Abstract
Alzheimer's disease (AD) is the most common progressive neurodegenerative disorder causing dementia. Massive deposition of amyloid β peptide (Aβ) as senile plaques in the brain is the pathological hallmark of AD, but oligomeric, soluble forms of Aβ have been implicated as the synaptotoxic component. The apolipoprotein E ε 4 (apoE ε4) allele is known to be a genetic risk factor for developing AD. However, it is still unknown how apoE impacts the process of Aβ oligomerization. Here, we found that the level of Aβ oligomers in APOE ε4/ε4 AD patient brains is 2.7 times higher than those in APOE ε3/ε3 AD patient brains, matched for total plaque burden, suggesting that apoE4 impacts the metabolism of Aβ oligomers. To test this hypothesis, we examined the effect of apoE on Aβ oligomer formation. Using both synthetic Aβ and a split-luciferase method for monitoring Aβ oligomers, we observed that apoE increased the level of Aβ oligomers in an isoform-dependent manner (E2 < E3 < E4). This effect appears to be dependent on the ApoE C-terminal domain. Moreover, these results were confirmed using endogenous apoE isolated from the TBS-soluble fraction of human brain, which increased the formation of Aβ oligomers. Together, these data show that lipidated apoE, especially apoE4, increases Aβ oligomers in the brain. Higher levels of Aβ oligomers in the brains of APOE ε4/ε4 carriers compared with APOE ε3/ε3 carriers may increase the loss of dendritic spines and accelerate memory impairments, leading to earlier cognitive decline in AD.
Introduction
The hallmark of Alzheimer's disease (AD) is deposition of fibrillar amyloid β peptide (Aβ) in senile plaques in the brain (Selkoe, 2001; Holtzman et al., 2011). Recent data, however, suggest that instead of, or in addition to, plaques, soluble oligomeric forms of Aβ are crucial for synaptic dysfunction, cognitive impairment and neurodegeneration (Lesné et al., 2006; Shankar et al., 2008; Koffie et al., 2009; Wu et al., 2010). However, it is still unknown what factors influence the kinetics of formation of soluble Aβ oligomers.
Apolipoprotein E (apoE) is a known Aβ binding protein and is a genetic risk factor for AD (Bu, 2009). There are 3 common alleles of the human APOE gene ε2, ε3, and ε4 (Zannis et al., 1982). Inheritance of two copies of the APOE ε4 allele is associated with a >10-fold increased risk for developing AD compared with the most common APOE ε3/ε3 genotype (Corder et al., 1993; Strittmatter et al., 1993a). However, the mechanisms whereby APOE ε4 promotes the development of AD remain controversial. There is strong evidence that apoE, and particularly apoE4, facilitates amyloid fibril deposition. Senile plaque density in the brains of APOE ε4/ε4 carrier AD patients is significantly higher than that in the brains of APOE ε3/ε3 carrier AD patients (Rebeck et al., 1993; Gomez-Isla et al., 1996). apoE-deficient mice crossed with APP (amyloid precursor protein)-transgenic mice exhibited a decrease in amyloid deposition in the brain, and human apoE4 overexpression increased fibrillar Aβ deposits compared with human apoE3 (Holtzman et al., 2000; Fagan et al., 2002). Moreover, recent studies support a role of apoE in Aβ metabolism. Using an in vivo microdialysis technique, the half-life of Aβ in the brain of apoE knock-out mice markedly decreased compared with wild-type mice (DeMattos et al., 2004). Importantly, it has now been shown that in APP-transgenic mice expressing apoE2, E3, and E4, apoE4 significantly slows Aβ clearance relative to E2 and E3 but has no effect on Aβ synthesis (Castellano et al., 2011).
Previous studies have revealed that apoE interacts with Aβ in vitro (Strittmatter et al., 1993b; LaDu et al., 1994) and in vivo, both in the CSF (Strittmatter et al., 1993a) and in the brain (Näslund et al., 1995). However, because of the difficulty in monitoring Aβ oligomers specifically and quantitatively, whether apoE increases the levels of native Aβ oligomers remains unknown. In this study we hypothesize that apoE, especially apoE4, impacts the formation of Aβ oligomers. We observed significantly increased levels of Aβ oligomers in APOE ε4/ε4 AD patient brains compared with APOE ε2/εx or APOE ε3/ε3 AD patient brains. We also found that apoE4 enhances the level of Aβ oligomers, using a split-luciferase complementation assay that enables quantitative monitoring of the formation of Aβ oligomers. Furthermore, endogenous apoE from human brain also increased the level of Aβ oligomers in vitro. These data suggest that apoE, especially apoE4, impacts Aβ oligomer levels by enhancing their formation and stabilizing them once formed.
Materials and Methods
Brain extraction and gel filtration.
Brains from human subjects with a diagnosis of Alzheimer's disease or no cognitive deficits were obtained through the Massachusetts Alzheimer's Disease Research Center. Cases were selected to have equivalent amyloid load (Ingelsson et al., 2004) and to be either APOE ε2/εx, APOE ε3/ε3, or APOE ε4/ε4; these data were not revealed during subsequent biochemical assays. The case number is 8 in control (4 males and 4 females), 6 in APOE ε2/εx AD (6 females), 10 in APOE ε3/ε3 AD (4 males and 6 females) or 10 in APOE ε4/ε4 AD (5 males and 5 females). Cortical gray matter from frontal lobe of AD patient brains or non-demented control brains was homogenized in 5 volumes of TBSI (Tris-buffered saline with Protease Inhibitor Cocktail; Roche) with 25 strokes on a mechanical Dounce homogenizer and centrifuged at 260,000 × g for 30 min at 4°C. The supernatant was used as a TBS-soluble fraction (Hashimoto et al., 2002). TBS-soluble fraction (750 μl) of human brains was separated by size-exclusion chromatography (SEC) on single or double Superdex 75 columns (GE Healthcare) in 50 mm ammonium acetate, pH 8.5, with an AKTA purifier 10 (GE Healthcare; Townsend et al., 2006). Conditioned medium from HEK293 cells (750 μl) was separated by size exclusion chromatography on a Superdex 200 column (GE Healthcare) in 50 mm ammonium acetate, pH 8.5, with an AKTA purifier 10 (GE Healthcare). The individual fractions separated by SEC were analyzed by immunoblotting and Aβ-specific sandwich ELISA.
cDNA plasmids.
Human ApoE2, ApoE3, and ApoE4 genes were a gift from Dr. Mary Jo LaDu at University of Illinois at Chicago. The human ApoA-I gene was purchased from the full-length mammalian gene collection (Invitrogen) and subcloned into pcDNA3.1 vector (Invitrogen) between HindIII site and BamHI site using the following primers: 5′-GAGAAGAAGCTTCCCCACGGCCCTTCAGG-3′ (forward), 5′-ATTCTGGGATCCGGGAAGGGGGGCGGCGG-3′ (reverse). The human ApoA-II gene was also purchased from the full-length mammalian gene collection and subcloned into pcDNA3.1 vector between HindIII site and BamHI site using the following primers: 5′-ACAGAGAAGCTTGCTAGGCCGCCCTCCCC-3′ (forward), 5′-GGGACAGGATCCCTAGGACTGGCCAGTGGG-3′ (reverse). The human ApoJ/clusterin gene was purchased from the full-length mammalian gene collection and subcloned into pcDNA3.1 vector between HindIII site and BamHI site using the following primers: 5′-TGACCGAAGCTTGCAAAGACTCCAGAATTGG-3′ (forward), 5′-AGTGCAGGATCCAGAGCGGGGAGAGG-3′ (reverse). For the apoE4 R61T mutant, we mutated Arg61 of apoE4 cDNA plasmid into Thr by in vitro site-directed mutagenesis using the following primers: 5′-CACCCAGGAGCTCACGGCGCTGATGG-3′ (forward), 5′-CCATCAGCGCCGTGAGCTCCTGGGTG-3′ (reverse). For the N-terminal fragments of apoE (apoE2 NTF, apoE3 NTF, and apoE4 NTF), we deleted apoE192–299 from the apoE2, apoE3, and apoE4 cDNA plasmids, respectively, using the following primers: 5′-TGAACGCCGAAGCCTGCAGCCATGCG-3′ (apoE1–191 forward), 5′-CCGCACGCGGCCCTGTTCCACCAGGGG-3′ (apoE1–191 reverse). For the C-terminal fragment of apoE (apoE CTF), we deleted apoE1–191 from the apoE2 cDNA plasmid using the following primers: 5′-GCCGCCACTGTGGGCTCCCTGGCC-3′ (apoE192–299 forward), 5′-CTTGGCCTGGCATCCTGCCAGGAATGTG-3′ (apoE192–299 reverse). The apoE signal sequence was retained before the apoE CTF. For the apoE231–299, we deleted apoE192–230 from the apoE CTF cDNA plasmid using the following primers: 5′-GAGGTGAAGGAGCAGGTGGCGGAGG-3′ (apoE231–299 forward) and apoE192–299 reverse primer. For apoE243–299, we deleted apoE192–242 from the apoE CTF cDNA plasmid using the following primers: 5′-CTGGAGGAGCAGGCCCAGCAGATACGCC-3′ (apoE243–299 forward) and apoE192–299 reverse primer. For apoE192–272, we deleted apoE273–299 from the apoE CTF cDNA plasmid using the following primers: apoE1–191 forward primer and 5′-CATGTCTTCCACCAGGGGCTCGAACC-3′ (apoE192–272 reverse). For apoE192–242, we deleted apoE243–299 from the apoE CTF cDNA plasmid using the following primers: apoE1–191 forward primer and 5′-CTTGGCGCGCACCTCCGCCACCTGC-3′ (apoE192–242 reverse). For apoE3 Δ243–272, we deleted apoE243–272 from the apoE3 cDNA plasmid using the following primers: 5′-CAGCGCCAGTGGGCCGGGCTGGTGG-3′ (apoE273–299 forward) and apoE192–242 reverse primer. For apoE3Δ273–299, we deleted apoE273–299 from the apoE3 cDNA plasmid using the following primers: apoE1–191 forward primer and apoE192–272 reverse primer. For apoE3Δ243–299, we deleted apoE243–299 from the apoE3 cDNA plasmid using the following primers: apoE1–191 forward primer and apoE192–242 reverse primer.
Cell culture and transient transfection.
Both N-terminal and C-terminal fragments of split-luciferase-tagged Aβ stably overexpressing HEK293 cells (doubly expressing HEK293 cells) were generated previously (Hashimoto et al., 2011). Doubly expressing HEK293 cells were cultured in Opti-MEM (Invitrogen) with 10% fetal bovine serum at 37°C in 5% CO2 atmosphere. Transient apoEs or apoE mutants expressing cell lines were generated by transfecting cDNA plasmids using Lipofectamine 2000 (Invitrogen) as suggested by the manufacturer. For luciferase assays of the conditioned medium, we incubated HEK293 cells 24 h after transfection, changed the medium to Opti-MEM without fetal bovine serum for 24 h at 37°C in 5% CO2 atmosphere, and collected conditioned medium. For luciferase assays of the cell lysate, we washed the cells with PBS and harvested them with Lysis Buffer (Promega).
Immunoblotting, sandwich ELISA, immunodepletion, immunoprecipitation.
Brain TBS-soluble fractions, individual SEC fractions or conditioned media from HEK293 cells were electrophoresed on 10–20% or 4–20% Novex Tris-Glycine gels (Invitrogen) in Tris-Glycine SDS running buffer for SDS-PAGE (Invitrogen). Gels were transferred to a polyvinylidene difluoride membrane (PolyScreen, PerkinElmer), and blocked for 30 min at room temperature (RT) in 5% nonfat skim milk/TBST (Tris-buffer saline with 0.1% Tween 20). Membranes were probed with 1 μg/ml monoclonal anti-Aβ antibody 6E10 (Signet) or 82E1 (IBL International), anti-apoE antibody 6C5 (Ottawa Heart Institute), or 3H1 (Ottawa Heart Institute) in TBST for 2 h at RT or for 12 h at 4°C. Following incubation with horseradish peroxidase-conjugated secondary antibody (Bio-Rad) for 1 h at RT, immunoreactive proteins were developed using an ECL kit (Western Lightning, PerkinElmer) and detected on Hyperfilm ECL (GE Healthcare; Jones et al., 2011). For the Aβ40 and Aβ42 quantification, individual SEC fractions were diluted and subjected to BNT77/BA27 for Aβ40, or BNT77/BC05 for Aβ42 using two-site ELISAs (WAKO Chemicals) and quantified as suggested by the manufacturer. For guanidine treatment, individual SEC fractions were incubated with 8 m guanidine-HCl (concentration of guanidine-HCl in the sample is 4 m) for 30 min at room temperature, diluted by 7 volumes of standard dilution buffer (final concentration of guanidine-HCl in the sample is 0.5 m) and subjected to ELISA (Yamada et al., 2009). For immunodepletion, we first incubated 200 μl of SEC-separated fractions with 30 μl of protein G magnetic beads (Millipore) for 1 h at 4°C and removed beads by using a magnet. We next incubated supernatants with or without 5 μg of anti-human apoE mAb 3H1 or anti-ApoA-I mAb 4H1 (Ottawa Heart Institute) for 12 h at 4°C. We further incubated samples with 30 μl of protein G magnetic beads for 2 h at 4°C, removed beads by using a magnet, and collected the supernatant as immunodepleted samples. For immunoprecipitation, we first incubated 200 μl of SEC-separated fractions with 30 μl of protein-G Sepharose beads (Invitrogen) for 1 h at 4°C and removed beads by centrifugation at 8000 rpm for 5 min at 4°C. The supernatants were incubated with anti-apoE (Millipore, Calbiochem), anti-Aβ (6E10), or control Ig (anti-p-glycoprotein) for 8 h at 4°C. Incubated samples were centrifuged at 8000 rpm for 5 min at 4°C and the pellets were washed by TBST 2 times, incubated with sample buffer for 10 min at 95°C, and applied to SDS-polyacrylamide gels.
Statistical analysis was performed by one-way ANOVA using Prism 5 for Mac OSX (GraphPad). Following ANOVA, Bonferroni's or Tukey's post hoc test was applied.
Aβ Immunohistochemistry and amyloid burden analysis.
Eight-micrometer-thick paraffin-embedded sections from the frontal association cortex (Brodmann's area 8,9) were obtained from the Massachusetts General Hospital Alzheimer Disease Research Center Brain Bank. Sections were deparaffinized with xylenes, rinsed in H2O2 0.3% in methanol for 20 min to block the endogenous peroxidase activity, and hydrated with decreasing concentrations of ethanol. Antigen retrieval before immunostaining was achieved by microwaving the sections in citrate buffer (0.01 m citric acid anhydrous, Tris-buffered saline, 0.05% Tween 20, NaOH to pH 6.0) at 95°C for 20 min, followed by a rinse in formic acid 90% for 5 min. After extensive washing, sections were blocked with 5% nonfat milk for 1 h to avoid nonspecific binding of the primary antibody. Sections were incubated overnight at 4°C with the N-terminal-specific anti-Aβ 10D5 mouse monoclonal antibody (1:50, Elan Pharmaceuticals). On the next day, sections were thoroughly washed, incubated with a goat anti-mouse-HRP-linked secondary antibody (1:200, Jackson ImmunoResearch) for 2 h at room temperature, and developed with 3–3′-diaminobenzidine. Finally, sections were lightly counterstained with Mayer's hematoxylin, dehydrated with increasing concentrations of ethanol, cleared with xylenes, and coverslipped with Permount mounting medium (Fisher Scientific).
The amyloid plaque burden (or amyloid load) was measured as the percentage of total cortical surface occupied by amyloid plaques. Plaque burden analysis was performed using the BIOQUANT system. Briefly, sections were placed on the motorized stage of an upright Leica DMRB microscope that was equipped with a CCD camera (model DC330, DAGE-MT) and coupled with BIOQUANT Nova Prime software (version 6.90.10.MBSR). An ≈1-cm-long strip of full-depth cortex was outlined under the 1.6× objective and amyloid plaques were thresholded under the 10× objective using the appropriate tool of the software (Ingelsson et al., 2004).
Purification of apoE from immortalized astrocytes.
Lipidated apoE particles were purified from culture media of human apoE2-, apoE3-, or apoE4-overexpressing immortalized astrocytes using an affinity column as described previously (Morikawa et al., 2005). Briefly, astrocytes were cultured in advanced DMEM (Invitrogen) with 10% FBS. After 90–95% confluency, cells were washed by PBS and further incubated in advanced DMEM with N-2 Supplement (Invitrogen) and 3 mm 25-hydroxycholesterol (Sigma) during 2∼3 d. Collected culture media were applied onto mouse monoclonal antibody against a human apoE (WU E-4) column. Lipidated apoE particles were eluted from the column with 3 m sodium thiocyanate, concentrated using Apollo centrifugal quantitative concentrators (QMWL: 150 kDa, Orbital Biosciences), and dialyzed against PBS.
In vitro Aβ oligomerization assay.
We incubated 0.1 mg/ml synthetic Aβ1–42 (Peptides International) with or without 10 μg of purified apoE2, apoE3, or apoE4 particles at 4°C for 0, 1.5, 6, 12, and 24 h and immediately applied the solution to SDS-PAGE (Hori et al., 2007).
Split-luciferase complementation assay.
HEK293 cells were stably transfected with two plasmids, each containing a complementary split-luciferase assay for Aβ oligomerization, as we have recently described (Hashimoto et al., 2011). Conditioned media from these cells were collected and centrifuged at 1200 rpm for 5 min to remove cell debris. After adding 17 μg/ml coelenterazine (NanoLight Technology) diluted by Opti-MEM into samples, luciferase activity was immediately measured using a Wallac 1420 (PerkinElmer) plate reader.
Statistical analysis was performed by one-way ANOVA using Prism 5 for Mac OSX (GraphPad). Following ANOVA, Bonferroni's post hoc test is applied.
Results
APOE ε4/ε4 AD patients have higher levels of Aβ oligomers in their brain than do APOE ε3/ε3 or APOE ε2/εx AD patients
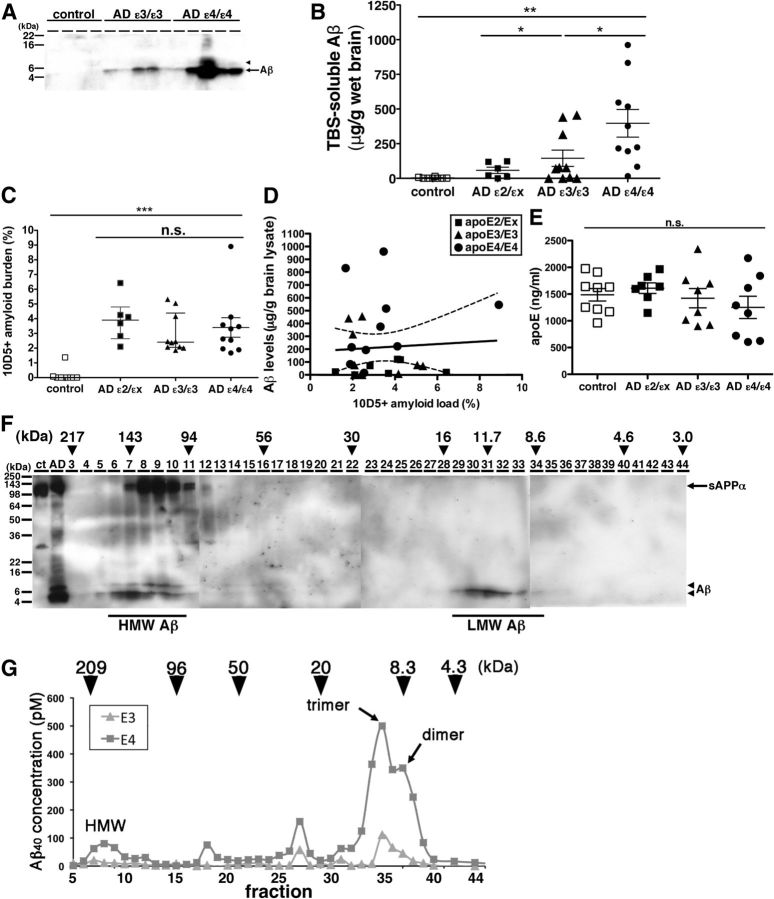
To investigate the effect of the different apoE isoforms on the metabolism of Aβ oligomers, we extracted the TBS-soluble fraction from the frontal associative neocortices of 8 non-demented controls, 6 AD patients with the APOE ε2/εx genotype, 10 AD patients with the APOE ε3/ε3 genotype, and 10 AD patients with the APOE ε4/ε4 genotype (Table 1). Because it is known that the level of senile plaque density in APOE ε4/ε4 carrier AD is on average significantly higher than that in APOE ε3/ε3 carrier AD (Rebeck et al., 1993), we carefully selected brains to have equal amyloid load in an adjacent cortical region by an immunohistochemical analysis using anti-Aβ antibody 10D5 (3.7 ± 1.4% in APOE ε2/εx AD patients, 3.0 ± 1.3% in APOE ε3/ε3 AD patients and 3.4 ± 2.1% in APOE ε4/ε4 AD patients, no significant difference; Fig. 1C). Selected brains also had a similar age at death, gender ratio, disease duration, and postmortem interval (Table 1). An equal amount of the TBS-soluble proteins from each of these brains was applied to SDS-polyacrylamide gels and visualized by anti-human Aβ-specific antibody 82E1 (Fig. 1A). While no bands were detectable in the TBS-soluble fraction from control brains, a 4 kDa band, corresponding (under these denaturing conditions) to monomeric Aβ, was observed in the TBS-soluble fraction from both APOE ε3/ε3 AD patient brains and APOE ε4/ε4 AD patient brains. We also detected an 8 kDa band corresponding to SDS-stable Aβ dimers, in the TBS-soluble fraction from several APOE ε4/ε4 AD patient brains. To quantitate the concentration of Aβ, we applied synthetic Aβ1–42 with known concentrations to the same gels and determined the concentration of Aβ in each TBS-soluble fraction. Despite the AD groups being matched for amyloid plaque burden (Fig. 1C), the level of TBS-soluble Aβ in APOE ε4/ε4 AD patient brains was 2.7 times higher than that in APOE ε3/ε3 AD patient brains, 6.9 times higher than that in APOE ε2/εx AD patient brains, and substantially times higher than the barely control brains' detectable levels (2.7 ± 5.0 μg/g brain lysate in control, 57.8 ± 54.6 μg/g brain lysate in APOE ε2/εX, 144.7 ± 185.8 μg/g brain lysate in APOE ε3/ε3 AD patient, and 396.9 ± 315.0 μg/g brain lysate in APOE ε4/ε4 AD patients; p < 0.05, ε4/ε4 vs ε3/ε3; p < 0.01, ε4/ε4 vs ε2/εx, control, ε3/ε3 vs control; Fig. 1B). We compared the amount of total soluble Aβ with the histochemically defined amounts of amyloid deposited in senile plaques, and found no correlation between plaque burden and levels of soluble Aβ (Fig. 1D, r = −0.01, p = 0.95). This suggests that the amount of TBS-soluble Aβ is independent of the amount of deposited amyloid plaques.
Table 1.
Information about the cases used in this study
| Number | Age at death (years) | Gender, n (% female) | Disease duration (years) | Postmortem interval (hours) | |
|---|---|---|---|---|---|
| Controla | 8 | 77.7 ± 10.5 | 4 (50.0) | NA | 24.3 ± 22.1 |
| AD (ϵ2/ϵx)b | 6 | 78.8 ± 8.7 | 6 (100.0) | 11.7 ± 5.2 | 20.0 ± 7.1 |
| AD (ϵ3/ϵ3) | 10 | 78.7 ± 10.5 | 6 (60.0) | 11.7 ± 4.7 | 14.7 ± 8.0 |
| AD (ϵ4/ϵ4) | 10 | 78.5 ± 8.0 | 5 (50.0) | 13.6 ± 4.6 | 19.6 ± 19.0 |
| p valuec | NA | 0.9977 | 0.8994 | 0.9830 | 0.6968 |
aOf these individuals, n = 2 (ϵ2/ϵ3), n = 5 (ϵ3/ϵ3), n = 1 (ϵ3/ϵ4).
bOf these individuals, n = 1 (ϵ2/ϵ2), n = 2 (ϵ2/ϵ3), n = 3 (ϵ2/ϵ4).
cOne-way Kruskal–Wallis ANOVA, except for gender, which was analyzed using χ2 test. Postmortem interval was not available for 2 controls and 1 AD (ϵ2/ϵx) subject.
NA, Not applicable.
Figure 1.
The level of Aβ oligomers in the brain of APOE ε4/ε4 AD patients was significantly higher compared with APOE ε3/ε3 AD patients. A, Immunoblotting of 50 μg of TBS-soluble fractions from 4 control, 5 APOE ε3/ε3 AD, and 5 APOE ε4/ε4 AD prefrontal brains. An anti-Aβ mAb 82E1 revealed Aβ monomers (arrow) and dimers (arrowhead). B, Quantification of TBS-soluble Aβ from 8 control (white squares), 6 APOE ε2/εx AD (dark squares), 10 APOE ε3/ε3 AD (dark triangles), and 10 APOE ε4/ε4 AD brains (black circles). The level of Aβ in APOE ε4/ε4 AD brains was significantly higher compared with control brains, APOE ε2/εx AD brains, and APOE ε3/ε3 AD brains. *p < 0.05, **p < 0.01, one-way ANOVA test (Tukey's post hoc test). C, Amyloid burden (%) in the prefrontal cortex of 8 control (white squares), 6 APOE ε2/εx AD (dark squares), 10 APOE ε3/ε3 AD (dark triangles), and 10 APOE ε4/ε4 AD brains (black circles) analyzed in this study. There is no significant difference among APOE ε2/εx AD, between APOE ε3/ε3 AD and APOE ε4/ε4 AD brains, one-way ANOVA test (Kruskal–Wallis test). D, Correlation analysis between the level TBS-soluble Aβ and the level of Aβ amyloid burden in 6 APOE ε2/εx AD (dark squares), 10 APOE ε3/ε3 AD (dark triangles), and 10 APOE ε4/ε4 AD brains (black circles). There is no significant difference. E, Quantification of apoE concentration in the TBS-soluble fraction of 8 control (white squares), 6 APOE ε2/εx AD (dark squares), 10 APOE ε3/ε3 AD (dark triangles), and 10 APOE ε4/ε4 AD brains (black circles). F, Immunoblotting of SEC-separated fractions from APOE ε4/ε4 AD brain. Anti-Aβ mAb 82E1 and 6E10 revealed Aβ (arrow) and sAPPα (arrowhead). Aβ eluted from 94 kDa to 217 kDa as HMW Aβ and eluted from 8.6 to 16 kDa as LMW Aβ. Estimated molecular weight (kDa) was indicated above (arrowheads). G, Representative data of the separation of 200 mg of TBS-soluble fractions of APOE ε3/ε3 AD (triangles) and APOE ε4/ε4 AD (squares) brains by double Superdex 75 SEC columns. The concentration of Aβ40 is measured by Aβ specific ELISA (BNT77-BA27) (Wako). Estimated molecular weight (kDa) was indicated above (arrowheads). Aβ in TBS-soluble fraction formed dimer, trimer and HMW oligomers.
It has also been reported that the level of apoE protein in the brains of APOE ε4/ε4 carriers is smaller than that of APOE ε3/ε3 AD carriers or of APOE ε2/ε2 carriers (Riddell et al., 2008). We measured the concentration of apoE in TBS-soluble fraction from the brains of control and AD cases by specific ELISA and found that it is similar among control and AD cases regardless of genotypes (Fig. 1E).
The finding of SDS-stable Aβ dimers in the TBS-soluble fraction of some APOE ε4/ε4 AD patients brains (Fig. 1A) prompted us to further characterize the presence of TBS-soluble Aβ oligomers under native conditions. We separated TBS-soluble fractions by SEC. We applied the TBS-soluble fraction of APOE ε4/ε4 AD patient brain onto two (tandem) Superdex 75 SEC columns (Townsend et al., 2006), collected fractions, applied the fractions into SDS-polyacrylamide gels, and detected Aβ by the anti-human Aβ antibody 6E10 (Fig. 1F). We found that fractions eluting from 94 kDa to 217 kDa strongly exhibited 4 kDa Aβ [hereafter called high-molecular weight (HMW) Aβ] and fractions eluting from 8.6 kDa to 16 kDa also exhibited 4 kDa Aβ [hereafter called low-molecular weight (LMW) Aβ]. To confirm these results and further refine this characterization, we applied the TBS-soluble fractions from 3 APOE ε3/ε3 and 6 APOE ε4/ε4 AD patients' brains onto two (tandem) Superdex 75 SEC columns, collected the eluted fractions, and measured the concentration of Aβ by an Aβ-specific ELISA (Fig. 1G). Similar to the result of immunoblotting above (Fig. 1E), we found that Aβ eluted into fractions from 100 to 200 kDa as HMW Aβ, fractions around 90 kDa, fractions around 30 kDa, and fractions from 6 to 20 kDa (the latter LMW Aβ, likely reflecting Aβ dimers and Aβ trimers). Remarkably, we found that the TBS-soluble fraction from APOE ε4/ε4 AD patient brains exhibited substantially higher amounts of Aβ in every peak compared with APOE ε3/ε3 AD patient brains. Together, the level of TBS-soluble Aβ oligomers in APOE ε4/ε4 AD patient brains was significantly higher than that in APOE ε3/ε3 AD patient brains.
ApoE forms HMW complex with Aβ oligomers in the brains of AD patients
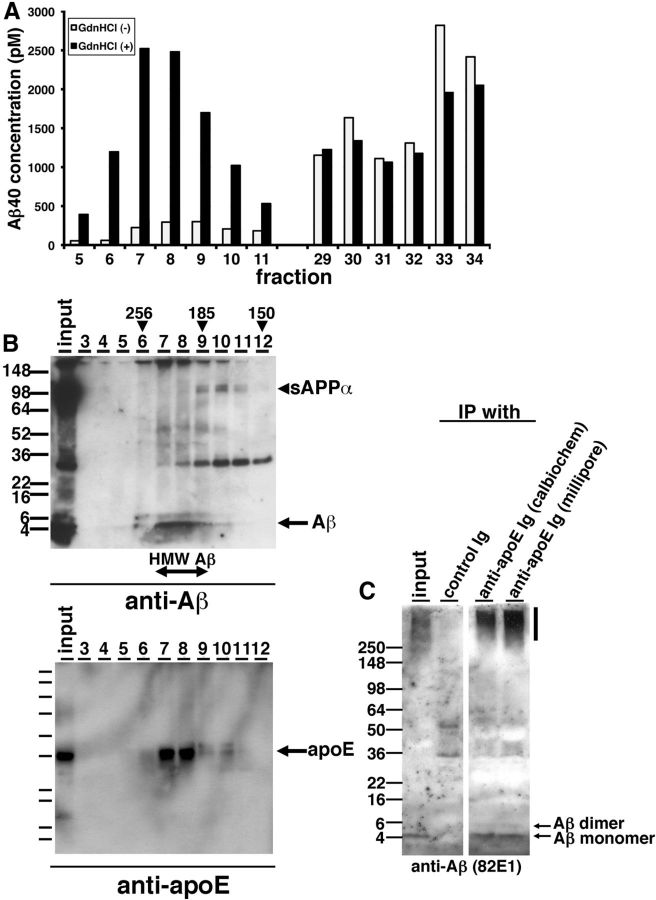
Compared with immunoblotting results (Fig. 1F), we did not detect a strong signal of Aβ in HMW Aβ fractions by ELISA (Fig. 1G). We postulated that the structure of highly oligomeric Aβ may inhibit the interaction between Aβ and anti-Aβ antibodies, or unidentified interacting molecules with Aβ in the HMW fraction may interfere with the detection of Aβ by anti-Aβ antibodies used in the ELISA. To examine these possibilities, we incubated individual SEC fractions with or without 8 m guanidine-HCl for 30 min and quantified Aβ concentration by ELISA. We found that the measured concentration of Aβ in HMW fractions dramatically increased, while the levels of Aβ in LMW fractions exhibited no difference (Fig. 2A), consistent with the idea that epitopes were masked in the HMW fraction.
Figure 2.
apoE forms HMW complex with Aβ oligomers in the brains of AD patients. A, Representative data from guanidine-HCl treatment for SEC-separated fractions. SEC-separated fractions from 5 to 11, from 29 to 34 were incubated with (black) or without (white) 8 m guanidine HCl and quantified the Aβ concentration by specific ELISA (BNT77-BA27). B, Immunoblotting of SEC-separated fractions (fraction 3–12) from an APOE ε4/ε4 AD brain. Top, Anti-mAbs 82E1 and 6E10 revealed Aβ monomers (arrow), dimers, and sAPPα (arrowhead). Bottom, Anti-mAb 3H1 revealed apoE (arrow). Estimated molecular weight (kDa) is indicated above (arrowheads). C, Immunoprecipitation using anti-apoE antibodies and control antibody from SEC-separated fraction 8 and immunoblotted by an anti-Aβ mAb 82E1. Aβ monomers and dimmers were detected (arrows).
ApoE is secreted in high-density lipoprotein (HDL) particles in the brain. To know whether apoE interacts with TBS-soluble Aβ oligomers and contributes to their apparent HMW, we immunoprobed Aβ and apoE protein in each SEC-separated fraction of TBS-soluble fraction from APOE ε4/ε4 AD patient brain using anti-Aβ and anti-apoE antibodies on SDS-polyacrylamide gels (Fig. 2B). We found that Aβ and apoE eluted in identical fractions, ranging from 185 to 256 kDa, suggesting that HMW Aβ may interact with apoE on the HDL particles. We also detected HMW Aβ and apoE in similar fractions from 185 to 256 kDa using SEC-separated samples from the TBS-soluble fraction of an APOE ε3/ε3 AD patient brain. Immunoprecipitation of apoE using each of two separate polyclonal antibodies (anti-apoE Ig, Calbiochem; and anti-apoE Ig, Millipore) pulled down Aβ from these fractions (Fig. 2C). These data suggest that apoE interacts with Aβ oligomers in human AD brain and thus may impact their oligomerization in the brain.
Purified apoE on HDL particles enhances synthetic Aβ1–42 oligomer formation in vitro
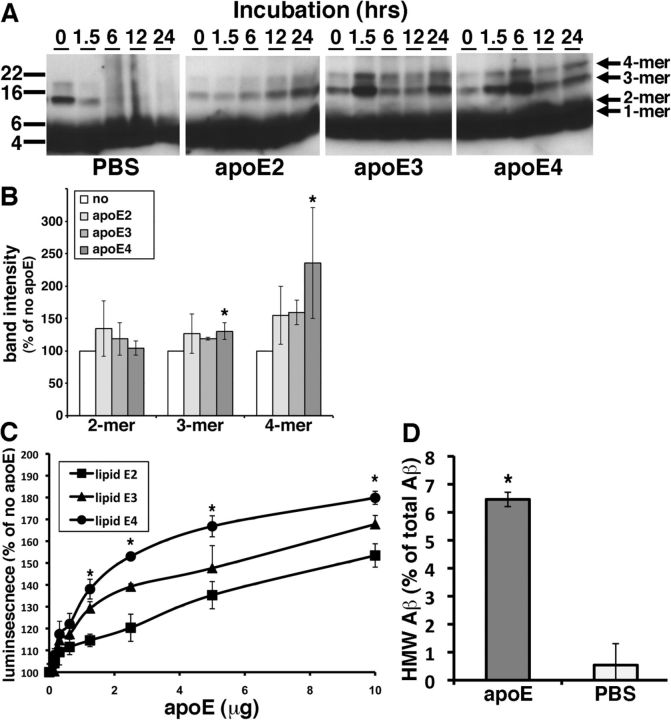
We hypothesized that apoE on HDL particles would affect the Aβ oligomerization in the brain in an isoform-dependent manner. In an in vitro Aβ fibrillization assay, apoE is known to inhibit Aβ fibrillization especially in the seeding phase of Aβ fibrillization (Evans et al., 1995; Naiki et al., 1997). Because these experiments used recombinant non-lipidated apoE and because they evaluated the level of Aβ fibrillization using the thioflavin T dye, which specifically interacts with β-sheeted structures but not oligomers, we reevaluated this interaction using physiologically relevant lipids and synthetic Aβ. We purified apoE lipid particles from immortalized astrocyte cell lines derived from human apoE2, apoE3, or apoE4 knock-in mice. These immortalized astrocyte cells are known to secrete human apoEs in HDL-like particles into conditioned media (Morikawa et al., 2005). We incubated 0.1 mg/ml (∼22 μm) synthetic Aβ1–42 with or without 10 μg of purified lipidated apoE2, apoE3, or apoE4 at 4°C for 0, 1.5, 6, 12, or 24 h in vitro and applied the samples to SDS-polyacrylamide gels (Fig. 3A). In the absence of apoE, the Aβ trimers and Aβ tetramers disappeared within 6 h and were replaced by smear bands after 6 h (Fig. 3A, PBS panel). In contrast, in the presence of lipidated apoE2, apoE3, or apoE4, the bands for Aβ trimers and Aβ tetramers gradually accumulated during the incubation period (Fig. 3A, apoE2, apoE3, apoE4 panels). Of note, qualitative inspection of the gels show that incubation of Aβ with apoE3 or apoE4 yielded higher levels of Aβ trimers and Aβ tetramers than incubation with apoE2. This result may suggest that lipidated apoE enhanced the oligomerization of Aβ or stabilized the Aβ oligomers. To elucidate whether lipidated apoE stabilize Aβ oligomers, we incubated synthetic Aβ1–42 oligomers with 5 μg/ml lipidated apoE2, apoE3, or apoE4 for 12 h in vitro, applied the samples to SDS-polyacrylamide gels, and quantified the level of remaining Aβ oligomers (Fig. 3B). Lipidated apoE4 significantly increased the level of Aβ trimers and tetramers compared with the sample incubated without apoE (130.0 ± 19.0% in trimers and 235.3 ± 85.4% in tetramers), suggesting that apoE4 may stabilize the Aβ oligomers. On the other hand, lipidated apoE2 and apoE3 did not exhibit significant increase of the level of the Aβ oligomers (Fig. 3B).
Figure 3.
Purified apoE-containing HDL particles enhanced oligomer formation of synthetic Aβ1–42 in vitro. A, Immunoblotting for Aβ after incubation of 0.1 mg/ml synthetic Aβ1–42 with PBS, 10 μg of purified apoE2, 10 μg of apoE3, or 10 μg of apoE4 for the indicated times (hours). Anti-Aβ mAb 6E10 revealed Aβ monomer, dimer, trimer, and tetramer (arrows). B, Band intensity of remaining Aβ in SDS-polyacrylamide gels after incubation of synthetic Aβ1–42 oligomers with PBS (no), 5 μg/ml purified lipidated apoE2, apoE3, or apoE4 for 12 h using an anti-Aβ mAb 6E10. Lipidated apoE4 significantly increased the level of Aβ trimer and tetramer compared with no lipidated apoE samples. N = 6, average ± SD, *p < 0.05, one-way ANOVA test (Bonferroni's test). C, Luminescence from conditioned media containing split-luciferase-tagged Aβ oligomers incubated with 0, 0.1, 0.3, 0.6, 1.25, 2.5, 5, or 10 μg of purified apoE2 (lipid apoE2, squares), purified apoE3 (lipid apoE3, triangles), or purified apoE4 (lipid apoE4, circles) for 24 h. N = 6, average ± SD, *p < 0.05, one-way ANOVA test (Bonferroni's test). D, Incubation of LMW Aβ isolated from TBS-soluble fractions of the AD brains with (apoE) or without (PBS) 5 μg of purified lipid apoE3 and separated the samples by double Superdex 75 SEC columns. The concentration of Aβ40 was measured by Aβ-specific ELISA (BNT77-BA27, WAKO Chemicals) and obtained the ratio of HMW Aβ measured (in fraction 7 and 8). N = 4, average ± SD, *p < 0.05, student' t test.
Because the concentration of Aβ oligomers is quite small, it is difficult to quantitatively monitor Aβ oligomers using these techniques. To evaluate the role of apoE in the formation of Aβ oligomers, we took advantage of a recently developed method using a split-luciferase complementation assay (Hashimoto et al., 2011). In this assay, the N- and C-terminal fragments of Gaussia luciferase are fused separately to Aβ, so that single split-luciferase-tagged Aβ does not exhibit luminescence. Once split-luciferase-tagged Aβ forms oligomers, the N- and C-terminal fragments of luciferase reconstitute into a functional molecule that exhibits luminescence (Hashimoto et al., 2011). This technique has the advantage of monitoring Aβ oligomers specifically and quantitatively without background from monomers. We incubated split-luciferase-tagged Aβ oligomers with 0, 0.1, 0.3, 0.6, 1.25, 2.5, 5, and 10 μg of purified lipidated apoE2, apoE3, or apoE4 for 24 h and measured the luminescence (Fig. 3C). We found that purified lipidated apoE in a dose-dependent manner enhanced the level of Aβ oligomers in an isoform-dependent manner (apoE2 < apoE3 < apoE4). Moreover, we isolated LMW Aβ from TBS-soluble fraction of the brains of APOE ε4/ε4 AD patients by SEC, incubated with or without 5 μg of purified lipidated apoE3, applied to two (tandem) Superdex 75 SEC columns again, and quantified the level of Aβ by the specific ELISA. We found that after incubation with purified lipidated apoE, ∼6.5% of LMW Aβ instead elutes in the HMW Aβ fraction (Fig. 3D, 6.5 ± 0.3% with apoE, 0.5 ± 0.7 without apoE), an increase of over tenfold. These qualitative and quantitative data suggest that lipidated apoE enhances Aβ oligomerization and inhibited further aggregation in vitro.
apoE enhances the level of Aβ oligomers in an isoform-dependent manner (apoE2 < apoE3 < apoE4)
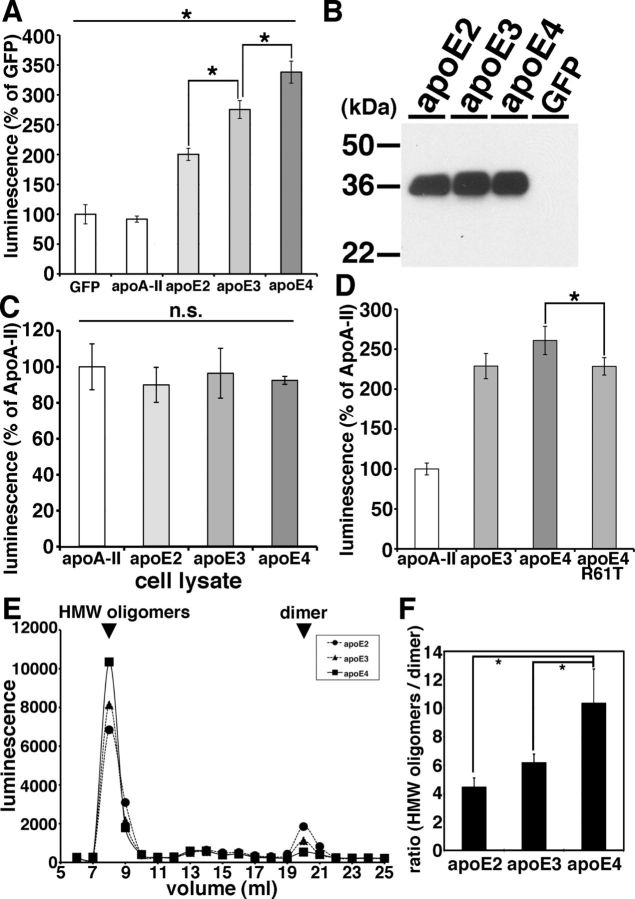
Using the split-luciferase complementation assay for monitoring Aβ oligomers, we further examined apoE's effect on Aβ oligomers. First we transiently transfected apoA-II, apoE2, apoE3, and apoE4 into doubly expressing HEK293 cells stably expressing both N- and C-terminal fragments of luciferase-tagged Aβ, collected their conditioned media after 24 h of incubation, and measured Aβ oligomers by measuring luminescence in the media (Fig. 4A,B). We found that apoE2, apoE3, or apoE4 each increased the luminescence. On the other hand, apoA-II, one of the other apolipoproteins in brain HDL particles, did not change the luminescence compared with GFP transfection (91.8 ± 5.1% in apoA-II, 200.3 ± 10.2% in apoE2, 275.5 ± 15.0% in apoE3, 338.1 ± 18.5% in apoE4; p < 0.05, E2 vs E3; E3 vs E4). apoA-II was therefore used as a control transfection for subsequent experiments.
Figure 4.
ApoE enhanced the level of Aβ oligomers in an isoform-dependent manner. A, Transient transfection of GFP (control), apoA-II, apoE2, apoE3, or apoE4 into double-expressing HEK293 cells. Luminescence of conditioned media was measured. apoE3 significantly increased the luminescence compared with apoE2 and apoE4 significantly increased the luminescence compared with apoE3. N = 6, average ± SD, *p < 0.05, one-way ANOVA test (Bonferroni's test). B, Immunoblotting of conditioned media from GFP (control), apoE2, apoE3, or apoE4 transiently transfected double-expressing HEK293 cells by an anti-apoE mAb 3H1. C, Transient transfection of apoA-II, apoE2, apoE3, or apoE4 into double-expressing HEK293 cells. Luminescence of cell lysates was measured. There is no significant difference of the luminescence among apoE2-, apoE3-, or apoE4-expressing cells. N = 6, average ± SD, one-way ANOVA test (Bonferroni's test). D, Transient transfection of apoA-II, apoE3, apoE4, or apoE4 R61T mutant into double-expressing HEK293 cells. Luminescence of conditioned media was measured. apoE4 significantly increased the luminescence compared with apoE4 R61T mutant. N = 6, average ± SD, *p < 0.05, one-way ANOVA test (Bonferroni's test). E, Transient transfection of apoE2, apoE3, or apoE4 into double-expressing HEK293 cells and separation of conditioned media by a SEC column Superdex 200. Representative data of the luminescence profile of the elutants from conditioned media of apoE2 (circles)-, apoE3 (triangles)-, or apoE4 (squares)-transfected cells. Two peaks, HMW oligomers and dimers (arrows) were observed. F, Average ratio between HMW oligomers and dimers. N = 3, average ± SD, *p < 0.01, one-way ANOVA test (Bonferroni's test).
apoE4 significantly increased the luminescence to a greater extent than apoE2 and apoE3, and apoE3 significantly increased the luminescence to a greater extent than apoE2, suggesting that apoE enhanced the level of Aβ oligomers in an isoform-dependent manner (apoE2 < apoE3 < apoE4). We did not see any difference in the levels of apoE in the conditioned media (Fig. 4B).
Although HEK293 cells transfected with human apoE3 and apoE4 naturally secrete apoE lipoparticles into the culture media (LaDu et al., 2006), we asked whether the induction of Aβ oligomerization by apoE might actually be taking place within the cells, before the secretion of apoE to the conditioned media. To test this, we transfected apoA-II, apoE2, apoE3, and apoE4 into doubly expressing HEK293 cells, collected cell lysates after 24 h incubation and measured the luminescence in cell lysates (Fig. 4C). We found that apoE2, apoE3, and apoE4 did not enhance the luminescence within cell lysates (100.0 ± 12.7% in apoA-II, 89.9 ± 9.7% in apoE2, 96.4 ± 13.9% in apoE3, 92.5 ± 2.2% in apoE4, no significant difference). Nevertheless they strongly increased the luminescence in the conditioned media (Fig. 4A), suggesting that apoE influences Aβ oligomers only in the extracellular compartment.
These data suggest that lipidated apoE2, 3, or 4 supports oligomeric Aβ generation to different extents, and we hypothesized that this was due to differences in their conformation (Jones et al., 2011). apoE2 and apoE3 prefer an open conformation, whereas apoE4 prefers a closed conformation due to a difference at amino acid residue 112 between apoE4 (Arg) and apoE2, E3 (Cys) (Dong et al., 1994; Mahley et al., 2009); indirectly this changes a salt bridge and alters the domain–domain interactions of the amino and carboxyl halves of apoE. To test the hypothesis that the difference of the tertiary structure of apoE is responsible for the observed isoform-dependent differences in apoE's facilitation of oligomer formation, we examined the effects of the apoE4 R61T mutation, which is known to change the apoE4 conformation so that it mimics the closed apoE3 conformation (Ye et al., 2005). We transiently transfected apoA-II, apoE3, apoE4, or apoE4 R61T into doubly expressing HEK293 cells, collected conditioned media after 24 h incubation, and measured its luminescence in the media (Fig. 4D). We found that apoE4 R61T increased the level of Aβ oligomers to the same extent as apoE3, but not as high as apoE4, suggesting that the tertiary structure of apoE is relevant to the apoE isoform-dependent effect on Aβ oligomerization (100.80 ± 7.4% in apoA-II, 228.8 ± 15.8% with apoE3, 260.8 ± 17.7% with apoE4, 228.4 ± 10.9% with apoE4 R61T; p < 0.05, E4 vs E4 R61T). We did not see any difference in the levels of apoE expression among apoE3, apoE4, and ApoE4 R61T.
We previously demonstrated that the split-luciferase-tagged Aβ oligomers consist of HMW 24∼36 mer and low-molecular weight dimers by SEC analyses (Hashimoto et al., 2011). To evaluate whether apoE shifts the molecular size of Aβ oligomers, we separated conditioned media from apoE2, apoE3, or apoE4 transiently transfected doubly expressing HEK293 cells using a single Superdex 200 SEC column, collected the eluted fractions, and measured their luminescence (Fig. 4E). We found that apoE4 significantly increased the level of HMW putative 24∼36 mer oligomers (or complexes of Aβ with other proteins) and decreased the level of dimers (Fig. 4E,F, p < 0.01). We observed no significant shift in the elution profile of Aβ oligomers by apoEs.
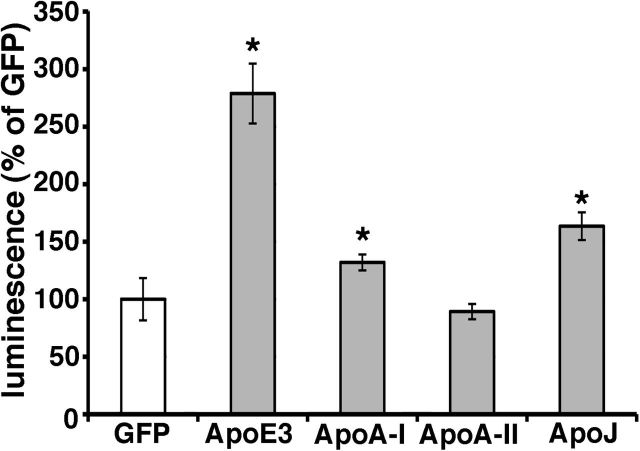
apoA-I and apoJ/clusterin also enhanced Aβ oligomerization
In addition to apoE, apoA-I, apoA-II, and apoJ/clusterin are also present on the HDL particles in the brain. Recently, several genome-wide association studies have identified a variant at CLU (gene of apoJ/clusterin) to be associated with Alzheimer's disease (Harold et al., 2009; Lambert et al., 2009). It has also been reported that apoJ/clusterin-deficient mice crossed with APP-transgenic mice exhibit significantly fewer fibrillar Aβ deposits in the brain compared with APP-transgenic mice (DeMattos et al., 2002). Hence we asked whether these other apolipoproteins might also modulate Aβ oligomerization. To address this question, we transiently transfected apoA-I, apoA-II, apoJ, and apoE3 into doubly expressing HEK293 cells, collected their conditioned media after 24 h incubation, and measured their luminescence (Fig. 5). Interestingly, apoA-I and apoJ/clusterin also enhanced the luminescence, although to a lesser extent than apoE3, whereas apoA-II did not change the luminescence from baseline (132.0 ± 7.0% in apoA-I, 89.3 ± 6.6% in apoA-II, 163.5 ± 12.1% in apoJ/clusterin, 278.8 ± 26.0% in apoE3, p < 0.05). This suggests that apoA-I and apoJ/clusterin may also modulate the metabolism of Aβ oligomers in the brain, and reinforces the idea that lipidated particles supported by several apolipoproteins may act as a scaffold for Aβ interactions.
Figure 5.
ApoA-I and apoJ enhanced the level of Aβ oligomers. Transient transfection of GFP (control), apoA-I, apoA-II, apoJ/clusterin, or apoE3 into double-expressing HEK293 cells. Luminescence of conditioned media was measured. apoA-I, apoJ/clusterin, or apoE3 significantly increased the luminescence. N = 6, average ± SD, *p < 0.05, one-way ANOVA test (Bonferroni's test).
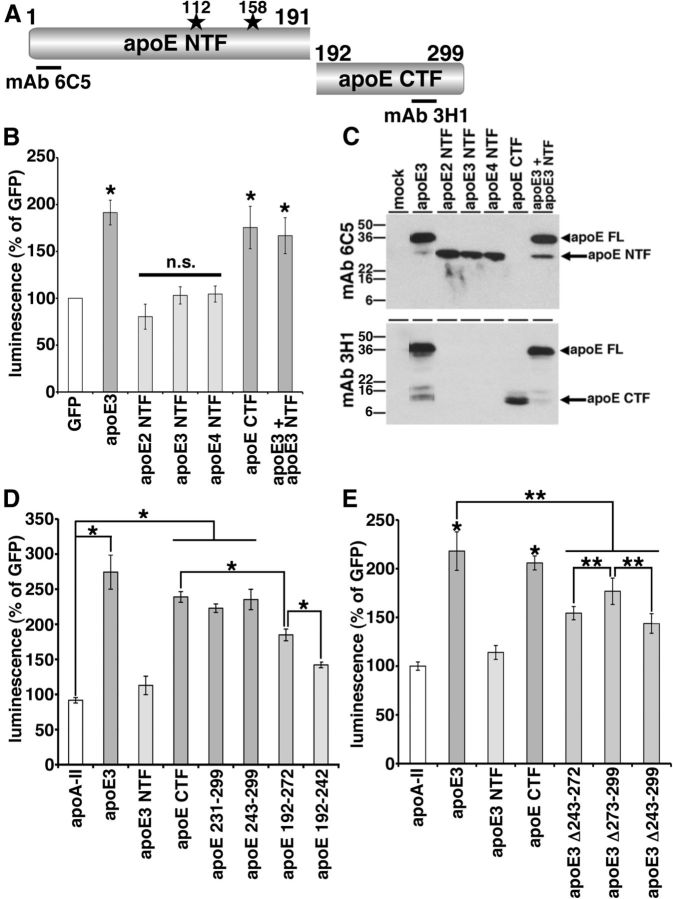
The lipid-binding domain of apoE is necessary for enhancement of Aβ oligomerization
apoE has a receptor-binding domain in the N-terminal region and a lipid-binding domain in the C-terminal region (Fig. 6A; Chou et al., 2005). To understand which domain of apoE is important in the enhancement of Aβ oligomerization, we expressed the apoE NTFs (apoE2 NTF, apoE3 NTF, apoE4 NTF) or apoE CTF in double-expressing HEK293 cells, collected conditioned media after 24 h incubation and measured their luminescence (Fig. 6B,C). We found that apoE2 NTF, apoE3 NTF, or apo4 NTF did not enhance the luminescence, whereas apoE CTF significantly enhanced the luminescence to an extent similar to that of full-length apoE3 (80.4 ± 13.4% for apoE2 NTF, 103.1 ± 9.2% for apoE3 NTF, 104.5 ± 8.6% for apoE4 NTF, compared with 175.5 ± 22.6% for apoE CTF and 191.5 ± 13.2% with full-length apoE3; p < 0.05, apoE3 and apoE3 CTF; no significant difference, apoE2 NTF, apoE3 NTF, and apoE4 NTF). This suggests that the C-terminal region of apoE is necessary and sufficient to induce Aβ oligomerization. We also coexpressed both apoE3 and apoE3 NTF together in doubly expressing HEK293 cells and found that apoE3 NTF did not inhibit the enhancing effect of apoE3, suggesting that the N-terminal fragments of apoE is a loss-of-function molecule regarding Aβ oligomerization (Fig. 6B, 166.9 ± 19.2% in apoE3 and apoE3 NTF, p < 0.05). In immunoblotting, we confirmed the expression of these apoE fragments using anti-human apoE antibodies. Mab 6C5, its epitope located in the N-terminal region of apoE, recognized full-length apoE3, apoE2 NTF, apoE3 NTF, and apoE4 NTF (Fig. 6A,C). Mab 3H1 (epitope located at 243–272 aa residues of apoE), recognized full-length apoE3 and apoE CTF (Fig. 6A,C).
Figure 6.
Lipid-binding domain of apoE was necessary for the enhancement of Aβ oligomers. A, Schematic structure of apoE and apoE fragments. The epitopes of mAb 6C5 and mAb 3H1 is illustrated. B, Transient transfection of GFP, apoE3, apoE2 NTF, apoE3 NTF, apoE4 NTF, apoE CTF, or both apoE3 and apoE3 NTF into double-expressing HEK293 cells. Luminescence of conditioned media was measured. apoE3 and apoE CTF significantly increased the luminescence, on the other hand, apoE2 NTF, apoE3 NTF, or apoE4 NTF did not increase the luminescence. N = 6, average ± SD, *p < 0.05, one-way ANOVA test (Bonferroni's test). C, Immunoblotting of conditioned media by the anti-apoE mAb 6C5 (top) and 3H1 (bottom panel). MAb 6C5 revealed 36 kDa band (full-length apoE, arrowhead) and 26 kDa band (apoE NTF, arrow). MAb 3H1 revealed also 36 kDa band (full-length apoE, arrowhead) and 10 kDa doublet band (apoE CTF, arrow). D, Transient transfection of GFP, apoA-II, apoE3, apoE3 NTF, apoE CTF, apoE 231–299, apoE 243–299, apoE 192–272, and apoE 192–242 into double-expressing HEK293 cells. Luminescence of conditioned media was measured. apoE3 significantly increased the luminescence compared with apoA-II, apoE CTF fragments. apoE CTF significantly increased the luminescence compared with apoE 192–272 and apoE 192–272 significantly increased the luminescence compared with apoE 192–242. N = 6, average ± SD, *p < 0.05, one-way ANOVA test (Bonferroni's test). E, Transient transfection of GFP, apoA-II, apoE3, apoE3 NTF, apoE CTF, apoE3 Δ243–272, apoE3 Δ273–299, and apoE3 Δ243–299 into double-expressing HEK293 cells. Luminescence of conditioned media was measured. apoE3 and apoE CTF significantly increased the luminescence compared with apoA-II. ApoE3 also significantly increased the luminescence compared with three apoE3 deletion mutants. ApoE3 Δ273–299 significantly increased the luminescence compared with apoE3 Δ243–272 or apoE3 Δ243–299. N = 6, average ± SD, *p < 0.01, **p < 0.05, one-way ANOVA test (Bonferroni's test).
The C-terminal domain of apoE contains the major lipid-binding region (243–272 aa residues; Hatters et al., 2006; Mahley et al., 2009). To find the C-terminal domain of apoE responsible for the enhancement of Aβ oligomerization, we next expressed apoE 231–299, apoE 243–299, apoE192–272, and apoE 192–242 in Aβ split-luciferase-expressing HEK293 cells, collected conditioned media after 24 h incubation, and measured the luminescence (Fig. 6D). We found that apoE 231–299 and apoE 243–299 increased the luminescence to the same level as apoE CTF, whereas apoE 192–272 did not increase the luminescence as strongly as apoE CTF. apoE 192–242 luminescence was even lower than that of apoE 192–272 (91.7 ± 3.9% in apoA-II, 274.4 ± 24.4% in apoE3, 112.9 ± 13.1% in apoE3 NTF, 239.0 ± 7.6% in apoE CTF, 222.9 ± 6.0% in apoE 231–299, 235.3 ± 14.5% in apoE 243–299, 184.9 ± 8.3% in apoE 192–272, 142.0 ± 4.0% in apoE 192–242; p < 0.05, apoA-II vs apoE3, apoE CTF, apoE 231–299, or apoE 243–299; apoE CTF vs apoE 192–272; apoE 192–272 vs apoE 192–242). Together, these data suggest that the 243–299 aa residues in the C-terminal region of apoE are especially important in the enhancement of Aβ oligomers. We confirmed similar expression levels of these apoE fragments by immunoblotting using a goat anti-apoE polyclonal antibody and mAb 3H1.
To further examine the effect of the 243–299 aa residues of apoE in the enhancement of the Aβ oligomerization, we also expressed the deletion mutants, apoE3 Δ243–272, apoE3 Δ273–299, and apoE3 Δ243–299, collected culture media after 24 h incubation, and measured their luminescence (Fig. 6E). We found that apoE3 Δ243–272, apoE3 Δ273–299, and apoE3 Δ243–299 significantly decreased the level of luminescence compared with full-length apoE3 and that apoE3 Δ243–299 significantly decreased the luminescence compared with apoE3 Δ273–299 (218.1 ± 19.7% in apoE3, 114.0 ± 7.1% in apoE3 NTF, 205.9 ± 7.2% in apoE CTF, 154.4 ± 6.8% in apoE3 Δ243–272, 176.8 ± 13.5% in apoE3 Δ273–299, 143.8 ± 10.0% in apoE3 Δ243–299; p < 0.01, apoA-II vs apoE3 or apoE CTF; p < 0.05, apoE3 vs apoE3 Δ243–272, apoE3 Δ273–299, or apoE3 Δ243–299; apoE3 Δ273–299 vs apoE3 Δ243–272 or apoE3 Δ243–299). We confirmed similar expression levels of these apoE mutants by immunoblotting using mAb 6C5 and mAb 3H1. Together, these results strongly argue that the C-terminal region of apoE, especially a domain within amino-acid residues 243–272, is essential for apoE's support of Aβ oligomers. This domain is the major lipid-binding domain of apoE; thus, the lipidation of apoE may be crucial in the enhancement of Aβ oligomerization, as this region may act to help catalyze oligomer formation.
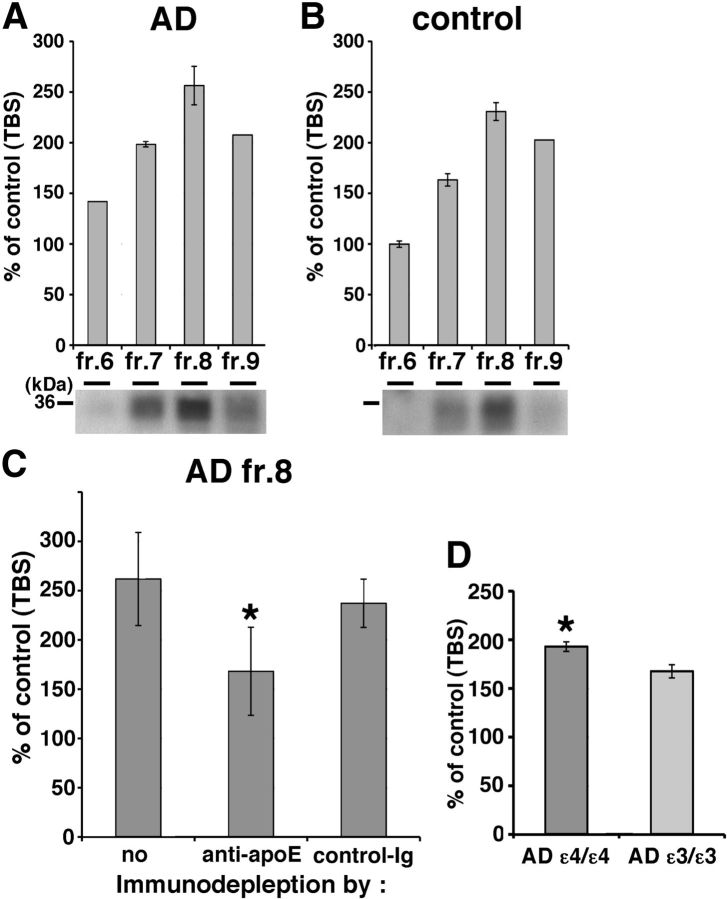
Human brain apoE promoted Aβ oligomerization
Since lipidated apoE particles generated in culture enhanced Aβ oligomerization, we next tested whether apoE isolated from human brain might have the same effect. We separated the TBS-extract of human brain by SEC and found both apoE and HMW Aβ oligomers are eluted into fraction 7 and 8 (Fig. 2B). We first incubated SEC-separated TBS-soluble fractions 6–9 from AD APOE ε3/ε3 brains, AD APOE ε4/ε4 brains (Fig. 7A), or control brains (Fig. 7B) with conditioned media from doubly expressing HEK293 cells for 24 h at 37°C, and measured the luminescence. We observed that all four fractions, but especially fraction 8 from both AD and control brains, increased the luminescence (141.8% in AD fraction 6, 198.4% in AD fraction 7, 256.4% in AD fraction 8, 207.6% in AD fraction 9, 99.8% in control fraction 6, 163.2% in control fraction 7, 230.8% in control fraction 8, 202.6% in control fraction 9). We also found that apoE eluted in fraction 8 from AD and control brain by immunoblotting using a goat anti-apoE antibody (Fig. 7A,B, bottom). Next, to test the hypothesis that apoE mediated the increase in luminescence observed due to fraction 8, we immunodepleted apoE from fraction 8 using an anti-human apoE mAb 3H1, incubated with conditioned media from doubly expressing HEK293 cells for 24 h at 37°C, and measured the luminescence (Fig. 7C). We found that apoE-immunodepleted sample significantly decreased the level of luminescence compared with immunodepletion using a control antibody (261.8 ± 47.3% with no antibody, 168.1 ± 44.6% with anti-apoE antibody, 237.1 ± 24.5% with control antibody, p < 0.05). Moreover, it is these fractions that were used for the immunoprecipitation of apoE and Aβ described above (Fig. 2C). Finally, we incubated fraction 8 from 4 AD patients with the APOE ε4/ε4 genotype and 4 AD patients with the APOE ε3/ε3 genotype with conditioned media from doubly expressing HEK293 cells for 24 h at 37°C, and measured the luminescence (Fig. 7D). We observed that fractions from APOE ε4/ε4 AD patients led to significantly increased levels of Aβ oligomers compared with that from APOE ε3/ε3 AD patients (193.0 ± 4.9% in APOE ε4/ε4 AD patients and 167.6 ± 6.8% in APOE ε3/ε3 AD patients, p < 0.05). These data indicate that endogenous apoE from human brain increased the level of Aβ oligomers, supporting the hypothesis that lipidated apoE derived from human brain also enhances Aβ oligomerization. The residual enhancement of luminescence after immunodepletion might be due to incomplete immunodepletion, the preservation of lipidated particles that do not contain apoE, or other non-apoE factors (including oligomeric Aβ itself) that might act as a nidus for oligomer formation.
Figure 7.
Endogenous apoE in the brain increases the level of Aβ oligomers. A, B, Luminescence of SEC-separated fraction 6, 7, 8, or 9 from TBS-soluble fraction of AD (A) or control (B) brain incubated with split-luciferase-tagged Aβ oligomers for 24 h. An anti-apoE antibody revealed 36 kDa apoE protein (bottom). C, Immunodepletion of fraction 8 of AD brains using no antibody, anti-apoE mAb 3H1 or control Ig. Luminescence from immunodepleted fraction 8 of AD brains incubated with split-luciferase-tagged Aβ oligomers for 24 h. Anti-apoE mAb 3H1 significantly reduced the luminescence compared with control Ig. N = 4, average ± SD, *p < 0.05, one-way ANOVA test (Bonferroni's test). D, Luminescence of SEC-separated fraction 8 from TBS-soluble fraction of 4 APOE ε4/ε4 AD brains and 4 APOE ε3/ε3 AD brains incubated with split-luciferase-tagged Aβ oligomers for 24 h. Fraction 8 from APOE ε4/ε4 AD brains significantly increased the level of Aβ oligomers compared with that APOE ε3/ε3 AD brains. Average ± SD, *p < 0.05, one-way ANOVA test (Bonferroni's test).
Discussion
In this study, we demonstrate that the levels of Aβ oligomers in TBS-soluble fraction of AD APOE ε4/ε4 brains are 2.7-fold higher compared with APOE ε3/ε3 patient brains and 6.9-fold higher compared with APOE ε2/εx patient brains, whereas brains from non-demented controls had negligible levels of Aβ oligomers (Fig. 1A,B). We also found that Aβ and apoE coeluted into HMW fractions in SEC-separated TBS-soluble fraction from AD brains, and coimmunoprecipitated (Fig. 2B,C), suggesting the possibility of an in vivo interaction between them. We confirmed that Aβ and apoE coeluted into HMW fractions in SEC-separated interstitial fluid from APP-PS mouse brains using a microdialysis techniques with a 1000 kDa molecular weight cutoff membrane probe (S. Takeda, T. Hashimoto, and B. T. Hyman, unpublished observation), suggesting that the apoE and Aβ HMW complex endogenously exists in the brain and is not a product caused during the mechanical homogenization steps.
Based on these data, we hypothesized that apoE would facilitate Aβ oligomerization, and tested the idea that the extent of oligomer formation would be isoform dependent. We performed Aβ oligomerization assays using three different preparations of apoE. First, apoE on HDL particles, purified from conditioned media of immortalized astrocytes expressing human apoE2, apoE3, or apoE4, promoted the oligomerization of synthetic Aβ, split-luciferase-tagged Aβ oligomers, or LMW Aβ isolated from TBS-soluble fraction of AD patients' brains Aβ (Fig. 3). Second, transient overexpression of apoE2, apoE3, or apoE4 in HEK293 cells stably expressing split-luciferase-tagged Aβ oligomers increased Aβ oligomers through apoE's C-terminal domain in an isoform-dependent manner (apoE2 < apoE3 < apoE4; Figs. 4, 6). Third, endogenous apoE extracted from TBS-soluble fraction of human AD and control brains also increased Aβ oligomers, again apoE3 < apoE4 (Fig. 7). Similarly, we assessed oligomerization using two preparations: synthetic Aβ and a quantitative split-luciferase assay, with confirmatory results. apoE, especially apoE4, appears to enhance Aβ oligomers.
The current view of AD pathophysiology emphasizes the deleterious effects of Aβ oligomers on synapses, leading to synaptic dysfunction, and progressive memory impairment in AD patients (Lesné et al., 2006; Shankar et al., 2008; Li et al., 2009; Wu et al., 2010). Recently we found that apoE colocalized with Aβ oligomers at synapse in the brain of AD patient brains using an array tomographic technique and found that APOE ε4/ε4 AD patients have a significantly higher level of colocalization of apoE and Aβ oligomers at synapse compared with APOE ε3/ε3 AD patients (Koffie et al., 2012). Our results suggest that apoE4 increases the level of Aβ oligomers in the brain, leading to increased synaptic localization with Aβ oligomers, synaptic dysfunction, and hastening the development of cognitive impairments.
Biochemical analyses of human AD revealed that the level of TBS-soluble Aβ oligomers in APOE ε4/ε4 brains are 2.7-fold higher compared with AD APOE ε3/ε3 brains and 6.9-fold higher compared with AD APOE ε2/εx brains (Fig. 1A,B). Importantly, these AD groups were matched for deposited amyloid burden. No significant correlation was observed between plaque burden and levels of TBS-soluble Aβ oligomers (r = −0.01, p = 0.95, Spearman's rank correlation test), so that the 2.7-fold difference in the levels of TBS-soluble Aβ oligomers between them cannot be attributed to the disruption of a higher amount of senile plaques in the APOE ε4/ε4 group during the homogenization of the specimens. Thus, these results indicate that apoE influences both plaque burden and also the levels of TBS-soluble Aβ oligomers in an isoform-differential manner.
Using both synthetic Aβ (Fig. 3A) and a split-luciferase complementation assay (Figs. 3C, 4), we found that apoE increased Aβ oligomerization in an isoform-dependent manner (apoE2 < apoE3 < apoE4). Interestingly, experiments using deletion mutants of apoE demonstrate that the C-terminal domain of apoE is necessary and sufficient to drive Aβ oligomerization, suggesting that apoE may directly interact with Aβ oligomers through its C-terminal region (Fig. 6B,D,E). We also found that apoE4 enhanced the ratio of HMW Aβ oligomers compared with apoE2 or apoE3 (Fig. 4E,F), suggesting that N-terminal domain might be important to modulate Aβ oligomerization. In a recent study using the anti-apoE mAb 3H1, which recognizes the amino acids 243–272, we observed significantly higher levels of apoE C-terminal fragments in APOE ε4/ε4 AD brains compared with APOE ε3/ε3 AD brains. In addition, using in situ fluorescence lifetime imaging–fluorescent resonance energy transfer in human AD brain specimens, we found that Aβ is closer to the apoE C-terminal region than to its N-terminal region within doubly labeled senile plaques (Jones et al., 2011). Together, these results argue that not only full-length apoE4 but also C-terminal fragments of apoE4 containing the region at 243–272 amino-acid residues may additionally enhance the formation of Aβ oligomers in APOE ε4/ε4 brains. Interestingly, the 243–272 aa residues of apoE are the major lipid-binding region of apoE, supporting the idea that apoE lipidation may be critical to facilitate Aβ oligomer formation. We hypothesize that lipidated apoE might concentrate Aβ monomer and provide a scaffold for oligomerization of Aβ. Alternatively, we found lipidated apoE4 increased the level of synthetic Aβ trimers and tetramers (Fig. 3B), suggesting that lipidated apoE, especially apoE4, might stabilize Aβ oligomers and inhibit the dissociation or further aggregation of Aβ. Furthermore, Aβ oligomers may be able to escape from Aβ degradation or phagocytosis by binding to apoE on HDL particles.
In addition to apoE, we found apoJ/clusterin and apoA-I, other apolipoproteins on the HDL particles, increased the level of Aβ oligomers (Fig. 5). Common variants in the apoJ/clusterin gene (CLU) have recently been linked to an increased risk of developing AD (Harold et al., 2009; Lambert et al., 2009), and apoJ/clusterin-deficient mice crossed with APP-transgenic mice exhibit significantly fewer fibrillar Aβ deposits in the brain compared with APP-transgenic mice (DeMattos et al., 2002), suggesting that apoJ/clusterin may modify Aβ oligomer formation in the human brain similarly to apoE. By contrast, crossing apoA-I-deficient mice with APP-transgenic mice did not alter Aβ deposition in the brain (Fagan et al., 2004), indicating a small or no effect of apoA-I on Aβ oligomerization and fibrillization. We observed that apoA-I only slightly increased the levels of Aβ oligomers (Fig. 5).
Recently, it was reported that the level of soluble oligomeric Aβ in frontal cortex of young adults is higher than that of elderly people or Alzheimer' disease patients using ELISA experiments (van Helmond et al., 2010), potentially calling into question a relationship between oligomeric Aβ (as measured by the ELISA) with Alzheimer disease pathogenesis. In this study we observed that the level of HMW Aβ from TBS-soluble fraction is under-estimated in ELISA experiments due to a possible masking effect either by itself or interacting molecules, and the treatment with 8 m Guanidine-HCl increases the measured concentration of Aβ in HMW Aβ fractions using our specific ELISA conditions (Fig. 2A). It would be interesting to evaluate the age-dependent ELISA results in the context of these technical issues of Aβ measurement.
In summary, we provide in vivo and in vitro evidence that apoE interacts with Aβ oligomers through its C-terminal region and that apoE, particularly apoE4, promotes and stabilizes Aβ oligomerization. Given the relatively large magnitude of these effects (e.g., a 2.7-fold increase in Aβ oligomers in APOE ε4/ε4 Alzheimer brains, compared with APOE E3/3 Alzheimer brains), it is plausible that these observations help explain the major risk for AD associated with APOE ε4 inheritance. Both the inhibition of the interaction between apoE and Aβ oligomers and the inhibition of the lipidation of apoE may be valuable therapeutic targets to prevent Aβ oligomerization and subsequent synaptic dysfunction.
Footnotes
This work was supported by NIH Grants AG12406 (B.T.H.), AG13956 (D.M.H.), and AG033670 (T.L.S.-J.), Ellison Medical Foundation/AFAR Grant 2009A059868 (T.H.), Fundación Alfonso Martín Escudero (A.S.-P.), and P50 AG005134 (Massachusetts Alzheimer's Disease Research Center). We thank Drs. Zhanyun Fan and Pamela J. McLean for the construction of split-luciferase-tagged Aβ cDNA plasmids. We also thank Dr. Eloise Hudry and Dr. Robert M. Koffie for valuable discussion.
References
- Bu G. Apolipoprotein E and its receptors in Alzheimer's disease: pathways, pathogenesis and therapy. Nat Rev Neurosci. 2009;10:333–344. doi: 10.1038/nrn2620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellano JM, Kim J, Stewart FR, Jiang H, DeMattos RB, Patterson BW, Fagan AM, Morris JC, Mawuenyega KG, Cruchaga C, Goate AM, Bales KR, Paul SM, Bateman RJ, Holtzman DM. Human apoE isoforms differentially regulate brain amyloid-β peptide clearance. Sci Transl Med. 2011;3:89ra57. doi: 10.1126/scitranslmed.3002156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou CY, Lin YL, Huang LC, Sheu SY, Lin TH, Tsay HJ, Chang GG, Shiao MS. Structural variation in human apolipoprotein E3 and E4: secondary structure, tertiary structure and size distribution. Biophys J. 2005;88:455–466. doi: 10.1529/biophysj.104.046813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corder EH, Saunders AM, Strittmatter WJ, Schmechel DE, Gaskell PC, Small GW, Roses AD, Haines JL, Pericak-Vance MA. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer's disease in late onset families. Science. 1993;261:921–923. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- DeMattos RB, O'dell MA, Parsadanian M, Taylor JW, Harmony JAK, Bales KR, Paul SM, Aronow BJ, Holtzman DM. Clusterin promotes amyloid plaque formation and critical for neuritic toxicity in a mouse model of Alzheimer's disease. Proc Natl Acad Sci U S A. 2002;99:10843–10848. doi: 10.1073/pnas.162228299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMattos RB, Cirrito JR, Parsadanian M, May PC, O'Dell MA, Taylor JW, Harmony JAK, Aronow BJ, Bales KR, Paul SM, Holtzman DM. ApoE and clusterin cooperatively suppress Aβ levels and deposition: evidence that apoE regulates extracellular Aβ metabolism in vivo. Neuron. 2004;41:193–202. doi: 10.1016/s0896-6273(03)00850-x. [DOI] [PubMed] [Google Scholar]
- Dong LM, Wilson C, Wardell MR, Simmons T, Mahley RW, Weisgraber KH, Agard DA. Human apolipoprotein E: Role of arginine 61 in mediating the lipoprotein preferences of the E3 and E4 isoforms. J Biol Chem. 1994;269:22358–22365. [PubMed] [Google Scholar]
- Evans KC, Berger EP, Cho CG, Weisgraber KH, Lansbury PT., Jr Apolipoprotein E is a kinetic but a thermodynamic inhibitor of amyloid formation: implications for the pathogenesis and treatment of Alzheimer disease. Proc Natl Acad Sci U S A. 1995;92:763–767. doi: 10.1073/pnas.92.3.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagan AM, Watson M, Parsadanian M, Bales KR, Paul SM, Holtzman DM. Human and murine ApoE markedly alters A beta metabolism before and after plaque formation in a mouse model of Alzheimer's disease. Neurobiol Dis. 2002;9:305–318. doi: 10.1006/nbdi.2002.0483. [DOI] [PubMed] [Google Scholar]
- Fagan AM, Christopher E, Taylor JW, Parsadanian M, Spinner M, Watson M, Fryer JD, Wahrle S, Bales KR, Paul SM, Holtzman DM. ApoAI deficiency results in marked reductions in plasma cholesterol but no alterations in amyloid-β pathology in a mouse model of Alzheimer's disease-like cerebral amyloidosis. Am J Pathol. 2004;165:1413–1422. doi: 10.1016/s0002-9440(10)63399-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Isla T, West HL, Rebeck GW, Harr SD, Growdon JH, Locascio JJ, Perls TT, Lipsitz LA, Hyman BT. Clinical and pathological correlates of apolipoprotein E ε4 in Alzheimer's disease. Ann Neurol. 1996;39:62–70. doi: 10.1002/ana.410390110. [DOI] [PubMed] [Google Scholar]
- Harold D, Abraham R, Hollingworth P, Sims R, Gerrish A, Hamshere ML, Pahwa JS, Moskvina V, Dowzell K, Williams A, Jones N, Thomas C, Stretton A, Morgan AR, Lovestone S, Powell J, Proitsi P, Lupton MK, Brayne C, Rubinsztein DC, et al. Genome-wide association study identifies variants at CLU and PICALM associated with Alzheimer's disease. Nat Genet. 2009;41:1088–1093. doi: 10.1038/ng.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto T, Wakabayashi T, Watanabe A, Kowa H, Hosoda R, Nakamura A, Kanazawa I, Arai T, Takio K, Mann DM, Iwatsubo T. CLAC: a novel Alzheimer amyloid plaque component derived from a transmembrane precursor, CLAC-P/collagen type XXV. EMBO J. 2002;21:1524–1534. doi: 10.1093/emboj/21.7.1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto T, Adams KW, Fan Z, McLean PJ, Hyman BT. Characterization of oligomer formation of amyloid-β peptide using a split-luciferase complementation assay. J Biol Chem. 2011;286:27081–27091. doi: 10.1074/jbc.M111.257378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatters DM, Peters-Libeu CA, Weisgraber KH. Apolipoprotein E structure: insights into function. Trends Biochem Sci. 2006;31:445–454. doi: 10.1016/j.tibs.2006.06.008. [DOI] [PubMed] [Google Scholar]
- Holtzman DM, Bales KR, Tenkova T, Fagan AM, Parsadanian M, Sartorius LJ, Mackey B, Olney J, McKeel D, Wozniak D, Paul SM. Apolipoprotein E isoform-dependent amyloid deposition and neuritic degeneration in a mouse model of Alzheimer's disease. Proc Natl Acad Sci U S A. 2000;97:2892–2897. doi: 10.1073/pnas.050004797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtzman DM, Morris JC, Goate AM. Alzheimer's disease: the Challenge of the second century. Sci Transl Med. 2011;3:77sr1. doi: 10.1126/scitranslmed.3002369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hori Y, Hashimoto T, Wakutani Y, Urakami K, Nakashima K, Condron MM, Tsubuki S, Saido TC, Teplow DB, Iwatsubo T. Tottori (D7N) and English (H6R) familial Alzheimer disease mutations accelerate Aβ fibril formation without increasing protofibril formation. J Biol Chem. 2007;282:4916–4923. doi: 10.1074/jbc.M608220200. [DOI] [PubMed] [Google Scholar]
- Ingelsson M, Fukumoto H, Newell KL, Growdon JH, Hedley-Whyte ET, Frosch MP, Albert MS, Hyman BT, Irizarry MC. Early Aβ Accumulation and progressive synaptic loss, gliosis, and tangle formation in AD brain. Neurology. 2004;62:925–931. doi: 10.1212/01.wnl.0000115115.98960.37. [DOI] [PubMed] [Google Scholar]
- Jones PB, Adams KW, Rozkalne A, Spires-Jones TL, Hshieh TT, Hashimoto T, von Armin CAF, Mielke M, Bacskai BJ, Hyman BT. Apolipoprotein E: isoform specific differences in tertiary structure and interaction with amyloid-β in human Alzheimer brain. PLoS One. 2011;6:e14586. doi: 10.1371/journal.pone.0014586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koffie RM, Meyer-Luehmann M, Hashimoto T, Adams KW, Mielke ML, Garcia-Alloza M, Micheva KD, Smith SJ, Kim ML, Lee VMY, Hyman BT, Spires-Jones TL. Oligomeric amyloid β associates with postsynaptic densities and correlates with excitatory synapse loss near senile plaques. Proc Natl Acad Sci U S A. 2009;106:4012–4017. doi: 10.1073/pnas.0811698106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koffie RM, Hashimoto T, Tai HC, Kay KR, Serrano-Pozo A, Joyner D, Hou S, Kopeikina KJ, Frosch MP, Lee VMY, Holtzman DM, Hyman BT, Spires-Jones TL. Apolipoprotein E4 effects in Alzheimer disease are mediated by synaptotoxic oligomeric amyloid-β. Brain. 2012;135:2155–2168. doi: 10.1093/brain/aws127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaDu MJ, Falduto MT, Manelli AM, Reardon CA, Getz GS, Frail DE. Isoform-specific binding of apolipoprotein E to β-amyloid. J Biol Chem. 1994;269:23403–23406. [PubMed] [Google Scholar]
- LaDu MJ, Stine WB, Jr, Narita M, Getz GS, Reardon CA, Bu G. Self-assembly of HEK cell-secreted apoE particles resembles apoE enrichment of lipoproteins as a ligand for the LDL receptor-related protein. Biochemistry. 2006;45:381–390. doi: 10.1021/bi051765s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert JC, Heath S, Even G, Campion D, Sleegers K, Hiltunen M, Combarros O, Zelenika D, Bullido MJ, Tavernier B, Letenneur L, Bettens K, Berr C, Pasquier F, Fiévet N, Barberger-Gateau P, Engelborghs S, De Deyn P, Mateo I, Franck A, et al. Genome-wide association study identifies variants at CLU and CR1 associated with Alzheimer's disease. Nat Genet. 2009;41:1094–1099. doi: 10.1038/ng.439. [DOI] [PubMed] [Google Scholar]
- Lesné S, Koh MT, Kotilinek L, Kayed R, Glabe CG, Yang A, Gallagher M, Ashe KH. A specific amyloid-β protein assembly in the brain impairs memory. Nature. 2006;440:352–357. doi: 10.1038/nature04533. [DOI] [PubMed] [Google Scholar]
- Li S, Hong S, Shepardson NE, Walsh DM, Shankar GM, Selkoe D. Soluble oligomers of amyloid β protein facilitate hippocampal long-term depression by disrupting neuronal glutamate uptake. Neuron. 2009;62:788–801. doi: 10.1016/j.neuron.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahley RW, Weisgraber KH, Huang Y. Apolipoprotein E: structure determines function, from atherosclerosis to Alzheimer's disease to AIDS. J Lipid Res. 2009;50:S183–S188. doi: 10.1194/jlr.R800069-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morikawa M, Fryer JD, Sullivan PM, Christopher EA, Wahrle SE, DeMattos RB, O'Dell MA, Fagan AM, Lashuel HA, Walz T, Asai K, Holtzman DM. Production and characterization of astrocyte-derived human apolipoprotein E isoforms from immortalized astrocytes and their interactions with amyloid-β. Neurobiol Dis. 2005;19:66–76. doi: 10.1016/j.nbd.2004.11.005. [DOI] [PubMed] [Google Scholar]
- Naiki H, Gejyo F, Nakakuki K. Concentration-dependent inhibitory effects of apolipoprotein E on Alzheimer's β-amyloid fibril formation in vitro. Biochemistry. 1997;36:6243–6250. doi: 10.1021/bi9624705. [DOI] [PubMed] [Google Scholar]
- Näslund J, Thyberg J, Tjernberg LO, Wernstedt C, Karlström AR, Bogdanovic N, Gandy SE, Lannfelt L, Terenius L, Nordstedt C. Characterization of stable complexes involving apolipoprotein E and the amyloid β peptide in Alzheimer's disease brain. Neuron. 1995;15:219–228. doi: 10.1016/0896-6273(95)90079-9. [DOI] [PubMed] [Google Scholar]
- Rebeck GW, Reiter JS, Strickland DK, Hyman BT. Apolipoprotein E in sporadic Alzheimer's disease: allelic variation and receptor interactions. Neuron. 1993;11:575–580. doi: 10.1016/0896-6273(93)90070-8. [DOI] [PubMed] [Google Scholar]
- Riddell DR, Zhou H, Atchison K, Warwick HK, Atkinson PJ, Jefferson J, Xu L, Aschmies S, Kirksey Y, Hu Y, Wagner E, Parratt A, Xu J, Li Z, Zaleska MM, Jacobsen JS, Pangalos MN, Reinhart PH. Impact of apolipoprotein E (ApoE) polymorphism on brain apoE levels. J Neurosci. 2008;28:11445–11453. doi: 10.1523/JNEUROSCI.1972-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selkoe DJ. Alzheimer's disease: genes, proteins and therapy. Physiol Rev. 2001;81:741–766. doi: 10.1152/physrev.2001.81.2.741. [DOI] [PubMed] [Google Scholar]
- Shankar GM, Li S, Mehta TH, Garcia-Munoz A, Shepardson NE, Smith I, Brett FM, Farrell MA, Rowan MJ, Lemere CA, Regan CM, Walsh DM, Sabatini BL, Selkoe DJ. Amyloid-β-protein dimers isolated directly from Alzheimer's brains impair synaptic plasticity and memory. Nat Med. 2008;14:837–842. doi: 10.1038/nm1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strittmatter WJ, Saunders AM, Schmechel D, Pericak-Vance M, Enghild J, Salvesen GS, Roses AD. Apolipoprotein E: high-avidity binding to β-amyloid and increased frequency of type 4 allele in late-onset familial Alzheimer disease. Proc Natl Acad Sci U S A. 1993a;90:1977–1981. doi: 10.1073/pnas.90.5.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strittmatter WJ, Weisgraber KH, Huang DY, Dong LM, Salvesen GS, Pericak-Vance M, Schmechel D, Saunders AM, Goldgaber D, Roses AD. Binding of human apolipoprotein E to synthetic amyloid β peptide: isoform-specific effects and implication for late-onset Alzheimer disease. Proc Natl Acad Sci U S A. 1993b;90:8098–8102. doi: 10.1073/pnas.90.17.8098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend M, Shankar GM, Mehta T, Walsh DM, Selkoe DJ. Effects of secreted oligomers of amyloid β-protein on hippocampal synaptic plasticity: a potent role for trimers. J Physiol. 2006;572:477–492. doi: 10.1113/jphysiol.2005.103754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Helmond Z, Miners JS, Kehoe PG, Love S. Higher soluble amyloid β concentration in frontal cortex of young adults than in normal elderly or Alzheimer's disease. Brain Pathol. 2010;20:787–793. doi: 10.1111/j.1750-3639.2010.00374.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu HY, Hudry E, Hashimoto T, Kuchibhotla K, Rozkalne A, Fan Z, Spires-Jones TL, Xie H, Arbel-Ornath M, Grosskreutz CL, Bacskai BJ, Hyman BT. Amyloid β induces the morphological neurodegenerative triad of spine loss, dendritic simplification, and neuritic dystrophies through calcineurin activation. J Neurosci. 2010;30:2636–2649. doi: 10.1523/JNEUROSCI.4456-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada K, Yabuki C, Schubert P, Schenk D, Hori Y, Ohtshuki S, Terasaki T, Hashimoto T, Iwatsubo T. Aβ immunotherapy: Intracerebral sequestration of Aβ by an anti-Aβ monoclonal antibody 266 with high affinity to soluble Aβ. J Neurosci. 2009;29:11393–11398. doi: 10.1523/JNEUROSCI.2021-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye S, Huang Y, Müllendorff K, Dong L, Giedt G, Meng EC, Cohen FE, Kuntz ID, Weisgraber KH, Mahley RW. Apolipoprotein (apo) E4 enhances amyloid β peptide production in cultured neuronal cells: ApoE structure as a potential therapeutic target. Proc Natl Acad Sci U S A. 2005;102:18700–18705. doi: 10.1073/pnas.0508693102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zannis VI, Breslow JL, Utermann G, Mahley RW, Weisgraber KH, Havel RJ, Goldstein JL, Brown MS, Schonfeld G, Hazzard WR, Blum C. Proposed nomenclature of apoE isoproteins, apoE genotypes, and phenotypes. J Lipid Res. 1982;23:911–914. [PubMed] [Google Scholar]