google Scholar]
- 7. De Vries L, Lou X, Zhao G, Zheng B, Farquhar MG (1998) GIPC, a PDZ domain containing protein, interacts specifically with the C terminus of RGS-GAIP. Proc Natl Acad Sci U S A 95: 12340–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chittenden TW, Claes F, Lanahan AA, Autiero M, Palac RT, et al. (2006) Selective regulation of arterial branching morphogenesis by synectin. Dev Cell 10: 783–95. [DOI] [PubMed] [Google Scholar]
- 9. Choi JS, Paek AR, Kim SY, You HJ (2010) GIPC mediates the generation of reactive oxygen species and the regulation of cancer cell proliferation by insulin-like growth factor-1/IGF-1R signaling. Cancer Lett 294: 254–63. [DOI] [PubMed] [Google Scholar]
- 10. Lou X, Yano H, Lee F, Chao MV, Farquhar MG (2001) GIPC and GAIP form a complex with TrkA: a putative link between G protein and receptor tyrosine kinase pathways. Mol Biol Cell 12: 615–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Varsano T, Dong MQ, Niesman I, Gacula H, Lou X, et al. (2006) GIPC is recruited by APPL to peripheral TrkA endosomes and regulates TrkA trafficking and signaling. Mol Cell Biol 26: 8942–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Naccache SN, Hasson T, Horowitz A (2006) Binding of internalized receptors to the PDZ domain of GIPC/synectin recruits myosin VI to endocytic vesicles. Proc Natl Acad Sci U S A 103: 12735–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lin DC, Quevedo C, Brewer NE, Bell A, Testa JR, et al. (2006) APPL1 associates with TrkA and GIPC1 and is required for nerve growth factor-mediated signal transduction. Mol Cell Biol 26: 8928–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Schenck A, Goto-Silva L, Collinet C, Rhinn M, Giner A, et al. (2008) The endosomal protein Appl1 mediates Akt substrate specificity and cell survival in vertebrate development. Cell 133: 486–97. [DOI] [PubMed] [Google Scholar]
- 15. Tan Y, You H, Wu C, Altomare DA, Testa JR (2010) Appl1 is dispensable for mouse development, and loss of Appl1 has growth factor-selective effects on Akt signaling in murine embryonic fibroblasts. J Biol Chem 285: 6377–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zoncu R, Perera RM, Balkin DM, Pirruccello M, Toomre D, et al. (2009) A phosphoinositide switch controls the maturation and signaling properties of APPL endosomes. Cell 136: 1110–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lanahan AA, Hermans K, Claes F, Kerley-Hamilton JS, Zhuang ZW, et al. (2010) VEGF receptor 2 endocytic trafficking regulates arterial morphogenesis. Dev Cell 18: 713–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hirakawa T, Galet C, Kishi M, Ascoli M (2003) GIPC binds to the human lutropin receptor (hLHR) through an unusual PDZ domain binding motif, and it regulates the sorting of the internalized human choriogonadotropin and the density of cell surface hLHR. J Biol Chem 278: 49348–57. [DOI] [PubMed] [Google Scholar]
- 19. Jeanneteau F, Diaz J, Sokoloff P, Griffon N (2004) Interactions of GIPC with dopamine D2, D3 but not D4 receptors define a novel mode of regulation of G protein-coupled receptors. Mol Biol Cell 15: 696–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Murph MM, Scaccia LA, Volpicelli LA, Radhakrishna H (2003) Agonist-induced endocytosis of lysophosphatidic acid-coupled LPA1/EDG-2 receptors via a dynamin2- and Rab5-dependent pathway. J Cell Sci 116: 1969–80. [DOI] [PubMed] [Google Scholar]
- 21. Urs NM, Kowalczyk AP, Radhakrishna H (2008) Different mechanisms regulate lysophosphatidic acid (LPA)-dependent versus phorbol ester-dependent internalization of the LPA1 receptor. J Biol Chem 283: 5249–57. [DOI] [PubMed] [Google Scholar]
- 22. Urs NM, Jones KT, Salo PD, Severin JE, Trejo J, et al. (2005) A requirement for membrane cholesterol in the beta-arrestin- and clathrin-dependent endocytosis of LPA1 lysophosphatidic acid receptors. J Cell Sci 118: 5291–304. [DOI] [PubMed] [Google Scholar]
- 23. Ishii I, Contos JJ, Fukushima N, Chun J (2000) Functional comparisons of the lysophosphatidic acid receptors, LP(A1)/VZG-1/EDG-2, LP(A2)/EDG-4, and LP(A3)/EDG-7 in neuronal cell lines using a retrovirus expression system. Mol Pharmacol 58: 895–902. [DOI] [PubMed] [Google Scholar]
- 24. Mitsuuchi Y, Johnson SW, Sonoda G, Tanno S, Golemis EA, et al. (1999) Identification of a chromosome 3p14.3–21.1 gene, APPL, encoding an adaptor molecule that interacts with the oncoprotein-serine/threonine kinase Akt2. Oncogene 18: 4891–8. [DOI] [PubMed] [Google Scholar]
- 25. Klemke RL, Leng J, Molander R, Brooks PC, Vuori K, et al. (1998) CAS/Crk coupling serves as a “molecular switch” for induction of cell migration. J Cell Biol 140: 961–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Akiyama SK (2002) Functional analysis of cell adhesion: quantitation of cell-matrix attachment. Methods Cell Biol 69: 281–96. [DOI] [PubMed] [Google Scholar]
- 27. Alderton F, Sambi B, Tate R, Pyne NJ, Pyne S (2001) Assessment of agonism at G-protein coupled receptors by phosphatidic acid and lysophosphatidic acid in human embryonic kidney 293 cells. Br J Pharmacol 134: 6–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. De Vries L, Mousli M, Wurmser A, Farquhar MG (1995) GAIP, a protein that specifically interacts with the trimeric G protein G alpha i3, is a member of a protein family with a highly conserved core domain. Proc Natl Acad Sci U S A 92: 11916–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ohta H, Sato K, Murata N, Damirin A, Malchinkhuu E, et al. (2003) Ki16425, a subtype-selective antagonist for EDG-family lysophosphatidic acid receptors. Mol Pharmacol 64: 994–1005. [DOI] [PubMed] [Google Scholar]
- 30. Hama K, Aoki J, Fukaya M, Kishi Y, Sakai T, et al. (2004) Lysophosphatidic acid and autotaxin stimulate cell motility of neoplastic and non-neoplastic cells through LPA1. J Biol Chem 279: 17634–9. [DOI] [PubMed] [Google Scholar]
- 31. Shida D, Kitayama J, Yamaguchi H, Okaji Y, Tsuno NH, et al. (2003) Lysophosphatidic acid (LPA) enhances the metastatic potential of human colon carcinoma DLD1 cells through LPA1. Cancer Res 63: 1706–11. [PubMed] [Google Scholar]
- 32. Yamada T, Sato K, Komachi M, Malchinkhuu E, Tobo M, et al. (2004) Lysophosphatidic acid (LPA) in malignant ascites stimulates motility of human pancreatic cancer cells through LPA1. J Biol Chem 279: 6595–605. [DOI] [PubMed] [Google Scholar]
- 33. Murph MM, Nguyen GH, Radhakrishna H, Mills GB (2008) Sharpening the edges of understanding the structure/function of the LPA1 receptor: expression in cancer and mechanisms of regulation. Biochim Biophys Acta 1781: 547–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yano H, Ninan I, Zhang H, Milner TA, Arancio O, et al. (2006) BDNF-mediated neurotransmission relies upon a myosin VI motor complex. Nat Neurosci 9: 1009–18. [DOI] [PubMed] [Google Scholar]
- 35. Yamada T, Yano S, Ogino H, Ikuta K, Kakiuchi S, et al. (2008) Lysophosphatidic acid stimulates the proliferation and motility of malignant pleural mesothelioma cells through lysophosphatidic acid receptors, LPA1 and LPA2. Cancer Sci 99: 1603–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Muders MH, Dutta SK, Wang L, Lau JS, Bhattacharya R, et al. (2006) Expression and regulatory role of GAIP-interacting protein GIPC in pancreatic adenocarcinoma. Cancer Res 66: 10264–8. [DOI] [PubMed] [Google Scholar]
- 37. Lee NY, Ray B, How T, Blobe GC (2008) Endoglin promotes transforming growth factor beta-mediated Smad 1/5/8 signaling and inhibits endothelial cell migration through its association with GIPC. J Biol Chem 283: 32527–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gao Y, Li M, Chen W, Simons M (2000) Synectin, syndecan-4 cytoplasmic domain binding PDZ protein, inhibits cell migration. J Cell Physiol 184: 373–9. [DOI] [PubMed] [Google Scholar]
- 39. Weiner JA, Chun J (1999) Schwann cell survival mediated by the signaling phospholipid lysophosphatidic acid. Proc Natl Acad Sci U S A 96: 5233–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Contos JJ, Ishii I, Chun J (2000) Lysophosphatidic acid receptors. Mol Pharmacol 58: 1188–96. [DOI] [PubMed] [Google Scholar]
- 41. Kingsbury MA, Rehen SK, Contos JJ, Higgins CM, Chun J (2003) Non-proliferative effects of lysophosphatidic acid enhance cortical growth and folding. Nat Neurosci 6: 1292–9. [DOI] [PubMed] [Google Scholar]
- 42. Hu LA, Chen W, Martin NP, Whalen EJ, Premont RT, et al. (2003) GIPC interacts with the beta1-adrenergic receptor and regulates beta1-adrenergic receptor-mediated ERK activation. J Biol Chem 278: 26295–301. [DOI] [PubMed] [Google Scholar]
- 43. Li C, Dandridge KS, Di A, Marrs KL, Harris EL, et al. (2005) Lysophosphatidic acid inhibits cholera toxin-induced secretory diarrhea through CFTR-dependent protein interactions. J Exp Med 202: 975–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Zhang H, Wang D, Sun H, Hall RA, Yun CC (2007) MAGI-3 regulates LPA-induced activation of Erk and RhoA. Cell Signal 19: 261–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Yamada T, Ohoka Y, Kogo M, Inagaki S (2005) Physical and functional interactions of the lysophosphatidic acid receptors with PDZ domain-containing Rho guanine nucleotide exchange factors (RhoGEFs). J Biol Chem 280: 19358–63. [DOI] [PubMed] [Google Scholar]
- 46. Shano S, Hatanaka K, Ninose S, Moriyama R, Tsujiuchi T, et al. (2008) A lysophosphatidic acid receptor lacking the PDZ-binding domain is constitutively active and stimulates cell proliferation. Biochim Biophys Acta 1783: 748–59. [DOI] [PubMed] [Google Scholar]
- 47. Chial HJ, Wu R, Ustach CV, McPhail LC, Mobley WC, et al. (2008) Membrane targeting by APPL1 and APPL2: dynamic scaffolds that oligomerize and bind phosphoinositides. Traffic 9: 215–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Dance AL, Miller M, Seragaki S, Aryal P, White B, et al. (2004) Regulation of myosin-VI targeting to endocytic compartments. Traffic 5: 798–813. [DOI] [PubMed] [Google Scholar]
- 49. Reed BC, Cefalu C, Bellaire BH, Cardelli JA, Louis T, et al. (2005) GLUT1CBP(TIP2/GIPC1) interactions with GLUT1 and myosin VI: evidence supporting an adapter function for GLUT1CBP. Mol Biol Cell 16: 4183–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Thomas RM, Nechamen CA, Mazurkiewicz JE, Ulloa-Aguirre A, Dias JA (2011) The Adapter Protein APPL1 Links FSH Receptor to Inositol 1,4,5-Trisphosphate Production and Is Implicated in Intracellular Ca2+ Mobilization. Endocrinology. [DOI] [PMC free article] [PubMed]
- 51. Blobe GC, Liu X, Fang SJ, How T, Lodish HF (2001) A novel mechanism for regulating transforming growth factor beta (TGF-beta) signaling. Functional modulation of type III TGF-beta receptor expression through interaction with the PDZ domain protein, GIPC. J Biol Chem 276: 39608–17. [DOI] [PubMed] [Google Scholar]
- 52. Gotthardt M, Trommsdorff M, Nevitt MF, Shelton J, Richardson JA, et al. (2000) Interactions of the low density lipoprotein receptor gene family with cytosolic adaptor and scaffold proteins suggest diverse biological functions in cellular communication and signal transduction. J Biol Chem 275: 25616–24. [DOI] [PubMed] [Google Scholar]
- 53. Lee JD, Hempel N, Lee NY, Blobe GC (2010) The type III TGF-beta receptor suppresses breast cancer progression through GIPC-mediated inhibition of TGF-beta signaling. Carcinogenesis 31: 175–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Characterization of HEK-LPA1 cell lines stably expressing FLAG-LPA1. A, Immunoblot of LPA1 from HEK-LPA1 cell lysates demonstrating receptor expression and glycosylation. A prominent broad band at ∼60 kD is seen in HEK-LPA1 cells (Lane 1) but not in HEK-pIRES controls stably expressing empty vector (lane 2). The electrophoretic mobility of FLAG-LPA1 shifts to the predicted theoretical molecular mass (38 kD) following treatment with PNGase-F (Lane 3) which removes N-glycans. The broad mobility and fuzziness of the 38 kD band most likely is due to remaining O-glycans. Lysates from HEK-LPA1 and HEK-pIRES cells were treated with PNGase (lanes 3–4) or sham treated (lanes 1–2), and proteins were immunoblotted with anti-FLAG IgG. B, LPA (0.01–1 µM) induces phosphorylation of Erk and Akt in HEK-LPA1 cells (lanes 2, 4, 6 and 8) but not in HEK-pIRES cells (lanes 1, 3, 5 and 7). HEK-LPA1 and HEK-pIRES cells were serum starved overnight, stimulated with the indicated amounts of LPA in 0.1% fatty acid free BSA for 5 min, lysed and analyzed by immunoblotting for LPA1 (FLAG), pErk, tErk, and pAkt. C, Phase contrast microscopy of HEK-pIRES and HEK-LPA1 cells showing that stable expression of LPA1 induces morphological changes in HEK293 cells. HEK-pIRES controls exhibit elongated processes (arrowheads, left panel) and overall morphology similar to the parental HEK293 cell line whereas HEK-LPA1 cells are flatter, more spread out and have shorter cell processes (right panel).
(TIF)
FLAG-LPA1 and GIPC colocalize at the plasma membrane in HeLa cells. A, Endogenous GIPC (red, in merged image) is widely distributed throughout the cytoplasm and is also concentrated along the plasma membrane whereas LPA1-FLAG (green) is mainly localized at the plasma membrane where it partially colocalizes with GIPC as demonstrated by yellow overlapping pixels (arrowheads, right lower panel). HeLa cells were transfected with FLAG-LPA1 and subsequently serum starved and processed for immunofluorescence using affinity purified rabbit anti-GIPC and mouse anti-FLAG IgG followed by goat anti-rabbit Alexa-593 and goat-anti-mouse Alexa-488 F(ab’)2 and examined with an Olympus FluoView 1000 confocal microscope using a 60X objective.
(TIF)
LPA1 receptor trafficking and its colocalization with GIPC at the PM. Upper panel: In serum starved cells GIPC (green) is concentrated at the plasma membrane were it colocalizes (yellow pixels, arrowheads) with LPA1 (red). Lower panel : At 30 min following stimulation, colocalization of LPA1 with GIPC is greatly diminished. Boxed regions are enlarged (3.2×) in the insets. HEK-LPA1 cells were stimulated with 10 µM LPA, processed for immunofluorescence, and images acquired exactly as for Fig. 2. Bar = 10 µm.
(TIF)
LPA1 traffics through APPL positive endosomes enroute to EEA1 positive early endosomes. Upper panel : 2 min following stimulation with LPA (10 µM), LPA1 (red) colocalizes (arrowheads) with APPL (green) in endocytic vesicles at the cell periphery. 30 min following LPA stimulation, LPA1 appears in internal vesicles and does not colocalize with APPL. Lower panel : 15 and 30 min following stimulation with LPA (10 µM), LPA1 (red) partially colocalizes (arrowheads) with EEA1 (green). Boxed regions are enlarged (3.2×) in the insets. HEK-LPA1 cells were stimulated, processed for immunofluorescence and images acquired as described for Fig. 2. Bar = 10 µm.
(TIF)
LPA1 and GIPC are internalized into clathrin coated vesicles. Upper panels : 3 min after stimulation with LPA (10 µM), LPA1 receptors (red) colocalize (arrowheads) with clathrin (green) on punctate structures at the plasma membrane and in endocytic vesicles immediately below the plasma membrane. Lower panels : GIPC (red) colocalizes (arrowheads) with clathrin (green) at the plasma membrane and on endocytic vesicles at 3 min after LPA stimulation. Boxed regions are enlarged (2.3×) in the insets. HEK-LPA1 cells were stimulated, processed for immunofluorescence and images acquired exactly as described for Fig. 2. Bar = 10 µm.
(TIF)