Abstract
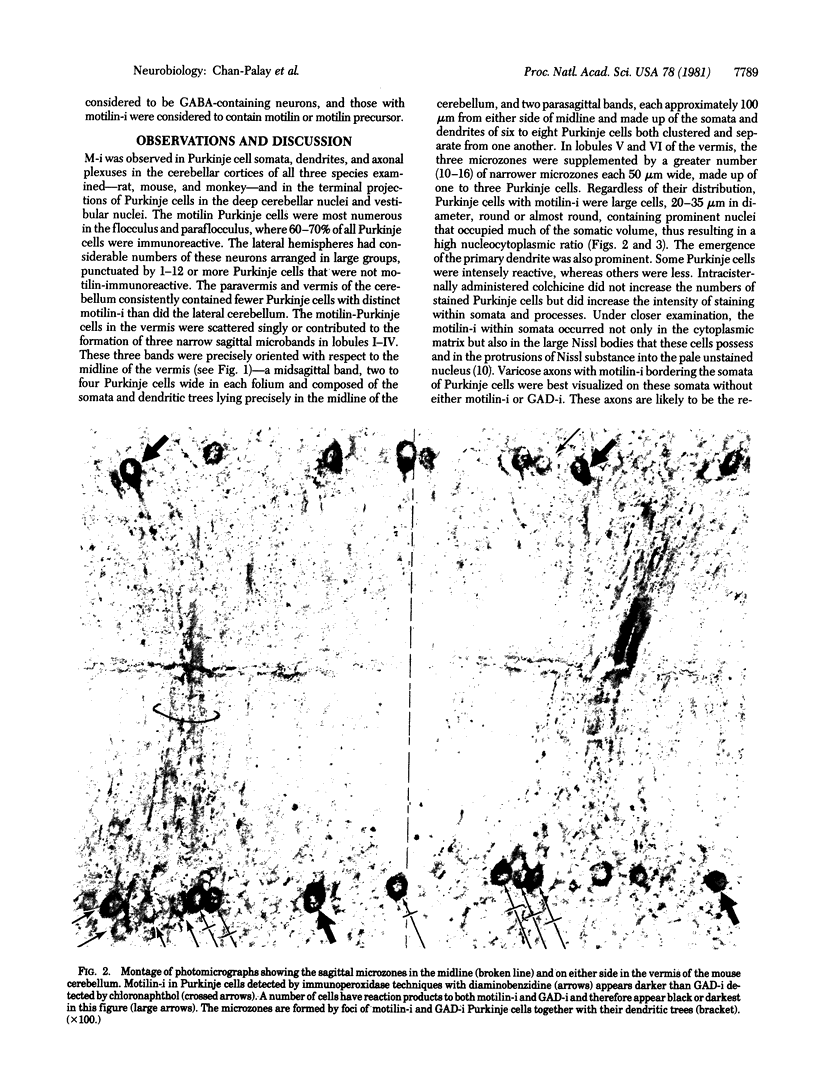
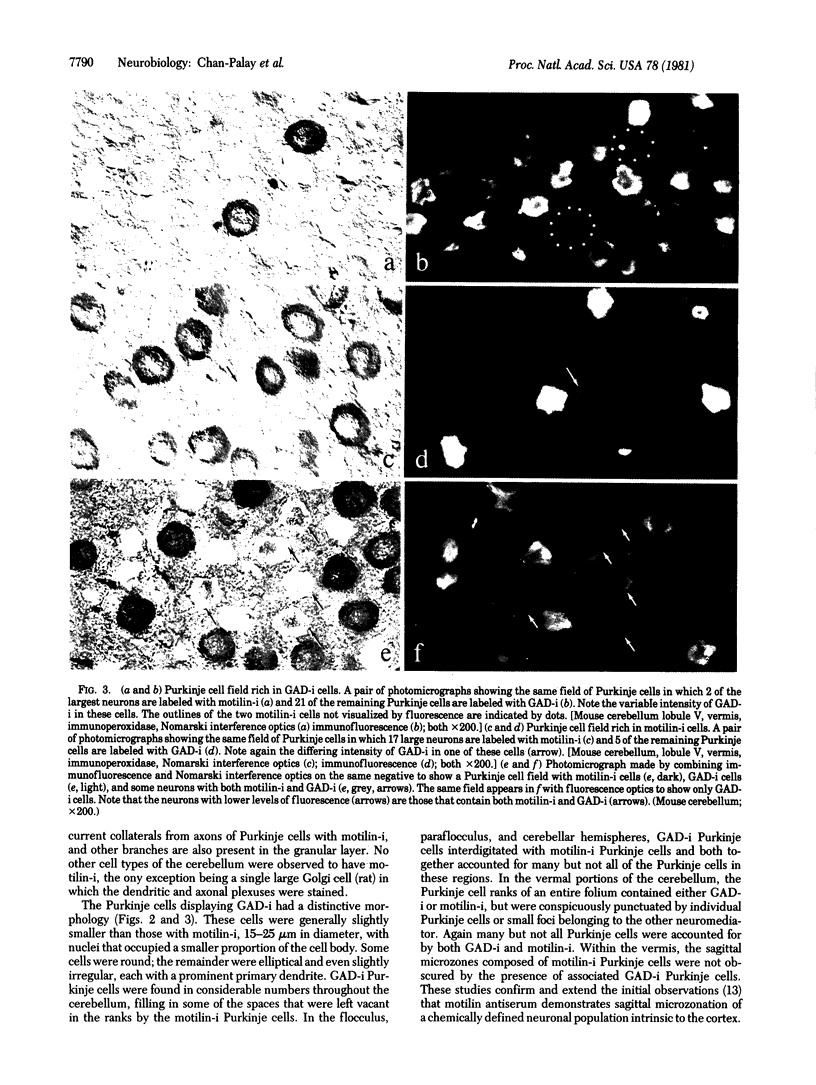
Purkinje neurons of the cerebellar cortex from a chemically and morphologically heterogeneous population containing some members that have gamma-aminobutyric acid (GABA), others that have immunoreactivity for motilin, and a small number that have both. The remaining 30-40% of all Purkinje cells have neither of these two neuroactive substances, leaving possibilities for other transmitter candidates. The evidence was compiled from double-staining immunocytochemical procedures performed on single sections of the cerebellum and brain stem in rat, mouse, and monkey. Two polyclonal antibodies were applied in succession, one directed against the midregion and COOH terminus of the 22-amino acid polypeptide motilin and the other against glutamic acid decarboxylase (glutamate decarboxylase; L-glutamate 1-carboxy-lyase, EC 4.1.1.15), the rate-limiting enzyme in the synthesis of the neurotransmitter GABA. The staining combinations employed the immunoperoxidase method, with different chromogens for distinguishing the motilin-like immunoreactivity from glutamic acid decarboxylase immunoreactivity by different colors, or the immunoperoxidase method for one antiserum and immunofluorescence for the other. The locations of both motilin and GABA cell types were mapped. The recognition of motilin in Purkinje cells calls for experimental definition of the role of this substance in the cerebellum and for reevaluation of the roles of Purkinje cells and of GABA in cerebellar function. The significant motilin representation in the flocculus, paraflocculus, and vermis suggests that it may be the Purkinje cell mediative chemical in the vestibular parts of the cerebellum. However, the presence of GABA as well in the same regions indicates that the chemical preference may be at least bimodal.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avrameas S., Ternynck T. The cross-linking of proteins with glutaraldehyde and its use for the preparation of immunoadsorbents. Immunochemistry. 1969 Jan;6(1):53–66. doi: 10.1016/0019-2791(69)90178-5. [DOI] [PubMed] [Google Scholar]
- Brown J. C., Mutt V., Dryburgh J. R. The further purification of motilin, a gastric motor activity stimulating polypeptide from the mucosa of the small intestine of hogs. Can J Physiol Pharmacol. 1971 May;49(5):399–405. doi: 10.1139/y71-047. [DOI] [PubMed] [Google Scholar]
- Brown J. C. Presence of a gastric motor-stimulating property in duodenal extracts. Gastroenterology. 1967 Feb;52(2):225–229. [PubMed] [Google Scholar]
- COONS A. H. Fluorescent antibody methods. Gen Cytochem Methods. 1958;1:399–422. [PubMed] [Google Scholar]
- Chan-Palay V. Autoradiographic localization of gamma-aminobutyric acid receptors in the rat central nervous system by using [3H]muscimol. Proc Natl Acad Sci U S A. 1978 Feb;75(2):1024–1028. doi: 10.1073/pnas.75.2.1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan-Palay V. Combined immunocytochemistry and autoradiography after in vivo injections of monoclonal antibody to substance P and 3H-serotonin: Coexistence of two putative transmitters in single raphe cells and fiber plexuses. Anat Embryol (Berl) 1979 Jul 26;156(3):241–254. doi: 10.1007/BF00299625. [DOI] [PubMed] [Google Scholar]
- Chan-Palay V., Palay S. L., Brown J. T., Van Itallie C. Sagittal organization of olivocerebellar and reticulocerebellar projections: autoradiographic studies with 35S-methionine. Exp Brain Res. 1977 Dec 19;30(4):561–576. doi: 10.1007/BF00237645. [DOI] [PubMed] [Google Scholar]
- Chan-Palay V., Palay S. L. Ultrastructural localization of gamma-aminobutyric acid receptors in the mammalian central nervous system by means of [3H]muscimol binding. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2977–2980. doi: 10.1073/pnas.75.6.2977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan-Palay V., Palay S. L., Wu J. Y. Gamma-aminobutyric acid pathways in the cerebellum studied by retrograde and anterograde transport of glutamic acid decarboxylase antibody after in vivo injections. Anat Embryol (Berl) 1979;157(1):1–14. doi: 10.1007/BF00315638. [DOI] [PubMed] [Google Scholar]
- Chan-Palay V., Wu J. Y., Palay S. L. Immunocytochemical localization of gamma-aminobutyric acid transaminase at cellular and ultrastructural levels. Proc Natl Acad Sci U S A. 1979 Apr;76(4):2067–2071. doi: 10.1073/pnas.76.4.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christofides N. D., Modlin I. M., Fitzpatrick M. L., Bloom S. R. Effect of motilin on the rate of gastric emptying and gut hormone release during breakfast. Gastroenterology. 1979 May;76(5 Pt 1):903–907. [PubMed] [Google Scholar]
- Dahlström A. Effect of colchicine on transport of amine storage granules in sympathetic nerves of rat. Eur J Pharmacol. 1968 Dec;5(1):111–113. doi: 10.1016/0014-2999(68)90165-9. [DOI] [PubMed] [Google Scholar]
- Ljungdahl A., Seiger A., Hökfelt T., Olson L. (3H)GABA uptake in growing cerebellar tissue: autoradiography of intraocular transplants. Brain Res. 1973 Oct 26;61:379–384. doi: 10.1016/0006-8993(73)90542-8. [DOI] [PubMed] [Google Scholar]
- McLaughlin B. J., Wood J. G., Saito K., Barber R., Vaughn J. E., Roberts E., Wu J. Y. The fine structural localization of glutamate decarboxylase in synaptic terminals of rodent cerebellum. Brain Res. 1974 Aug 23;76(3):377–391. doi: 10.1016/0006-8993(74)90815-4. [DOI] [PubMed] [Google Scholar]
- McLaughlin B. J., Wood J. G., Saito K., Roberts E., Wu J. Y. The fine structural localization of glutamate decarboxylase in developing axonal processes and presynaptic terminals of rodent cerebellum. Brain Res. 1975 Mar 7;85(3):355–371. doi: 10.1016/0006-8993(75)90813-6. [DOI] [PubMed] [Google Scholar]
- Nakane P. K. Simultaneous localization of multiple tissue antigens using the peroxidase-labeled antibody method: a study on pituitary glands of the rat. J Histochem Cytochem. 1968 Sep;16(9):557–560. doi: 10.1177/16.9.557. [DOI] [PubMed] [Google Scholar]
- Phillis J. W., Kirkpatrick J. R. Motilin excites neurons in the cerebral cortex and spinal cord. Eur J Pharmacol. 1979 Oct 15;58(4):469–472. doi: 10.1016/0014-2999(79)90318-2. [DOI] [PubMed] [Google Scholar]